Morpho-Physiological and Biochemical Responses in Prosopis laevigata Seedlings to Varied Nitrogen Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growing Conditions, and Experimental Design
2.2. Plant Harvest and Morphology Assessment
2.3. Physiological Traits Calculation
2.4. Biochemical Determinations
2.5. Resprouting Capacity Test
2.6. Statistical Analyses
3. Results
3.1. Morphological Responses
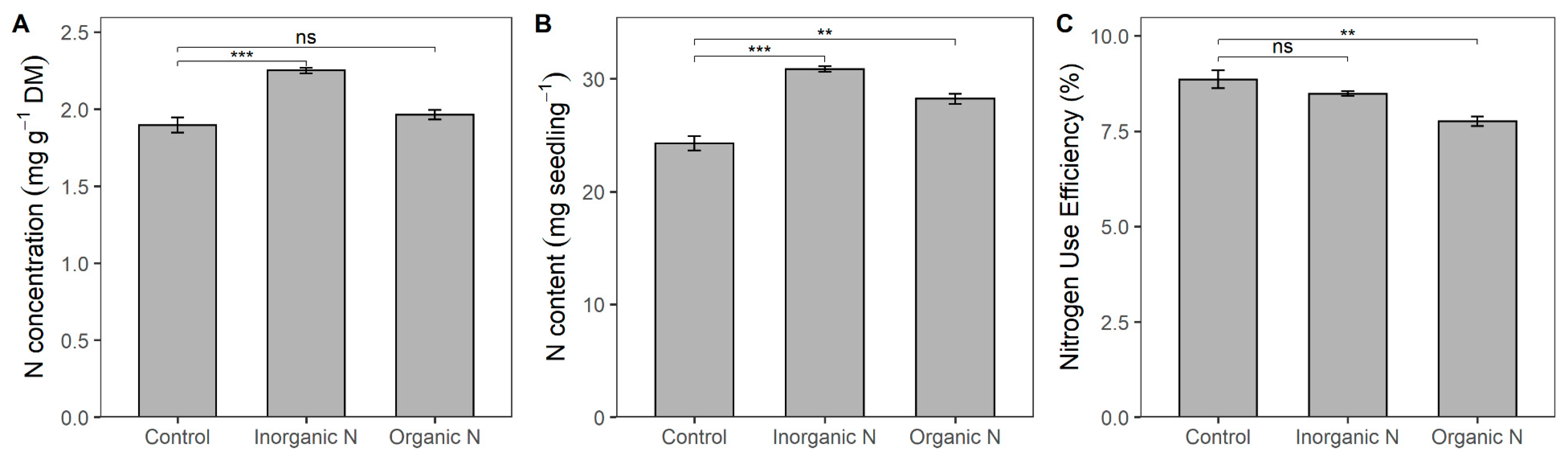
3.2. Physiological Responses
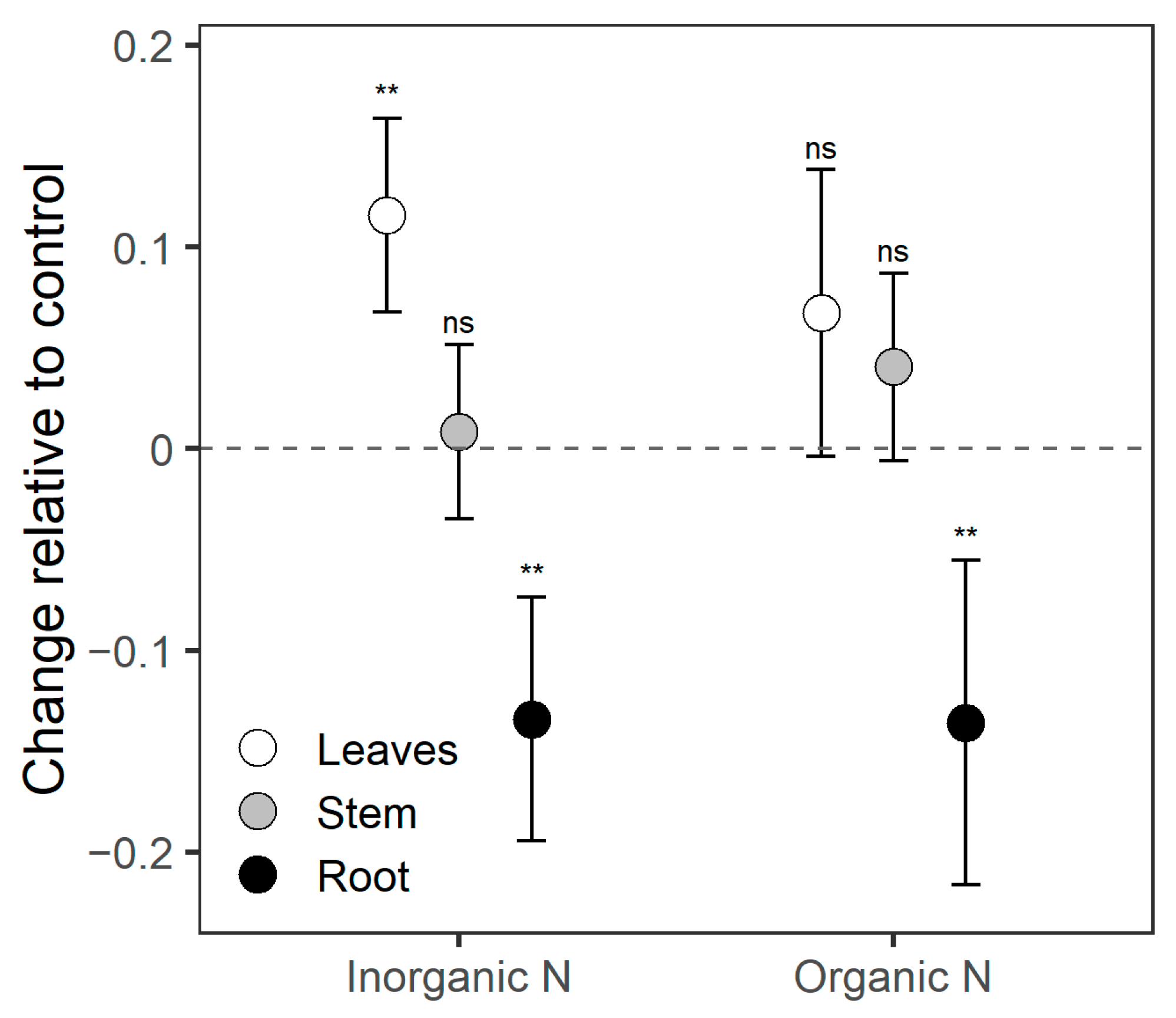
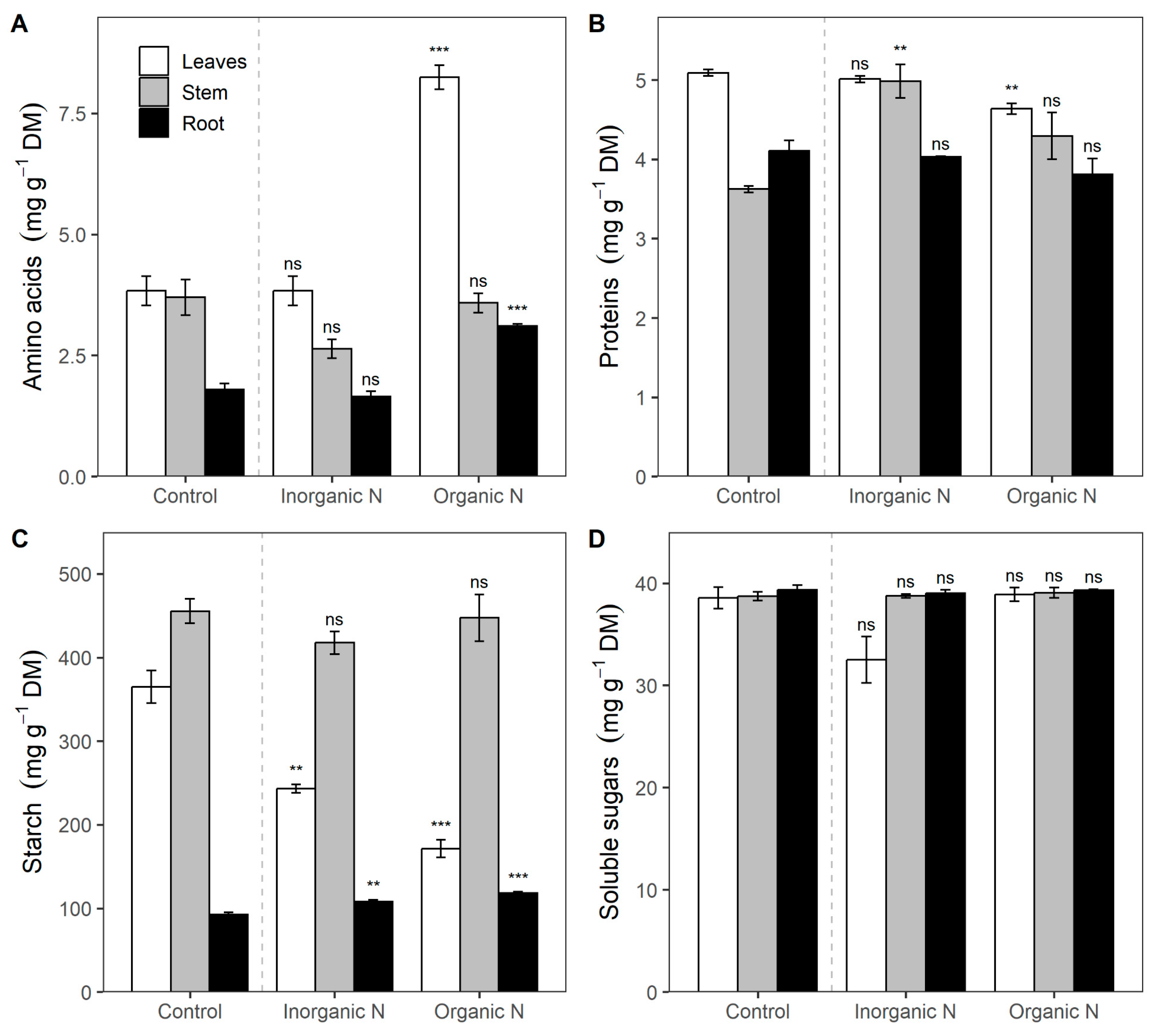
3.3. Biochemical Responses
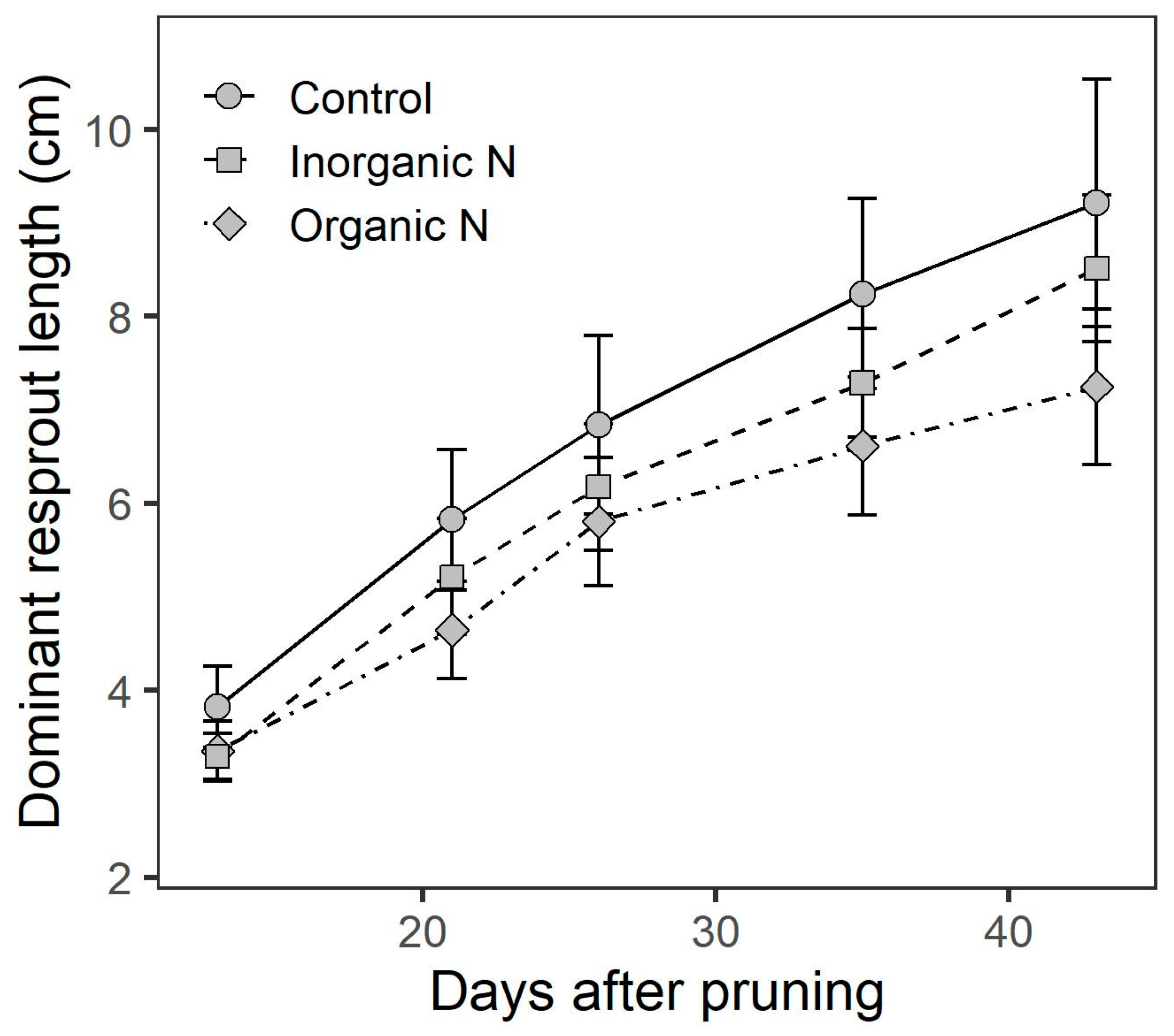
3.4. Resprouting Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Control | Additional Supply of N | ANOVA Results | ||
|---|---|---|---|---|---|
| N Inorganic | N Organic | F Value | Pr (>F) | ||
| Stem length (cm) | 27.2 ± 1.0 | 31.1 ± 1.5 ns | 29.1 ± 1.0 ns | 0.5875 | 0.5602 |
| Stem diameter (mm) | 2.7 ± 0.1 | 2.6 ± 0.1 ns | 2.8 ± 0.1 ns | 1.4216 | 0.2527 |
| Leaf area (cm2) | 59.6 ± 2.9 | 71.1 ± 5 ns | 71.1 ± 3.5 ns | 2.896 | 0.0663 |
| Leaf biomass (g) | 0.36 ± 0.02 | 0.43 ± 0.03 ns | 0.43 ± 0.02 ns | 2.8960 | 0.0663 |
| Stem biomass (g) | 0.57 ± 0.04 | 0.63 ± 0.06 ns | 0.67 ± 0.05 ns | 1.0077 | 0.3737 |
| Root biomass(g) | 0.35 ± 0.02 | 0.32 ± 0.02 ns | 0.34 ± 0.02 ns | 0.5875 | 0.5602 |
| Total biomass (g) | 1.28 ± 0.07 | 1.37 ± 0.10 ns | 1.44 ± 0.07 ns | 0.9587 | 0.4069 |
| LMF (%) | 28.3 ± 0.7 | 31.6 ± 0.8 * | 30.2 ± 1.1 ns | 3.4869 | 0.0397 |
| SMF (%) | 44.4 ± 0.9 | 44.8 ± 1.1 ns | 46.2 ± 1.2 ns | 0.8165 | 0.4489 |
| RMF (%) | 27.3 ± 0.7 | 23.6 ± 0.9 * | 23.6 ± 1.2 * | 4.5415 | 0.0164 |
| NAR (mg cm2 day−1) | 0.18 ± 0.01 | 0.16 ± 0.01 * | 0.17 ± 0.01 ns | 3.3527 | 0.0446 |
| RGR (mg g−1 day−1) | 30 ± 0.4 | 30.4 ± 0.6 ns | 31 ± 0.4 ns | 0.9557 | 0.3928 |
References
- Berendse, F.; de Kroon, H.; Braakhekke, W.G. Acquisition, Use, and Loss of Nutrients. In Functional Plant Biology; Pugnaire, F., Valladares, F., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 259–284. [Google Scholar]
- Sage, R.F.; Pearcy, R.W.; Seemann, J.R. The Nitrogen Use Efficiency of C3 and C4 Plants: III. Leaf Nitrogen Effects on the Activity of Carboxylating Enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 1987, 85, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition, 2nd ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; ISBN 978-0-12-384905-2. [Google Scholar]
- Acevedo, M.; Rubilar, R.; Dumroese, R.K.; Ovalle, J.F.; Sandoval, S.; Chassin-Trubert, R. Nitrogen Loading of Eucalyptus Globulus Seedlings: Nutritional Dynamics and Influence on Morphology and Root Growth Potential. New For. 2021, 52, 31–46. [Google Scholar] [CrossRef]
- Cortina, J.; Vilagrosa, A.; Trubat, R. The Role of Nutrients for Improving Seedling Quality in Drylands. New For. 2013, 44, 719–732. [Google Scholar] [CrossRef]
- Rocha, J.S.; Calzavara, A.K.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Oliveira, H.C. Nitrogen Supplementation Improves the High-Light Acclimation of Guazuma Ulmifolia Lam. Seedlings. Trees 2019, 33, 421–431. [Google Scholar] [CrossRef]
- Villar-Montero, R.; Ruiz-Robleto, J.; Quero-Pérez, J.L.; Poorter, H.; Valladares, F.; Marañón, T. Tasas de Crecimiento En Especies Leñosas: Aspectos Funcionales e Implicaciones Ecológicas. In Ecología del Bosque Mediterráneo en un Mundo Cambiante; Valladares, F., Ed.; Ministerio de Medio Ambiente y Medio Rural y Marino (España): Madrid, Spain, 2008; pp. 193–230. [Google Scholar]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of Organic Nitrogen by Plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Warren, C.R. Does Nitrogen Concentration Affect Relative Uptake Rates of Nitrate, Ammonium, and Glycine? J. Plant Nutr. Soil Sci. 2009, 172, 224–229. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological Significance and Complexity of N-Source Preference in Plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef]
- Bueno, A.; Greenfield, L.; Pritsch, K.; Schmidt, S.; Simon, J. Responses to Competition for Nitrogen between Subtropical Native Tree Seedlings and Exotic Grasses Are Species-Specific and Mediated by Soil N Availability. Tree Physiol. 2018, 39, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Møller, I.M.; Murphy, A.; Zeiger, E. Plant Physiology and Development, 7th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2022; ISBN 9780197577240. [Google Scholar]
- Zerihun, A.; McJenzie, B.A.; Morton, J.D. Photosynthate Costs Associated with the Utilization of Different Nitrogen–Forms: Influence on the Carbon Balance of Plants and Shoot–Root Biomass Partitioning. New Phytol. 1998, 138, 1–11. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of Ammonium Toxicity and the Quest for Tolerance. Plant Sci 2016, 248, 92–101. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; GK, S.; Vadivel, R.; TK, D.; et al. Nitrogen Use Efficiency—A Key to Enhance Crop Productivity under a Changing Climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef] [PubMed]
- Franklin, O.; Cambui, C.A.; Gruffman, L.; Palmroth, S.; Oren, R.; Näsholm, T. The Carbon Bonus of Organic Nitrogen Enhances Nitrogen Use Efficiency of Plants. Plant Cell Environ. 2017, 40, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Gruffman, L.; Ishida, T.; Nordin, A.; Näsholm, T. Cultivation of Norway Spruce and Scots Pine on Organic Nitrogen Improves Seedling Morphology and Field Performance. For. Ecol. Manag. 2012, 276, 118–124. [Google Scholar] [CrossRef]
- Sigala, J.A.; Oliet, J.A.; Uscola, M. Assessing Growth, Frost Tolerance, and Acclimation of Pine Seedlings with Contrasted Dormancy Strategies as Influenced by Organic Nitrogen Supply. Physiol. Plant 2021, 173, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Sigala, J.A.; Uscola, M.; Oliet, J.A.; Jacobs, D.F. Drought Tolerance and Acclimation in Pinus Ponderosa Seedlings: The Influence of Nitrogen Form. Tree Physiol. 2020, 40, 1165–1177. [Google Scholar] [CrossRef]
- Palacios Romero, A.; Rodríguez Laguna, R.; de la Hernández Flores, M.L.; Jiménez Muñoz, E.; Tirado Torres, D. Distribución Potencial de Prosopis Laevigata (Humb. et Bonpl. Ex Willd) MC Johnston Basada En Un Modelo de Nicho Ecológico. Rev. Mex. Cienc. For. 2016, 7, 35–46. [Google Scholar] [CrossRef]
- Lopez-Lozano, N.E.; Carcaño-Montiel, M.G.; Bashan, Y. Using Native Trees and Cacti to Improve Soil Potential Nitrogen Fixation during Long-Term Restoration of Arid Lands. Plant Soil 2016, 403, 317–329. [Google Scholar] [CrossRef]
- Trucios-Caciano, R.; Valenzuela-Nuñez, L.M.; Ríos-Saucedo, J.C.; Rivera-González, M.; Estrada-Ávalos, J. Cambio de Uso de Suelo En Coahuila y Durango. Rev. Chapingo Ser. Zonas 2012, 11, 68–74. [Google Scholar]
- Landis, T.D.; Tinus, R.W.; McDonald, S.E.; Bartlett, J.P. Containers and Growing Media. In The Container Tree Nursery Manual; Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 2, pp. 41–92. [Google Scholar]
- Wright, R.D.; Grueber, K.L.; Leda, C. Medium Nutrient Extraction with the Pour-through and Saturated Medium Procedures for Poinsettia. HortScience 1990, 25, 658–660. [Google Scholar] [CrossRef]
- Prieto-Ruíz, J.Á.; Ríos-Saucedo, J.C.; Monárrez-González, J.C.; García-Rodríguez, J.L.; Mejía-Bojorquez, J.; Bustamante-García, V. Recomendaciones Para La Producción de Mezquite En Condiciones de Vivero; INIFAP: Durango, México, 2012. [Google Scholar]
- Li, X.; Schmid, B.; Wang, F.; Paine, C.E.T. Net Assimilation Rate Determines the Growth Rates of 14 Species of Subtropical Forest Trees. PLoS ONE 2016, 11, e0150644. [Google Scholar] [CrossRef]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A Modern Tool for Classical Plant Growth Analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.R.; Chandler, R.A.; Dumroese, R.K. Growth, Nitrogen Use Efficiency, and Leachate Comparison of Subirrigated and Overhead Irrigated Pale Purple Coneflower Seedlings. HortScience 2008, 43, 897–901. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- van Handel, E. Direct Microdetermination of Sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- del Morales-Espinoza, I.C.; Ortiz-Solorio, C.A.; Gutiérrez-Castorena, M.D.C.; Gutiérrez-Castorena, E.V. Estudio Etnoedafológico Para El Reconocimiento de Tipos de Usos Asociados Con Cadenas Productivas En El Ejido de Santa Cruz, Durango. Terra Latinoam 2021, 39, e853. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Signorell, A. DescTools: Tools for Descriptive Statistics; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means; The R Foundation: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Cambui, C.A.; Svennerstam, H.; Gruffman, L.; Nordin, A.; Ganeteg, U.; Näsholm, T. Patterns of Plant Biomass Partitioning Depend on Nitrogen Source. PLoS ONE 2011, 6, e19211. [Google Scholar] [CrossRef]
- Kiba, T.; Krapp, A. Plant Nitrogen Acquisition under Low Availability: Regulation of Uptake and Root Architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef]
- Xie, T.; Shan, L.; Zhang, W. N Addition Alters Growth, Non-Structural Carbohydrates, and C:N:P Stoichiometry of Reaumuria Soongorica Seedlings in Northwest China. Sci. Rep. 2022, 12, 15390. [Google Scholar] [CrossRef]
- Soares, C.B.; de Freitas, E.C.S.; de Paiva, H.N.; Neves, J.C.L. Nitrogen Sources and Doses on Growth and Quality of Seedlings of Cassia Grandis and Peltophorum Dubium. Rev. Árvore 2017, 41, e410214. [Google Scholar] [CrossRef][Green Version]
- Aliarab, A.; Vazifekhah, O.E.; Sadati, S.-E. Effect of Soil Moisture Content and Nitrogen Fertilizer on Survival, Growth and Some Physiological Characteristics of Platycladus Orientalis Seedlings. J. For. Sci. 2020, 66, 511–523. [Google Scholar] [CrossRef]
- Bardy, L.R.; Debiasi, T.V.; Sanada, K.; Rondina, A.B.; Torezan, J.M.; Stolf-Moreira, R.; Bianchini, E.; Pimenta, J.A.; Oliveira, H.C. Effect of Nitrogen Addition to the Soil on Atlantic Forest Tree Seedlings. Forests 2023, 14, 1111. [Google Scholar] [CrossRef]
- Terradas, J. Ecología de La Vegetación: De La Ecofisiología de Las Plantas a La Dinámica de Comunidades y Paisajes, 1st ed.; Ediciones Omega: Barcelona, Spain, 2001. [Google Scholar]
- Lim, H.; Jämtgård, S.; Oren, R.; Gruffman, L.; Kunz, S.; Näsholm, T. Organic Nitrogen Enhances Nitrogen Nutrition and Early Growth of Pinus Sylvestris Seedlings. Tree Physiol. 2022, 42, 513–522. [Google Scholar] [CrossRef]
- Garnett, T.; Conn, V.; Kaiser, B.N. Root Based Approaches to Improving Nitrogen Use Efficiency in Plants. Plant Cell Environ. 2009, 32, 1272–1283. [Google Scholar] [CrossRef]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved Organic Nitrogen Uptake by Plants—An Important N Uptake Pathway? Soil Biol. Biochem. 2005, 37, 413–423. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic Substrate for Transplant Production in Organic Nurseries. A Review. Agron. Sustain. Dev. 2018, 38, 35. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Geisseler, D.; Wu, Z.; Gong, P.; Xue, Y.; Yu, C.; Juan, Y.; Horwath, W.R. Available C and N Affect the Utilization of Glycine by Soil Microorganisms. Geoderma 2016, 283, 32–38. [Google Scholar] [CrossRef]
- Garcia, A.L.; Madrid, R.; Gimeno, V.; Rodriguez-Ortega, W.M.; Nicolas, N.; Garcia-Sanchez, F. The Effects of Amino Acids Fertilization Incorporated to the Nutrient Solution on Mineral Composition and Growth in Tomato Seedlings. Span. J. Agric. Res. 2011, 9, 852–861. [Google Scholar] [CrossRef]
- Owen, A.G.; Jones, D.L. Competition for Amino Acids between Wheat Roots and Rhizosphere Microorganisms and the Role of Amino Acids in Plant N Acquisition. Soil Biol. Biochem. 2001, 33, 651–657. [Google Scholar] [CrossRef]
- Rosado-Souza, L.; Yokoyama, R.; Sonnewald, U.; Fernie, A.R. Understanding Source–Sink Interactions: Progress in Model Plants and Translational Research to Crops. Mol. Plant 2023, 16, 96–121. [Google Scholar] [CrossRef]
- Bihmidine, S.; Hunter, C.T.; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of Assimilate Import into Sink Organs: Update on Molecular Drivers of Sink Strength. Front. Plant Sci. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Nambara, E.; Yamagishi, K.; Goto, D.B.; Naito, S. Storage Proteins. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2002; Volume 1, p. e0020. [Google Scholar]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a Key Functional Trait: How Buds, Protection and Resources Drive Persistence after Fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Trumbore, S. Understanding the Roles of Nonstructural Carbohydrates in Forest Trees—From What We Can Measure to What We Want to Know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basave-Villalobos, E.; Valenzuela-Núñez, L.M.; García-Rodríguez, J.L.; Sarmiento-López, H.; García-Pérez, J.L.; Calixto-Valencia, C.G.; Sigala, J.A. Morpho-Physiological and Biochemical Responses in Prosopis laevigata Seedlings to Varied Nitrogen Sources. Nitrogen 2024, 5, 857-870. https://doi.org/10.3390/nitrogen5040055
Basave-Villalobos E, Valenzuela-Núñez LM, García-Rodríguez JL, Sarmiento-López H, García-Pérez JL, Calixto-Valencia CG, Sigala JA. Morpho-Physiological and Biochemical Responses in Prosopis laevigata Seedlings to Varied Nitrogen Sources. Nitrogen. 2024; 5(4):857-870. https://doi.org/10.3390/nitrogen5040055
Chicago/Turabian StyleBasave-Villalobos, Erickson, Luis Manuel Valenzuela-Núñez, José Leonardo García-Rodríguez, Homero Sarmiento-López, José Luis García-Pérez, Celi Gloria Calixto-Valencia, and José A. Sigala. 2024. "Morpho-Physiological and Biochemical Responses in Prosopis laevigata Seedlings to Varied Nitrogen Sources" Nitrogen 5, no. 4: 857-870. https://doi.org/10.3390/nitrogen5040055
APA StyleBasave-Villalobos, E., Valenzuela-Núñez, L. M., García-Rodríguez, J. L., Sarmiento-López, H., García-Pérez, J. L., Calixto-Valencia, C. G., & Sigala, J. A. (2024). Morpho-Physiological and Biochemical Responses in Prosopis laevigata Seedlings to Varied Nitrogen Sources. Nitrogen, 5(4), 857-870. https://doi.org/10.3390/nitrogen5040055






