Impact of Nodulation Efficiency and Concentrations of Soluble Sugars and Ureides on Soybean Water Deficit During Vegetative Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Experimental Design and Imposition of Treatments
2.3. Water Status, Specific Leaf Area and Chlorophyll Index
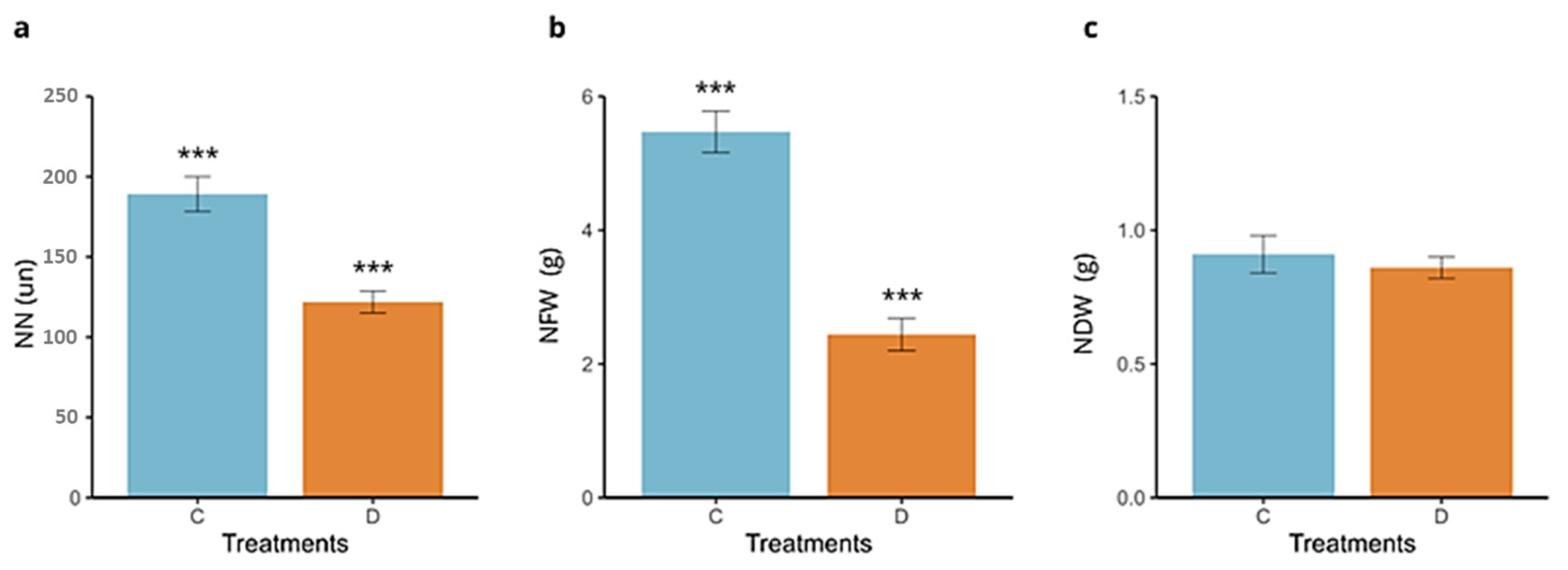
2.4. Nodulation
2.5. Concentration of Total Soluble Sugars in Leaves and Roots
2.6. Concentration of Ureides in Leaves and Roots (Nodules)
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic Stress Responses in Legumes: Strategies Used to Cope with Environmental Challenges. Crit. Rev. Plant Sci. 2014, 34, 237–280. [Google Scholar] [CrossRef]
- Hirakuri, M.H. Economic losses generated by biotic and abiotic stresses in Brazilian soybean production in the 2016-2020 period. Embrapa Centro Nacional de Pesquisa de Soja, Londrina, PR, 169, 7, 2021. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1131745 (accessed on 27 July 2023).
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2017, 24, 37–50. [Google Scholar] [CrossRef]
- CONAB—Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Grãos, Brasília, DF, 10, safra 2022/23, 7, 2023. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos/item/download/47017_2cc607edd5428e14b76bd6e988cd358c (accessed on 27 April 2023).
- Rodrigues, R.d.A.R.; de Mello, W.Z.; da Conceição, M.C.G.; de Souza, P.A.; da Silva, J.J.N. Nitrogen Dynamics in Tropical Agricultural and Forest Systems and their Impact on Climate Change. Rev. Virtual De Quimica 2017, 9, 1868–1886. [Google Scholar] [CrossRef]
- Guo, K.; Yang, J.; Yu, N.; Luo, L.; Wang, E. Biological nitrogen fixation in cereal crops: Progress, strategies, and perspectives. Plant Commun. 2022, 4, 100499. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Mendes, I.C. A importância Do Processo de Fixação Biológica Do Nitrogênio Para a Cultura da Soja: Componente Essencial Para a Competitividade Do Produto Brasileiro. Embrapa Soja-Documentos (INFOTECA-E), 2007. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/468512?locale=en (accessed on 27 April 2023).
- Zilli, J.; Pacheco, R.S.; Gianluppi, V.; Smiderle, O.J.; Urquiaga, S.; Hungria, M. Biological N2 fixation and yield performance of soybean inoculated with Bradyrhizobium. Nutr. Cycl. Agroecosystems 2021, 119, 323–336. [Google Scholar] [CrossRef]
- Telles, T.S.; Nogueira, M.A.; Hungria, M. Economic value of biological nitrogen fixation in soybean crops in Brazil. Environ. Technol. Innov. 2023, 31, 103158. [Google Scholar] [CrossRef]
- Atkins, C.A.; Smith, P.M.C. Translocation in Legumes: Assimilates, Nutrients, and Signaling Molecules. Plant Physiol. 2007, 144, 550–561. [Google Scholar] [CrossRef]
- Lu, S.; Jia, Z.; Meng, X.; Chen, Y.; Wang, S.; Fu, C.; Yang, L.; Zhou, R.; Wang, B.; Cao, Y. Combined Metabolomic and Transcriptomic Analysis Reveals Allantoin Enhances Drought Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 14172. [Google Scholar] [CrossRef]
- Cunha, M.L.O.; de Oliveira, L.C.A.; Silva, V.M.; Montanha, G.S.; dos Reis, A.R. Selenium increases photosynthetic capacity, daidzein biosynthesis, nodulation and yield of peanuts plants (Arachis hypogaea L.). Plant Physiol. Biochem. 2022, 190, 231–239. [Google Scholar] [CrossRef]
- Baral, B.; da Silva, J.A.T.; Izaguirre-Mayoral, M.L. Early signaling, synthesis, transport and metabolism of ureides. J. Plant Physiol. 2016, 193, 97–109. [Google Scholar] [CrossRef]
- Ladeira, R.; Marino, D.; Larrainzar, E.; González, E.M.; Arrese-Igor, C. Reduced Carbon Availability to Bacteroids and Elevated Ureides in Nodules, But Not in Shoots, Are Involved in the Nitrogen Fixation Response to Early Drought in Soybean. Plant Physiol. 2007, 145, 539–546. [Google Scholar] [CrossRef]
- Cerezini, P.; Fagotti, D.d.S.L.; Pípolo, A.E.; Hungria, M.; Nogueira, M.A. Water restriction and physiological traits in soybean genotypes contrasting for nitrogen fixation drought tolerance. Sci. Agricola 2017, 74, 110–117. [Google Scholar] [CrossRef]
- King, C.A.; Purcell, L.C. Inhibition of N2 Fixation in Soybean Is Associated with Elevated Ureides and Amino Acids. Plant Physiol. 2005, 137, 1389–1396. [Google Scholar] [CrossRef]
- Purcell, L.C.; Serraj, R.; Sinclair, T.R.; De, A. Soybean N2 Fixation Estimates, Ureide Concentration, and Yield Responses to Drought. Crop. Sci. 2004, 44, 484–492. [Google Scholar] [CrossRef]
- Cooper, J.E.; Scherer, H.W. Nitrogen Fixation. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: San Diego, CA, USA, 2012; pp. 389–408. [Google Scholar] [CrossRef]
- Cerezini, P.; Kuwano, B.H.; Grunvald, A.K.; Hungria, M.; Nogueira, M.A. Soybean tolerance to drought depends on the associated Bradyrhizobium strain. Braz. J. Microbiol. 2020, 51, 1977–1986. [Google Scholar] [CrossRef]
- Vadez, V.; Sinclair, T.R. Leaf ureide degradation and N2 fixation tolerance to water deficit in soybean1. J. Exp. Bot. 2001, 52, 153–159. [Google Scholar] [CrossRef]
- Schneider, J.R.; Müller, M.; Klein, V.A.; Rossato-Grando, L.G.; Barcelos, R.P.; Dalmago, G.A.; Chavarria, G. Soybean Plant Metabolism under Water Deficit and Xenobiotic and Antioxidant Agent Application. Biology 2020, 9, 266. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van der Drift, C. Degradation of purines and pyrimidines by microorganisms. Bacteriol. Rev. 1976, 40, 403–468. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org (accessed on 1 August 2022).
- Gálvez, L.; González, E.M.; Arrese-Igor, C. Evidence for carbon flux shortage and strong carbon/nitrogen interactions in pea nodules at early stages of water stress. J. Exp. Bot. 2005, 56, 2551–2561. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R.; Purcell, L.C. Symbiotic N2 fixation response to drought. J. Exp. Bot. 1999, 50, 143–155. [Google Scholar]
- Alamillo, J.M.; Díaz-Leal, J.L.; Sánchez-Moran, M.V.; Pineda, M. Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant Cell Environ. 2010, 33, 1828–1837. [Google Scholar] [CrossRef]
- Cerezini, P.; Pípolo, A.E.; Hungria, M.; Nogueira, M.A. Gas Exchanges and Biological Nitrogen Fixation in Soybean under Water Restriction. Am. J. Plant Sci. 2014, 5, 4011–4017. [Google Scholar] [CrossRef]
- Sprent, J.I.E. Root nodule anatomy, type of export product and evolutionary origin in some Leguminosae. Plant Cell Envi-Ronment 1980, 3, 35–43. [Google Scholar] [CrossRef]
- Marquez-Garcia, B.; Shaw, D.; Cooper, J.W.; Karpinska, B.; Quain, M.D.; Makgopa, E.M.; Kunert, K.; Foyer, C.H. Redox markers for drought-induced nodule senescence, a process occurring after drought-induced senescence of the lowest leaves in soybean (Glycine max). Ann. Bot. 2015, 116, 497–510. [Google Scholar] [CrossRef]
- de Freitas, V.F.; Cerezini, P.; Hungria, M.; Nogueira, M.A. Strategies to deal with drought-stress in biological nitrogen fixation in soybean. Appl. Soil Ecol. 2021, 172, 104352. [Google Scholar] [CrossRef]
- Liyanage, D.K.; Torkamaneh, D.; Belzile, F.; Balasubramanian, P.; Hill, B.; Thilakarathna, M.S. The Genotypic Variability among Short-Season Soybean Cultivars for Nitrogen Fixation under Drought Stress. Plants 2023, 12, 1004. [Google Scholar] [CrossRef]
- Gil-Quintana, E.; Larrainzar, E.; Seminario, A.; Díaz-Leal, J.L.; Alamillo, J.M.; Pineda, M.; Arrese-Igor, C.; Wienkoop, S.; González, E.M. Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. J. Exp. Bot. 2013, 64, 2171–2182. [Google Scholar] [CrossRef]
- Vance, C.P. Carbon and nitrogen metabolism in legume nodules. In Nitrogen-Fixing Leguminous Symbioses; Springer: Berlin/Heidelberg, Germany, 2008; Volume 7, pp. 293–320. [Google Scholar] [CrossRef]
- Streeter, J.G. Effects of drought on nitrogen fixation in soybean root nodules. Plant Cell Environ. 2003, 26, 1199–1204. [Google Scholar] [CrossRef]
- Ohyama, T.; Matsumoto, K.; Goto, H.; Saito, A.; Higuchi, K. Nitrogen Metabolism in Non-Nodulated and Nodulated Soybean Plants Related to Ureide Synthesis. Nitrogen 2023, 4, 209–222. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Interactive effect of temperature and water stress on physiological and biochemical processes in soybean. Physiol. Mol. Biol. Plants 2019, 25, 667–681. [Google Scholar] [CrossRef]
- Lavres, J.; Franco, G.C.; Câmara, G.M.d.S. Soybean Seed Treatment with Nickel Improves Biological Nitrogen Fixation and Urease Activity. Front. Environ. Sci. 2016, 4. [Google Scholar] [CrossRef]
- Zilli, J.; Marson, L.C.; Marson, B.F.; Gianluppi, V.; Campo, R.J.; Hungria, M. Inoculação de Bradyrhizobium em soja por pulverização em cobertura. Pesqui. Agropecu. Bras. 2008, 43, 541–544. [Google Scholar] [CrossRef][Green Version]

| Parameter | C | D | p |
|---|---|---|---|
| RWC | 67.07 ± 1.26 | 40.97 ± 4.43 | <0.0001 *** |
| SLA | 43.79 ± 0.8 | 57.32 ± 1.74 | <0.0001 *** |
| CI | 33.17 ± 0.49 | 38.89 ± 1.19 | 0.0001 *** |
| Parameter | C | D | p |
|---|---|---|---|
| TSSL | 8.55 ± 0.31 | 9.57 ± 0.89 | 0.58 |
| TSSR | 5.27 ± 1.3 | 5.4 ± 0.53 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasca, H.C.; Posso, D.A.; Mossi, A.J.; Bayer, C.; Cansian, R.L.; Chavarria, G.; Sausen, T.L. Impact of Nodulation Efficiency and Concentrations of Soluble Sugars and Ureides on Soybean Water Deficit During Vegetative Growth. Nitrogen 2024, 5, 992-1000. https://doi.org/10.3390/nitrogen5040063
Tasca HC, Posso DA, Mossi AJ, Bayer C, Cansian RL, Chavarria G, Sausen TL. Impact of Nodulation Efficiency and Concentrations of Soluble Sugars and Ureides on Soybean Water Deficit During Vegetative Growth. Nitrogen. 2024; 5(4):992-1000. https://doi.org/10.3390/nitrogen5040063
Chicago/Turabian StyleTasca, Helena Chaves, Douglas Antônio Posso, Altemir José Mossi, Cimélio Bayer, Rogério Luís Cansian, Geraldo Chavarria, and Tanise Luisa Sausen. 2024. "Impact of Nodulation Efficiency and Concentrations of Soluble Sugars and Ureides on Soybean Water Deficit During Vegetative Growth" Nitrogen 5, no. 4: 992-1000. https://doi.org/10.3390/nitrogen5040063
APA StyleTasca, H. C., Posso, D. A., Mossi, A. J., Bayer, C., Cansian, R. L., Chavarria, G., & Sausen, T. L. (2024). Impact of Nodulation Efficiency and Concentrations of Soluble Sugars and Ureides on Soybean Water Deficit During Vegetative Growth. Nitrogen, 5(4), 992-1000. https://doi.org/10.3390/nitrogen5040063







