Abstract
An experiment was conducted in spring 2024 to investigate the effects of biochar, biogas slurry, and dicyandiamide (DCD) on N2O emissions from soil in protected tomato cultivation. Five treatments were applied: conventional fertilization (CK1), biogas slurry alone (CK2), biochar combined with biogas slurry (T1), DCD combined with biogas slurry (T2), and the combination of biochar, biogas slurry, and DCD (T3). The study aimed to assess the response of the soil physicochemical properties and nitrifying ammonia-oxidizing microorganisms in the tomato root zone to these treatments and to determine their impact on soil N2O emissions. The results showed that adding biochar and biogas slurry increased the soil pH, organic matter content, and levels of nitrate-N and ammonium-N, without affecting ammonia-oxidizing archaea (AOA) but inhibiting ammonia-oxidizing bacteria (AOB). The inclusion of DCD raised the soil pH and ammonium-N levels, enhanced AOA growth, did not alter organic matter content, and significantly reduced nitrate-N levels and AOB activity. Compared to CK1, treatments CK2, T1, T2, and T3 decreased the average N2O emission flux by 5.83%, 8.24%, 15.27%, and 16.16%, respectively. The application of biochar, biogas slurry, and DCD enhanced the physicochemical properties of the root zone soil and notably reduced N2O emissions in protected tomato cultivation, with T3 showing the most effective results. The biochar and biogas slurry used in this study, both derived from agricultural waste, promote sustainable agricultural development and enhance economic benefits. However, this study only considered the short-term effects of biochar, biogas slurry, and DCD, necessitating further research to explore their long-term impacts and mechanisms.
1. Introduction
Climate change represents a significant threat to human health and ecological systems, attracting worldwide scholarly attention [1]. Agricultural ecosystems are major sources of greenhouse gas emissions, accounting for 21–37% of the total [2]. Consequently, the reduction in greenhouse gases from agricultural sources has become a prevalent topic of international and domestic research. In these ecosystems, the widespread use of nitrogen fertilizers leads to increased emissions of nitrous oxide (N2O) [3]. Unlike open-field farming, protected vegetable cultivation is characterized by its high intensification and substantial economic advantages. However, the rate of nitrogen fertilizer application in protected cultivation is 3–4 times higher than in cereal crops [4], and water usage is 2–7 times greater [5]. The continuous overuse of nitrogen fertilizers results in soil acidification and salinization [6,7] and significantly heightens N2O emissions [8], making protected vegetable cultivation especially vulnerable to higher N2O emission risks compared to open-field farming.
Prior studies have indicated that organic fertilization, as opposed to no fertilization or inorganic fertilization, increased N2O emissions by 208.14% and decreased them by −3.66%, respectively [9]. Biogas slurry, a liquid organic fertilizer with over 90% water content and low nutrient concentration, is rich in essential plant nutrients such as nitrogen, phosphorus, and potassium, along with proteins, amino acids, and other beneficial substances [10]. The use of biogas slurry also helps retain other soil nutrients, moderates soil pH, boosts microbial activity, and mitigates soil heavy metal toxicity [11]. Nevertheless, reports are scarce regarding the effects of biogas slurry on N2O emissions in agricultural fields.
Biochar is a solid product obtained through high-temperature pyrolysis of organic materials under anaerobic or oxygen-limited conditions [12]. It is characterized by a high carbon content and resistance to microbial degradation, making it suitable for increasing soil carbon stocks [13]. It has been applied in various fields such as agricultural soil and environmental ecology [14,15]. Research on biochar’s effect on N2O emissions has produced mixed results, including promotion [16,17], no effect [18], and inhibition [19,20]. Dong [21] demonstrated that increasing the amount of biochar added to soil correspondingly increases the reduction in soil N2O emissions, achieving maximum effect at an addition rate of 40 tons per hectare. Comparative studies [22] on the impact of biochar on N2O emissions from acidic and alkaline soils have shown that corn cob biochar can decrease N2O emissions from acidic soils by 26.9%, while olive fruit biochar can reduce emissions by 68.4% in acidic soils and 34.3% in alkaline soils. Adding biochar made from spruce debris, particularly under soil moisture conditions ranging from 20% to 50%, can increase N2O emissions in soils where legumes are planted [23]. Furthermore, if the pyrolysis syngas is not purified, biochar production may emit N2O, with emission levels approximately 2% to 4% of the nitrogen [21]. In soils with low organic carbon content (5 g/kg), the impact of biochar on N2O emissions is minimal and statistically insignificant due to the limited carbon available for heterotrophic processes, which promotes nitrification in low-organic-carbon soils [21]. Biochar produced from manure or pyrolyzed at temperatures below 350 °C has a negligible effect on reducing soil N2O emissions, due to its weaker aromatic ring structure, reduced surface area, lower electrical conductivity, and fewer surface functional groups [21]. In summary, the impact of biochar on soil N2O emissions is influenced by various factors, including the soil and climatic conditions of the study area, experimental conditions (laboratory/field) and duration, type of biochar (feedstock source), application rate, and pyrolysis conditions. These varying outcomes highlight the need for additional research on biochar’s efficacy in mitigating N2O emissions and its potential impacts under different soil physicochemical properties. DCD is a nitrification inhibitor that effectively reduces the conversion rate of ammonium nitrogen to nitrate nitrogen, thus diminishing the production and accumulation of and consequently decreasing leaching and denitrification losses [24]. Furthermore, DCD has been shown to reduce N2O emissions by 30.4% according to a meta-analysis [25], although some studies indicate that it does not significantly affect N2O emissions in protected vegetable fields [26]. Current research is focused on the combined application of biochar and DCD [27], but the effects of their co-application with biogas slurry on N2O emissions remain to be clarified.
Therefore, this study uses soil from a tomato greenhouse as the subject. By establishing treatments of traditional fertilization (CK1), biogas slurry (CK2), biochar + biogas slurry (T1), DCD + biogas slurry (T2), and biochar + biogas slurry + DCD (T3), it aims to investigate the response patterns of soil physicochemical properties and nitrifying microorganisms in the root zone of greenhouse tomatoes under these treatments. The objective is to elucidate the effects of the combined application of biochar, biogas slurry, and DCD on soil N2O emissions, providing a theoretical foundation for reducing N2O emissions in greenhouse tomato production.
2. Materials and Methods
2.1. Overview of the Research Area
The experiment was conducted from March to July 2024 in a greenhouse on Gouyashan Mountain, Weiling Township, Qilihe District, Lanzhou City, Gansu Province (104°13′35′′ E, 36°58′12′′ N). The region experiences a temperate semi-arid climate, with an average annual temperature of 9.6 °C and precipitation of 344 mm, at an altitude of 1871.6 m. The greenhouse, oriented east–west, measures 50 m in length, 11 m in width, and 4.5 m in height. The soil’s physical and chemical properties in the experimental site’s top 50 cm layer are detailed in Table 1.

Table 1.
Physicochemical properties of soil in the study area.
2.2. Experimental Materials
The tomato variety “Fenyan 347” was used. The biogas slurry, derived from the anaerobic fermentation of vegetable residuals by Lanzhou Xinsu Eco-Energy Co., Ltd. (Lanzhou, China), contained less than 2% total solids, with a pH of 8.06, organic matter content of 1.7 g·kg−1, total nitrogen of 0.956 g·kg−1, phosphorus of 0.054 g·kg−1, and potassium of 1.588 g·kg−1. The biochar used in the experiment was straw-derived biochar, with the following physicochemical properties: fixed carbon of 650 g·kg⁻¹, available phosphorus of 10.2 g·kg−1, potassium of 55.65 g·kg−1, a bulk density of 0.19 g·cm−3, a specific surface area of 9 m2·g−1, total porosity of 67.03%, air-filled porosity of 12.87%, water-holding porosity of 61.10%, a pH of 10.24, and cation exchange capacity of 60.8 mol·kg−1. DCD (67%N) was used as an analytically pure reagent.
2.3. Experimental Design
The field experiment spanned from 13 March to 10 July 2024, divided into three growth stages: seedling, flowering and fruiting, and fruit ripening. Five treatments were implemented: conventional fertilization (CK1), biogas slurry (CK2), biochar + biogas slurry (T1), DCD + biogas slurry (T2), and biochar + biogas slurry + DCD (T3). Each utilized single-ridge plastic film covering technology, with ridges 20 cm high and row spacing of 60 cm, 8 seedlings per ridge at 50 cm apart, and replicated three times in a randomized block design. To prevent water infiltration, a 1 m deep impermeable geomembrane was installed around the plots. The integrated water–biogas slurry hole irrigation method involved two holes of 5 cm diameter and 7 cm depth, positioned 5 cm from the root zone on each side of the ridge [28]. The total fertilization was consistent across treatments, with nitrogen applied at 390 kg·hm−2 [29]. The initial application included 130 kg·hm−2 of nitrogen for each treatment. For CK1, potassium and phosphorus were applied at 216 kg·hm−2 and 7 kg·hm−2, respectively, with subsequent amounts applied in two topdressings. For other treatments, deficiencies were supplemented with biogas slurry. Biochar was added at 2% soil weight, and DCD at 10% of the total nitrogen used. The irrigation volume for each treatment was calculated using the formula:
In the formula, KP is the pan coefficient, set at 0.85; A is the root zone area, set at 1800 cm2 (30 cm × 60 cm); and EP is the evaporation from the pan (cm). Irrigation was conducted daily. During this period, for all treatments except CK1, the water requirement of the crops was adjusted by subtracting the water content in the biogas slurry from the total irrigation volume to ensure uniform irrigation across all treatments.
W = KP × A × EP.
2.4. Measurement Indicators and Methods
2.4.1. Collection and Measurement of N2O Gas
Soil N2O was collected using a static chamber constructed from 5 mm thick PVC, measuring 32 cm in diameter and 40 cm in height. The chamber was insulated with sponge and covered with reflective film. A thermometer and a fan were installed at the top to measure the temperature and circulate the gas, respectively. The chamber base was embedded in the ridge on the day of tomato transplantation, devoid of crops. The base featured a 3 cm deep groove for placing the chamber body, sealed with water during sampling. Samples were taken on the 1st, 3rd, and 7th days and then biweekly between 9:00 and 10:00 AM, using a syringe fitted with a three-way valve to collect 60 mL of gas. Gas concentrations were analyzed using an Agilent gas chromatograph on the same day, and the results were converted into N2O gas emission flux using the formula:
where F is the N2O gas emission flux (μg N2O-N m−2·h−1); ρ is the gas density under standard conditions (mg·m−3); T is the temperature inside the static chamber during sampling (°C); dC/dt is the rate of concentration change over time; and h is the height of the static chamber (m).
F = ρ × h × 273.15/(273.15 + T)dC/dt,
2.4.2. Physicochemical Properties of Soil
Three representative plants from each treatment were selected at every growth stage. Soil samples were extracted using a soil auger from five different depths, each 10 cm thick, located 10 cm from the plant stem base along the vertical ridge. Samples were air-dried, ground, and sieved to determine the pH and organic matter content, among other parameters. Organic matter was quantified using the potassium dichromate method; soil pH was measured with a DDSJ-308F detector; nitrate nitrogen and ammonium nitrogen levels were determined using a spectrophotometer.
2.4.3. Measurements of Ammonia-Oxidizing Microorganisms in Soil
The gene copy numbers of ammonia-oxidizing archaea (AOA) and bacteria (AOB) were quantified using real-time fluorescent quantitative PCR (qPCR). Soil DNA was extracted using the Fast DNA Spin Kit (MP Biomedicals, LLC) and assessed for quality via 1% agarose gel electrophoresis. Qualified samples were preserved at −80 °C. The gene copy numbers for AOA and AOB were measured using a LineGene9600plus PCR instrument (Bioer Technology, Hangzhou, China), with genes amplified on the Illumina NovaSeq PE250 platform (Auwisen, China).
2.5. Statistical Analysis
Experimental data were organized and analyzed using Excel 2021; graphs were generated using OriginPro 2024.
3. Results
3.1. Soil pH Value
As illustrated in Figure 1, the pH trends for tomato plants remained consistent across all treatments throughout the growth period, with pH values initially rising then falling with increased soil depth, peaking in the 10–20 cm layer. At the same depth, the pH decreased in the order of T3 > T1 > T2 > CK2 > CK1. Compared to CK1, increases in soil pH were observed in CK2, T1, T2, and T3 by 0.12–0.13, 0.14–0.17, 0.17–0.18, and 0.20–0.21, respectively, during each growth stage. Furthermore, the pH in T1, T2, and T3 was 0.02–0.04, 0.04–0.05, and 0.07–0.09 higher than in CK2, respectively. These findings indicate that the application of biochar, biogas slurry, and DCD tends to elevate soil pH in the crop root zone.

Figure 1.
Soil pH in the tomato root zone.
3.2. Soil Organic Matter
As depicted in Figure 2, the soil organic matter content at various depths increased throughout the tomato growth period, peaking in the 10–20 cm layer. Compared to CK1, increases in the 10–20 cm layer’s organic matter content were 38.037–49.849% and 45.405–52.904% for T1 and T3, respectively. CK2 and T2 also showed increases of 24.056–27.695% and 20.081–24.415%, respectively. These results demonstrate that the combined application of biochar and biogas slurry significantly enhances the soil organic matter content, with less pronounced effects observed from the sole application of biogas slurry (CK2). The addition of DCD had no significant impact on the soil organic matter content.
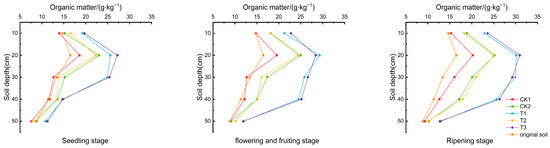
Figure 2.
Organic matter content in the soil of the tomato root zone.
3.3. NH4+-N
As shown in Figure 3, the content at various soil depths increased throughout the tomato growth period, reaching its highest levels in the 10–20 cm layer. The ranking of the content from highest to lowest was T3 > T2 > T1 > CK2 > CK1. Compared to CK1, the increases in content in the 10–20 cm layer were 5.693–6.21%, 6.313–15.106%, 29.791–23.737%, and 21.459–29.105% for CK2, T1, T2, and T3, respectively. These findings suggest that the use of biochar, biogas slurry, and DCD enhances the accumulation of in the soil.
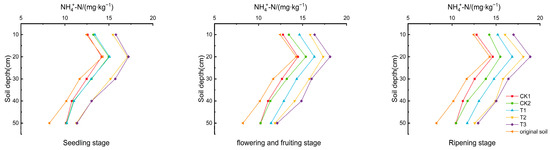
Figure 3.
content in the soil of the tomato root zone.
3.4. NO3−-N
As depicted in Figure 4, throughout the tomato growth period, content in the soil at various depths showed a pattern of increase followed by a decrease, peaking at a depth of 10–20 cm. Compared to CK1, the content in the 10–20 cm layer decreased by 37.402–40.085% and 38.232–41.795% in T2 and T3, respectively, while it increased by 2.972–6.335% and 2.803–7.766% in CK2 and T1, respectively. These results suggest that biochar and biogas slurry enhance the accumulation of , whereas DCD significantly inhibits it.
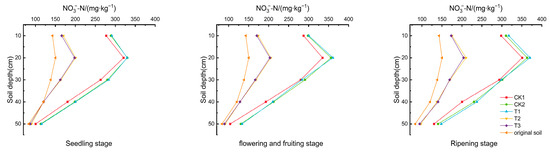
Figure 4.
content in the soil of the tomato root zone.
3.5. Ammonia-Oxidizing Microorganisms
Figure 5 illustrates that the functional gene copy numbers of AOA and AOB varied among treatments, ranging from 1.83 × 105(g−1 dry soil) to 3.38 × 105 (g−1 dry soil) for AOA and 1.24 × 105 (g−1 dry soil) and 3.12 × 105 (g−1 dry soil) for AOB. Compared to CK1, CK2 and T1 had no significant impact on AOA but reduced AOB by 6.40–10.42% and 5.89–10.89%, respectively. Conversely, T2 and T3 increased AOA by 37.707–40.628% and 38.073–44.440%, respectively, while reducing AOB by 54.792–55.035% and 54.605–55.495%, respectively. This indicates that while biochar and biogas slurry had no significant effect on AOA, they reduced AOB, and DCD increased AOA but decreased AOB.
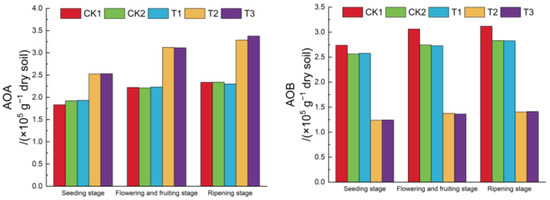
Figure 5.
Changes in the copy numbers of functional genes of AOA and AOB.
3.6. N2O Emission Flux
As shown in Figure 6, the N2O emission flux across treatments fluctuated over time, initially increasing and then decreasing, with distinct phases corresponding to the growth stages of the tomatoes. The emission flux ranged from 64.29 to 180.44 μg·m−2·h−1 throughout the growth period. The N2O emission was lower during the seedling stage, likely due to lower temperatures, increased during the flowering and fruit-setting stage, and decreased during the fruit ripening stage. Following top-dressing, a significant peak in emissions was observed. The order of N2O emission peaks among the treatments was CK1 > CK2 > T1 > T2 > T3, with T3 having the lowest average emission at only 89.08 μg·m−2·h−1. Compared to CK1, the average N2O emission flux of CK2, T1, T2, and T3 decreased by 5.83%, 8.24%, 15.27%, and 16.16%, respectively. These findings indicate that biochar, biogas slurry, and DCD exert inhibitory effects on N2O emissions, with DCD demonstrating a more pronounced effect.
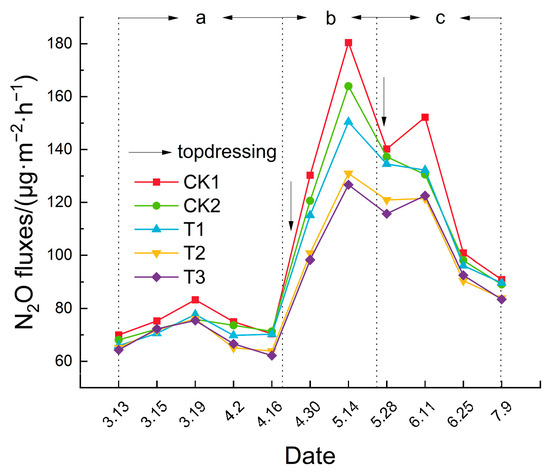
Figure 6.
Variation curve of soil N2O emission flux (where a, b, and c in the figure represent the seedling stage, flowering and fruiting stage, and ripening stage, respectively).
4. Discussion
4.1. The Effects of Biochar, Biogas Slurry, and DCD Application on Soil Physicochemical Properties
Soil, a critical component of ecosystems, influences the growth, species diversity, and nutritional status of microorganisms and plants [30,31]. Its physicochemical properties significantly affect N2O emissions and the abundance of nitrifying bacterial genes [32]. This study demonstrated that the addition of biochar and biogas slurry increases the pH, organic matter content, and the contents of soil nitrate and ammonium nitrogen, aligning with previous findings [33,34]. The inherently high pH values of biogas slurry and biochar effectively inhibit soil acidification. Biochar’s porous structure and large specific surface area provide strong adsorption capabilities for organic matter, , and , reducing the leaching of these components [35]. Biogas slurry, containing organic matter, , and , when used with biochar, enhances the soil nutrient utilization efficiency, decreases the nutrient loss, and increases the content of soil organic matter, , and . This combination effectively retains organic matter, , and in the soil of the crop root zone. Treatments with DCD (T2, T3) exhibited higher levels and lower levels throughout all growth stages, consistent with prior research [36]. This is primarily due to DCD’s ability to inhibit the initial stage of nitrification, where is oxidized to , thus slowing the acidification process, increasing pH and levels, and reducing levels [33].
4.2. The Effects of Biochar, Biogas Slurry, and DCD Application on N2O Emissions
This study demonstrated that the addition of biochar (T1) reduced the average N2O emission flux by 7.052% compared to traditional fertilization (CK1). This reduction may be attributed to biochar facilitating the conversion of N2O to N2, a process associated with the nosZ gene in soil [37]. In neutral and alkaline soils, the nosZ gene regulates N2O emissions. The N2O reductase encoded by this gene converts N2O to N2 during denitrification [38]. Biochar also adsorbs , the substrate for soil nitrification, thereby decreasing its availability and reducing N2O emissions from the soil [39]. The addition of biogas slurry (CK2) also inhibited N2O emission flux, achieving a reduction of 5.83% compared to CK1. This effect is primarily due to the substitution of part of the nitrogen fertilizer with biogas slurry, which reduces the copy number of AOB functional genes. A lower level of AOB is a critical factor in decreasing N₂O emissions [40]. Furthermore, the addition of DCD (T2, T3) significantly impacted N2O emission flux. During the growth stages, levels were high and increased as the tomatoes matured. The copy number of AOA functional genes was significantly higher, while that of AOB was considerably lower compared to traditional fertilization. This indicates that AOB, the primary driver of nitrification, is inhibited by DCD. A high-ammonium environment inhibits AOA function, and while some AOA can oxidize ammonia in environments with a pH below 5.0 [41], such acidic conditions prevent AOB from undergoing normal growth and metabolism [42].
This study was conducted under greenhouse conditions, not in outdoor field trials. Differences in biochar characteristics, experimental conditions, soil properties, and the long-term weathering of biochar and soil may result in discrepancies between the results of outdoor field trials and greenhouse studies. Additionally, this study only addressed the short-term effects of biochar and DCD on N2O emissions, without assessing their long-term impacts. Future research should focus on the long-term effects of biochar and DCD application on N2O emissions in outdoor field settings. Furthermore, determining the lifespan of biochar and DCD is crucial to provide a theoretical basis for N2O emission reduction.
5. Conclusions
- The addition of biochar and biogas slurry increased the soil pH, organic matter, ammonium nitrogen, and nitrate nitrogen contents in the tomato root zone’s soil layer, while decreasing the copy number of AOB functional genes without affecting those of AOA. The introduction of DCD raised the soil pH, ammonium nitrogen, and the copy number of AOA functional genes in the 0–50 cm soil layer of the tomato root zone, while lowering the nitrate nitrogen content and the copy number of AOB functional genes, without impacting organic matter.
- The application of biochar, biogas slurry, and DCD effectively reduced N2O emissions. DCD exerted the most significant effect on reducing N2O emissions, followed by biochar, with biogas slurry having the least impact. The T3 treatment, combining biochar, biogas slurry, and DCD, yielded the best results.
Author Contributions
Conceptualization, J.Z.; methodology, Z.L.; software, Q.S. and Y.W.; validation, J.Z. and Z.L.; formal analysis, Z.L.; investigation, Z.L.; resources, Y.W.; data curation, Z.L.; writing—original draft preparation, Z.L.; writing—review and editing, J.Z.; visualization, Q.S., Y.W.; supervision, J.Z.; project administration, J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52469011, 51969012.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the NSFC for its support.
Conflicts of Interest
The authors declare no conflicts of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Kang, S.; Eltahir, E.A.B. North China Plain threatened by deadly heatwaves due to climate change and irrigation. Nat. Commun. 2018, 9, 2894. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Q.; Guo, J.; Li, C.; Li, J.; Niu, K.; Jin, S.; Fu, C.; Gaffney, P.P.J.; Xu, Y.; et al. Inequality in agricultural greenhouse gas emissions intensity has risen in rural China from 1993 to 2020. Nat. Food 2024, 5, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]
- Wu, L.; Wu, X.; Lin, S.; Wu, Y.; Tang, S.; Zhou, M.; Shaaban, M.; Zhao, J.; Hu, R.; Kuzyakov, Y.; et al. Carbon budget and greenhouse gas balance during the initial years after rice paddy conversion to vegetable cultivation. Sci. Total Environ. 2018, 627, 46–56. [Google Scholar] [CrossRef]
- Xin, Y.; Tao, F. Developing climate-smart agricultural systems in the North China Plain. Agric. Ecosyst. Environ. 2020, 291, 106791. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberan, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ. Sci. Pollut. Res. Int. 2015, 22, 2976–2986. [Google Scholar] [CrossRef]
- Homyak, P.M.; Kamiyama, M.; Sickman, J.O.; Schimel, J.P. Acidity and organic matter promote abiotic nitric oxide production in drying soils. Glob. Chang. Biol. 2017, 23, 1735–1747. [Google Scholar] [CrossRef]
- Sa, Q.; Zheng, J.; Li, Z.; Wang, Y. A Meta-analysis of the Impact of Organic Fertilizer Application on Greenhouse Gas Emissions from Global Agricultural Soils. Environ. Sci. 2025, 46, 148–161. [Google Scholar] [CrossRef]
- Zhong, Y.; Ragauskas, A.J.; Zheng, Y.; Meng, X.; Zhou, Y.; Lin, Y. A review on the pretreatment of straw biomass by using biogas slurry. Process Saf. Environ. Prot. 2025, 195, 106843. [Google Scholar] [CrossRef]
- Zhou, W.; Qi, Y.; Xiao, N. Research progress and development suggestions on harmless treatment and resource utilization of biogas slurry. Trans. Chin. Soc. Agric. Eng. 2018, 34, 115–122. [Google Scholar]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The ‘Terra Preta’ phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, X.; Gao, Y.; Liu, G.; Liu, Z.; Zhang, Q.; Liu, E.; Sun, S.; Ren, X.; Jia, Z.; et al. Environment and agricultural practices regulate enhanced biochar-induced soil carbon pools and crop yield: A meta-analysis. Sci. Total Environ. 2023, 905, 167290. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Saarnio, S.; Heimonen, K.; Kettunen, R. Biochar addition indirectly affects N2O emissions via soil moisture and plant N uptake. Soil Biol. Biochem. 2013, 58, 99–106. [Google Scholar] [CrossRef]
- Verhoeven, E.; Six, J. Biochar does not mitigate field-scale N2O emissions in a Northern California vineyard: An assessment across two years. Agric. Ecosyst. Environ. 2014, 191, 27–38. [Google Scholar] [CrossRef]
- Yamamoto, A.; Akiyama, H.; Kojima, M.; Osaki, A. Nitrous oxide emissions from an Andosol upland field amended with four different types of biochars. Nutr. Cycl. Agroecosystems 2019, 113, 323–335. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Stott, A.W.; Grant, H.K.; Whitaker, J. Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biol. Biochem. 2015, 81, 178–185. [Google Scholar] [CrossRef]
- Lentz, R.D.; Ippolito, J.A.; Spokas, K.A. Biochar and Manure Effects on Net Nitrogen Mineralization and Greenhouse Gas Emissions from Calcareous Soil under Corn. Soil Sci. Soc. Am. J. 2014, 78, 1641–1655. [Google Scholar] [CrossRef]
- Cheng, D.; ZhiYong, C.; Xie, Y.X.; YangYang, Z.; Gou, P.X.; JiaHeng, Y.; DongYun, M.A.; ChenYang, W.; Guo, T.C. Effects of Successive Biochar Addition to Soil on Nitrogen Functional Microorganisms and Nitrous Oxide Emission. Sci. Agric. Sin. 2020, 53, 4024–4034. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.-M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Pan, G.; Hussain, Q.; Li, L.; Zheng, J.; Zhang, X. Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 2012, 351, 263–275. [Google Scholar] [CrossRef]
- Fan, C.; Wang, D.; Duan, P.; Gao, W.; Liu, Y.; Wu, X.; Liu, H.; Ning, Z.; Li, Q.; Chen, M. Mechanistic insights into mitigating N2O emissions by the nitrification inhibitor dicyandiamide (DCD) in a tropical sandy soil after six years of manure amendment. Pedosphere 2024, in press. [Google Scholar] [CrossRef]
- Gao, J.; Luo, J.; Lindsey, S.; Shi, Y.; Wang, L. Benefits and Risks for the Environment and Crop Production with Application of Nitrification Inhibitors in China. J. Soil Sci. Plant Nutr. 2021, 21, 497–512. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, C.H.; Li, Q.L.; Li, B.; Zhu, Y.Y.; Xiong, Z.Q. A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agric. Ecosyst. Environ. 2015, 201, 43–50. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, W.; Wen, J.; Wu, R.; Wu, J.; Zhang, W.; Zhang, M. Non-additive effects of bamboo-derived biochar and dicyandiamide on soil greenhouse gas emissions, enzyme activity and bacterial community. Ind. Crops Prod. 2023, 194, 116385. [Google Scholar] [CrossRef]
- He, S.; Zheng, J. Effects of different water/biogas slurry integrated irrigation methods on tomato growth, yield and quality. China Rural. Water Hydropower 2022, 118–122+129. [Google Scholar]
- Shan, N.; Chuan, L.; Li, M.; Liu, J. Effect of fertilizer application recommended by nutrient expert system on tomatoes. China Cucurbits Veg. 2022, 35, 45–50. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef]
- Oyonarte, C.; Aranda, V.; Durante, P. Soil surface properties in Mediterranean mountain ecosystems: Effects of environmental factors and implications of management. For. Ecol. Manag. 2008, 254, 156–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, T.; Zhang, G.; Bahn, M.; Mo, F.; Han, J. Biochar strategy for long-term N2O emission reduction: Insights into soil physical structure and microbial interaction. Soil Biol. Biochem. 2025, 202, 109685. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Dempster, D.N.; Jones, D.L.; Murphy, D.V. Clay and biochar amendments decreased inorganic but not dissolved organic nitrogen leaching in soil. Soil Res. 2012, 50, 216. [Google Scholar] [CrossRef]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Han, S.; Zeng, L.; Luo, X.; Xiong, X.; Wen, S.; Wang, B.; Chen, W.; Huang, Q. Shifts in Nitrobacter- and Nitrospira-like nitrite-oxidizing bacterial communities under long-term fertilization practices. Soil Biol. Biochem. 2018, 124, 118–125. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the Effects of Biochar on Manure Composting: Evidence Supporting the Relationship between N2O Emission and Denitrifying Community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- Harter, J.; Weigold, P.; El-Hadidi, M.; Huson, D.H.; Kappler, A.; Behrens, S. Soil biochar amendment shapes the composition of N2O-reducing microbial communities. Sci. Total Environ. 2016, 562, 379–390. [Google Scholar] [CrossRef]
- Hyodo, A.; Malghani, S.; Zhou, Y.; Mushinski, R.M.; West, J.B. Biochar amendment suppresses N2O emissions but has no impact on 15N site preference in an anaerobic soil. Rapid Commun. Mass Spectrom. 2019, 33, 165–175. [Google Scholar] [CrossRef]
- Shi, Y.; Rahaman, M.A.; Zhang, Q.; Zhan, X.; Zheng, L. Effects of partial substitution of chemical fertilizer with biogas slurry on nitrous oxide emissions and the related nitrifier and denitrifier in a saline–alkali soil. Environ. Technol. Innov. 2022, 28, 102900. [Google Scholar] [CrossRef]
- Jia, Z.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E.; Stoecker, K.; Vilcinskas, A.; Prosser, J.I.; Nicol, G.W. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl. Acad. Sci. USA 2011, 108, 15892–15897. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).