Rapid Permafrost Thaw Removes Nitrogen Limitation and Rises the Potential for N2O Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Sampling and Sample Preparation
2.3. Soil Analysis
2.4. Nitrogen Transformation
2.5. Greenhouse Gas Production in Anaerobic Laboratory Incubations
2.6. Nitrate Addition
2.7. In–Situ N2O Fluxes
2.8. Statistics
3. Results
3.1. Soil Properties
3.2. Nitrogen Pool Composition
3.3. Nitrogen Transformation
3.4. Anaerobic N2O Production and In–Situ N2O Fluxes
3.5. Anaerobic CO2 and CH4 Production
3.6. Anerobic Production of CO2 Equivalents
4. Discussion
4.1. Nitrogen Availability
4.2. Anaerobic N2O Production
4.3. Effects of Nitrate on Greenhouse Gas Production
4.4. Role of Revegetation on Greenhouse Gas Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Turetsky, M.R.; Abbott, B.W.; Jones, M.C.; Anthony, K.W.; Olefeldt, D.; Schuur, E.A.G.; Grosse, G.; Kuhry, P.; Hugelius, G.; Koven, C.; et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 2020, 13, 138–143. [Google Scholar] [CrossRef]
- Marushchak, M.E.; Pitkämäki, A.; Koponen, H.; Biasi, C.; Seppälä, M.; Martikainen, P.J. Hot spots for nitrous oxide emissions found in different types of permafrost peatlands. Glob. Chang. Biol. 2011, 17, 2601–2614. [Google Scholar] [CrossRef]
- Abbott, B.W.; Jones, J.B. Permafrost collapse alters soil carbon stocks, respiration, CH4, and N2O in upland tundra. Glob. Chang. Biol. 2015, 21, 4570–4587. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.C.; Abbott, B.W.; Zhao, Q.; Su, H.; Wang, S.F.; Wu, Q.B.; Zhang, T.J.; Wu, X.D. Permafrost collapse shifts alpine tundra to a carbon source but reduces N2O and CH4 release on the northern Qinghai-Tibetan Plateau. Geophys. Res. Lett. 2017, 44, 8945–8952. [Google Scholar] [CrossRef]
- Yang, G.; Peng, Y.; Marushchak, M.E.; Chen, Y.; Wang, G.; Li, F.; Zhang, D.; Wang, J.; Yu, J.; Liu, L.; et al. Magnitude and Pathways of Increased Nitrous Oxide Emissions from Uplands Following Permafrost Thaw. Environ. Sci. Technol. 2018, 52, 9162–9169. [Google Scholar] [CrossRef]
- Marushchak, M.E.; Kerttula, J.; Diáková, K.; Faguet, A.; Gil, J.; Grosse, G.; Knoblauch, C.; Lashchinskiy, N.; Martikainen, P.J.; Morgenstern, A.; et al. Thawing Yedoma permafrost is a neglected nitrous oxide source. Nat. Commun. 2021, 12, 7107. [Google Scholar] [CrossRef]
- Voigt, C.; Marushchak, M.E.; Abbott, B.W.; Biasi, C.; Elberling, B.; Siciliano, S.D.; Sonnentag, O.; Stewart, K.J.; Yang, Y.; Martikainen, P.J. Nitrous oxide emissions from permafrost-affected soils. Nat. Rev. Earth Environ. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Lacroix, F.; Zaehle, S.; Caldararu, S.; Schaller, J.; Stimmler, P.; Holl, D.; Kutzbach, L.; Göckede, M. Mismatch of N release from the permafrost and vegetative uptake opens pathways of increasing nitrous oxide emissions in the high Arctic. Glob. Chang. Biol. 2022, 28, 5973–5990. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen Mineralization: Challenges of a Changing Paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Ramm, E.; Liu, C.; Ambus, P.; Butterbach-Bahl, K.; Hu, B.; Martikainen, P.J.; Marushchak, M.E.; Mueller, C.W.; Rennenberg, H.; Schloter, M.; et al. A review of the importance of mineral nitrogen cycling in the plant-soil-microbe system of permafrost-affected soils—Changing the paradigm. Environ. Res. Lett. 2022, 17, 13004. [Google Scholar] [CrossRef]
- Ramm, E.; Liu, C.; Wang, X.; Yue, H.; Zhang, W.; Pan, Y.; Schloter, M.; Gschwendtner, S.; Mueller, C.W.; Hu, B.; et al. The Forgotten Nutrient—The Role of Nitrogen in Permafrost Soils of Northern China. Adv. Atmos. Sci. 2020, 37, 793–799. [Google Scholar] [CrossRef]
- Prosser, J.I.; Hink, L.; Gubry-Rangin, C.; Nicol, G.W. Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Chang. Biol. 2019, 26, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Myhre, G.D.; Shindell, F.-M.; Bréon, W.; Collins, J.; Fuglestvedt, J.; Huang, D.; Koch, J.-F.; Lamarque, D.; Lee, B.; Mendoza, T.; et al. Anthropogenic and Natural Radiative Forcing in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 659–740. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Harms, T.K.; Jones, J. Thaw depth determines reaction and transport of inorganic nitrogen in valley bottom permafrost soils: Nitrogen cycling in permafrost soils. Glob. Chang. Biol. 2012, 18, 2958–2968. [Google Scholar] [CrossRef] [PubMed]
- Harms, T.K.; Abbott, B.W.; Jones, J.B. Thermo-erosion gullies increase nitrogen available for hydrologic export. Biogeochemistry 2014, 117, 299–311. [Google Scholar] [CrossRef]
- Louiseize, N.L.; Lafrenière, M.J.; Hastings, M.G. Stable isotopic evidence of enhanced export of microbially derived NO3− following active layer slope disturbance in the Canadian High Arctic. Biogeochemistry 2014, 121, 565–580. [Google Scholar] [CrossRef]
- Abbott, B.W.; Jones, J.B.; Godsey, S.E.; Larouche, J.R.; Bowden, W.B. Patterns and persistence of hydrologic carbon and nutrient export from collapsing upland permafrost. Biogeosciences 2015, 119, 2049–2063. [Google Scholar] [CrossRef]
- Tanski, G.; Lantuit, H.; Ruttor, S.; Knoblauch, C.; Radosavljevic, B.; Strauss, J.; Wolter, J.; Irrgang, A.M.; Ramage, J.; Fritz, M. Transformation of terrestrial organic matter along thermokarst-affected permafrost coasts in the Arctic. Sci. Total Environ. 2017, 581–582, 434–447. [Google Scholar] [CrossRef]
- Schirrmeister, L.; Froese, D.; Tumskoy, V.; Grosse, G.; Wetterich, S. Yedoma: Late Pleistocene Ice-Rich Syngenetic Permafrost of Beringia. In The Encyclopedia of Quaternary Science; Elias, S.A., Ed.; Elsevier: Amsterdam, the Netherlands, 2013; Volume 3, pp. 542–552. [Google Scholar]
- Segal, R.A.; Lantz, T.C.; Kokelj, S.V. Acceleration of thaw slump activity in glaciated landscapes of the Western Canadian Arctic. Environ. Res. Lett. 2016, 11, 034025. [Google Scholar] [CrossRef]
- Strauss, J.; Abbott, B.W.; Beermann, F.; Biasi, C.; Fuchs, M.; Grosse, G.; Horn, M.A.; Liebner, S.; Sanders, T.; Schirrmeister, L.; et al. The nitrogen stock of the ice-rich yedoma domain. In Proceedings of the 5th European Conference on Permafrost, Chamonix Mont-Blanc, France, 23 June–1 July 2018; pp. 784–785. [Google Scholar]
- Hugelius, G.; Loisel, J.; Chadburn, S.; Jackson, R.B.; Jones, M.; MacDonald, G.; Marushchak, M.; Olefeldt, D.; Packalen, M.; Siewert, M.B.; et al. Large stocks of peatland carbon and nitrogen are vulnerable to permafrost thaw. Proc. Natl. Acad. Sci. USA 2020, 117, 20438–20446. [Google Scholar] [CrossRef]
- Fuchs, M.; Nitze, I.; Strauss, J.; Günther, F.; Wetterich, S.; Kizyakov, A.; Fritz, M.; Opel, T.; Grigoriev, M.N.; Maksimov, G.T.; et al. Rapid Fluvio-Thermal Erosion of a Yedoma Permafrost Cliff in the Lena River Delta. Front. Earth Sci. 2020, 8, 00336. [Google Scholar] [CrossRef]
- Grosse, G.; Schirrmeister, L.; Malthus, T.J. Application of Landsat-7 satellite data and a DEM for the quantification of thermokarst-affected terrain types in the periglacial Lena–Anabar coastal lowland. Polar Res. 2006, 25, 51–67. [Google Scholar] [CrossRef][Green Version]
- Knoblauch, C.; Beer, C.; Schuett, A.; Sauerland, L.; Liebner, S.; Steinhof, A.; Rethemeyer, J.; Grigoriev, M.N.; Faguet, A.; Pfeiffer, E.-M. Carbon Dioxide and Methane Release Following Abrupt Thaw of Pleistocene Permafrost Deposits in Arctic Siberia. J. Geophys. Res.-Biogeo. 2021, 126, e2021JG006543. [Google Scholar] [CrossRef]
- Wolfe, S.; Kotler, E.; Dallimore, S. Surficial Characteristics and the Distribution of Thaw Landforms (1970–1999), Shingle Point to Kay Point, Yukon Territory; Geological Survey of Canada Open File 4115; Terrain Sciences Division, Geological Survey of Canada: Ottawa, ON, Canada, 2001. Available online: https://emrlibrary.gov.yk.ca/gsc/open_files/4115/OF4115.pdf (accessed on 15 November 2022).
- Lantuit, H.; Pollard, W.H. Fifty years of coastal erosion and retrogressive thaw slump activity on Herschel Island, southern Beaufort Sea, Yukon Territory, Canada. Geomorphology 2008, 95, 84–102. [Google Scholar] [CrossRef]
- Sanders, T.; Fiencke, C.; Fuchs, M.; Haugk, C.; Juhls, B.; Mollenhauer, G.; Ogneva, O.; Overduin, P.; Palmtag, J.; Povazhniy, V.; et al. Seasonal nitrogen fluxes of the Lena River Delta. Ambio 2022, 51, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Peng, Y.; Olefeldt, D.; Chen, Y.; Wang, G.; Li, F.; Zhang, D.; Wang, J.; Yu, J.; Liu, L.; et al. Changes in Methane Flux along a Permafrost Thaw Sequence on the Tibetan Plateau. Environ. Sci. Technol. 2018, 52, 1244–1252. [Google Scholar] [CrossRef]
- Monteux, S.; Keuper, F.; Fontaine, S.; Gavazov, K.; Hallin, S.; Juhanson, J.; Krab, E.J.; Revaillot, S.; Verbruggen, E.; Walz, J.; et al. Carbon and nitrogen cycling in Yedoma permafrost controlled by microbial functional limitations. Nat. Geosci. 2020, 13, 794–798. [Google Scholar] [CrossRef]
- Yergeau, E.; Hogues, H.; Whyte, L.G.; Greer, C.W. The functional potential of high Arctic permafrost revealed by metagenomic sequencing, qPCR and microarray analyses. ISME J. 2010, 4, 1206–1214. [Google Scholar] [CrossRef]
- Wild, B.; Schnecker, J.; Alves, R.J.E.; Barsukov, P.; Bárta, J.; Capek, P.; Gentsch, N.; Gittel, A.; Guggenberger, G.; Lashchinskiy, N.; et al. Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biol. Biochem. 2014, 75, 143–151. [Google Scholar] [CrossRef]
- Wild, B.; Gentsch, N.; Čapek, P.; Diáková, K.; Alves, R.J.E.; Bárta, J.; Gittel, A.; Hugelius, G.; Knoltsch, A.; Kuhry, P.; et al. Plant-derived compounds stimulate the decomposition of organic matter in arctic permafrost soils. Sci. Rep. 2016, 6, 25607. [Google Scholar] [CrossRef]
- Boike, J.; Nitzbon, J.; Anders, K.; Grigoriev, M.; Bolshiyanov, D.; Langer, M.; Lange, S.; Bornemann, N.; Morgenstern, A.; Schreiber, P.; et al. A 16-year record (2002–2017) of permafrost, active-layer, and meteorological conditions at the Samoylov Island Arctic permafrost research site, Lena River delta, northern Siberia: An opportunity to validate remote-sensing data and land surface, snow, and permafrost models. Earth Syst. Sci. Data 2019, 11, 261–299. [Google Scholar] [CrossRef]
- Boike, J.; Kattenstroth, B.; Abramova, K.; Bornemann, N.; Chetverova, A.; Fedorova, I.; Fröb, K.; Grigoriev, M.; Grüber, M.; Kutzbach, L.; et al. Baseline characteristics of climate, permafrost and land cover from a new permafrost observatory in the Lena River Delta, Siberia (1998–2011). Biogeosciences 2013, 10, 2105–2128. [Google Scholar] [CrossRef]
- Wetterich, S.; Kuzmina, S.; Andreev, A.A.; Kienast, F.; Meyer, H.; Schirrmeister, L.; Kuznetsova, T.; Sierralta, M. Palaeoenvironmental dynamics inferred from late Quaternary permafrost deposits on Kurungnakh Island, Lena Delta, Northeast Siberia, Russia. Quat. Sci. Rev. 2008, 27, 1523–1540. [Google Scholar] [CrossRef]
- Laschinskiy, N.; Faguet, A.; Biasi, C. Primary plant succession on freshly degraded yedoma (ice complex) in Lena delta (Eastern Siberia). BIO Web Conf. 2020, 24, 00047. [Google Scholar] [CrossRef]
- Haney, R.L.; Haney, E.B. Simple and Rapid Laboratory Method for Rewetting Dry Soil for Incubations. Commun. Soil Sci. Plant Anal. 2010, 41, 1493–1501. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Jones, D.L.; Willet, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Surey, R.; Schimpf, C.M.; Sauheitl, L.; Mueller, C.W.; Rummel, P.S.; Dittert, K.; Kaiser, K.; Böttcher, J.; Mikutta, R. Potential denitrification stimulated by water-soluble organic carbon from plant residues during initial decomposition. Soil Biol. Biochem. 2020, 147, 107841. [Google Scholar] [CrossRef]
- Riehm, H. Die Bestimmung der laktatlöslichen Phosphorsäure im Boden unter Verwendung eines lichtelektrischen Kolorimeters. Bodenk. Pflanzenernaehr. 1938, 9, 30–50. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Carroll, J.J.; Slupsky, J.D.; Mather, A.E. The Solubility of Carbon Dioxide in Water at Low Pressure. J. Phys. Chem. Ref. Data 1991, 20, 1201–1209. [Google Scholar] [CrossRef]
- Millero, F.; Huang, F.; Graham, T.; Pierrot, D. The dissociation of carbonic acid in NaCl solutions as a function of concentration and temperature. Geochim. Cosmochim. Acta 2007, 71, 46–55. [Google Scholar] [CrossRef]
- Yamamoto, S.; Alcauskas, J.B.; Crozier, T.E. Solubility of methane in distilled water and seawater. J. Chem. Eng. Data 1976, 21, 78–80. [Google Scholar] [CrossRef]
- Weiss, R.F.; Price, B.A. Nitrous oxide solubility in water and seawater. Mar. Chem. 1980, 8, 347–359. [Google Scholar] [CrossRef]
- Repo, M.E.; Susiluoto, S.; Lind, S.E.; Jokinen, S.; Elsakov, V.; Biasi, C.; Virtanen, T.; Martikainen, P.J. Large N2O emissions from cryoturbated peat soil in tundra. Nat. Geosci. 2009, 2, 189–192. [Google Scholar] [CrossRef]
- Voigt, C.; Lamprecht, R.E.; Marushchak, M.E.; Lind, S.E.; Novakovskiy, A.; Aurela, M.; Martikainen, P.J.; Biasi, C. Warming of subarctic tundra increases emissions of all three important greenhouse gases—Carbon dioxide, methane, and nitrous oxide. Glob. Chang. Biol. 2017, 23, 3121–3138. [Google Scholar] [CrossRef]
- Wild, B.; Schnecker, J.; Bárta, J.; Čapek, P.; Guggenberger, G.; Hofhansl, F.; Kaiser, C.; Lashchinsky, N.; Mikutta, R.; Mooshammer, M.; et al. Nitrogen dynamics in Turbic Cryosols from Siberia and Greenland. Soil Biol. Biochem. 2013, 67, 85–93. [Google Scholar] [CrossRef]
- Beermann, F.; Teltewskoi, A.; Fiencke, C.; Pfeiffer, E.-M.; Kutzbach, L. Stoichiometric analysis of nutrient availability (N, P, K) within soils of polygonal tundra. Biogeochemistry 2015, 122, 211–227. [Google Scholar] [CrossRef]
- Wild, B.; Schnecker, J.; Knoltsch, A.; Takriti, M.; Mooshammer, M.; Gentsch, N.; Mikutta, R.; Alves, R.J.E.; Gittel, A.; Lashchinskiy, N.; et al. Microbial nitrogen dynamics in organic and mineral soil horizons along a latitudinal transect in western Siberia. Glob. Biogeochem. Cycles 2015, 29, 567–582. [Google Scholar] [CrossRef]
- Wickland, K.P.; Waldrop, M.P.; Aiken, G.R.; Koch, J.C.; Jorgenson, M.T.; Striegl, R.G. Dissolved organic carbon and nitrogen release from boreal Holocene permafrost and seasonally frozen soils of Alaska. Environ. Res. Lett. 2018, 13, 65011. [Google Scholar] [CrossRef]
- Nordin, A.; Schmidt, I.K.; Shaver, G.R. Nitrogen uptake by Arctic soil microbes and plant in relation to soil nitrogen supply. Ecology 2004, 85, 955–962. [Google Scholar] [CrossRef]
- Oulehle, F.; Rowe, E.C.; Myška, O.; Chuman, T.; Evans, C.D. Plant functional type affects nitrogen use efficiency in high-Arctic tundra. Soil Biol. Biochem. 2016, 94, 19–28. [Google Scholar] [CrossRef]
- Morison, M.Q.; Macrae, M.L.; Petrone, R.M.; Fishback, L. Climate-induced changes in nutrient transformations across landscape units in a thermokarst subarctic peatland. Arct. Antarct. Alp. Res. 2018, 50, e1519366. [Google Scholar] [CrossRef]
- Fiencke, C.; Marushchak, M.E.; Sanders, T.; Wegner, R.; Beer., C. Microbiogeochemical Traits to Identify Nitrogen Hotspots in Permafrost Regions. Nitrogen 2022, 3, 458–501. [Google Scholar] [CrossRef]
- Ma, W.K.; Schautz, A.; Fishback, L.A.E.; Bedard-Haughn, A.; Farrell, R.E.; Siciliano, S.D. Assessing the potential of ammonia oxidizing bacteria to produce nitrous oxide in soils of a high arctic lowland ecosystem on Devon Island, Canada. Soil Biol. Biochem. 2007, 39, 2001–2013. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Gleeson, D.B.; Müller, C.; Banerjee, S.; Ma, W.; Siciliano, S.D.; Murphy, D.V. Response of ammonia oxidizing archaea and bacteria to changing water filled pore space. Soil Biol. Biochem. 2010, 42, 1888–1891. [Google Scholar] [CrossRef]
- Mao, C.; Kou, D.; Wang, G.; Peng, Y.; Yang, G.; Liu, F.; Zhang, J.; Yang, Y. Trajectory of Topsoil Nitrogen Transformations Along a Thermo-Erosion Gully on the Tibetan Plateau. J. Geophys. Res.-Biogeo. 2019, 124, 1342–1354. [Google Scholar] [CrossRef]
- Weber, D.F.; Gainey, P.L. Relative sensitivity of nitrifying organisms to hydrogen ions in soils and in solutions. Soil Sci. 1961, 94, 138–145. [Google Scholar] [CrossRef]
- Prinčič, A.; Mahne, I.; Megušar, F.; Paul, E.A.; Tiedje, J.M. Effects of pH and Oxygen and Ammonium Concentrations on the Community Structure of Nitrifying Bacteria from Wastewater. Appl. Environ. Microbiol 1998, 64, 3584–3590. [Google Scholar] [CrossRef]
- Li, Z.; Tian, D.; Wang, B.; Wang, J.; Wang, S.; Chen, H.Y.H.; Xu, X.; Wang, C.; He, N.; Niu, S. Microbes drive global soil nitrogen mineralization and availability. Glob. Chang. Biol. 2018, 25, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.A.; Marushchak, M.E.; Rütting, T.; Baggs, E.M.; Pérez, T.; Novakovskiy, A.; Trubnikova, T.; Kaverin, D.; Martikainen, P.J.; Biasi, C. Sources of nitrous oxide and fate of mineral nitrogen in sub-Arctic permafrost peat soils. Biogeosciences 2022, 19, 2683–2698. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, S.; Zhang, Z.; Du, Y.; Yao, B.; Xie, H.; Liu, P.; Zhang, Y.; Wang, W.; Zhou, H. Soil Moisture but Not Warming Dominates Nitrous Oxide Emissions During Freeze–Thaw Cycles in a Qinghai–Tibetan Plateau Alpine Meadow With Discontinuous Permafrost. Front. Ecol. Evol. 2021, 9, 676027. [Google Scholar] [CrossRef]
- Voigt, C.; Marushchak, M.E.; Lamprecht, R.E.; Jackowicz-Korczyński, M.; Lindgren, A.; Mastepanov, M.; Granlund, L.; Christensen, T.R.; Tahvanainen, T.; Martikainen, P.J.; et al. Increased nitrous oxide emissions from Arctic peatlands after permafrost thaw. Proc. Natl. Acad. Sci. USA 2017, 114, 6238–6243. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Schaeffer, S.M.; Schimel, J.P. Vegetation Leachate During Arctic Thaw Enhances Soil Microbial Phosphorus. Ecosystems 2016, 19, 477–489. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Mao, C.; Qin, S.; Wang, J.; Liu, F.; Blagodatsky, S.; Yang, G.; Zhang, Q.; Zhang, D.; et al. Nitrogen availability regulates topsoil carbon dynamics after permafrost thaw by altering microbial metabolic efficiency. Nat. Commun. 2018, 9, 13951. [Google Scholar] [CrossRef]
- Klüber, H.D.; Conrad, R. Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil. FEMS Microbiol. Ecol. 1998, 25, 301–318. [Google Scholar] [CrossRef]
- Roy, R.; Conrad, R. Effect of methanogenic precursors (acetate, hydrogen, propionate) on the suppression of methane production by nitrate in anoxic rice field soil. FEMS Microbiol. Ecol. 1999, 28, 49–61. [Google Scholar] [CrossRef]
- Wang, J.; Xu, T.; Yin, L.; Han, C.; Deng, H.; Jiang, Y.; Zhong, W. Nitrate addition inhibited methanogenesis in paddy soils under long-term managements. Plant Soil Environ. 2018, 64, 393–399. [Google Scholar] [CrossRef]
- Yang, G.; (State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China). Personal communication, 2022.
- Marushchak, M.E.; (Department of Environmental and Biological Sciences, University of Eastern Finland, Kuopio, Finland). Personal communication, 2022.
- Liu, W.; Qiao, C.; Yang, S.; Bai, W.; Liu, L. Microbial carbon use efficiency and priming effect regulate soil carbon storage under nitrogen deposition by slowing soil organic matter decomposition. Geoderma 2018, 332, 37–44. [Google Scholar] [CrossRef]
- Melchert, J.O.; Wischhöfer, P.; Knoblauch, C.; Eckhardt, T.; Liebner, S.; Rethemeyer, J. Sources of CO2 Produced in Freshly Thawed Pleistocene-Age Yedoma Permafrost. Front. Earth Sci. 2022, 9, 737237. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J.; Qin, S.; Liu, L.; Fang, K.; Xu, Y.; Ding, J.; Li, F.; Luo, Y.; Yang, Y. Determinants of carbon release from the active layer and permafrost deposits on the Tibetan Plateau. Nat. Commun. 2016, 7, 13046. [Google Scholar] [CrossRef] [PubMed]
- Gentsch, N.; Wild, B.; Mikutta, R.; Čapek, P.; Diáková, K.; Schrumpf, M.; Turner, S.; Minnich, C.; Schaarschmidt, F.; Shibistova, O.; et al. Temperature response of permafrost soil carbon is attenuated by mineral protection. Glob. Chang. Biol. 2018, 24, 3401–3415. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, C.; Beer, C.; Liebner, S.; Grigoriev, M.N.; Pfeiffer, E.-M. Methane production as key to the greenhouse gas budget of thawing permafrost. Nat. Clim. Chang. 2018, 8, 309–312. [Google Scholar] [CrossRef]
- Holm, S.; Walz, J.; Horn, F.; Yang, S.; Grigoriev, M.N.; Wagner, D.; Knoblauch, C.; Liebner, S. Methanogenic response to long-term permafrost thaw is determined by paleoenvironment. FEMS Microbiol. Ecol. 2020, 96, fiaa021. [Google Scholar] [CrossRef]
- Davidson, E.A.; Keller, M.; Erickson, H.E.; Vercholt, L.V.; Veldkamp, E. Testing a Conceptual Model of Soil Emissions of Nitrousand Nitric Oxides. BioScience 2000, 50, 677–680. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Rousk, K.; Michelsen, A.; Rousk, J. Microbial control of soil organic matter mineralization responses to labile carbon in subarctic climate change treatments. Glob. Chang. Biol. 2016, 22, 4150–4161. [Google Scholar] [CrossRef]
- Hicks, L.C.; Leizeaga, A.; Rousk, K.; Michelsen, A.; Rousk, J. Simulated rhizosphere deposits induce microbial N-mining that may accelerate shrubification in the subarctic. Ecology 2020, 101, e03094. [Google Scholar] [CrossRef]
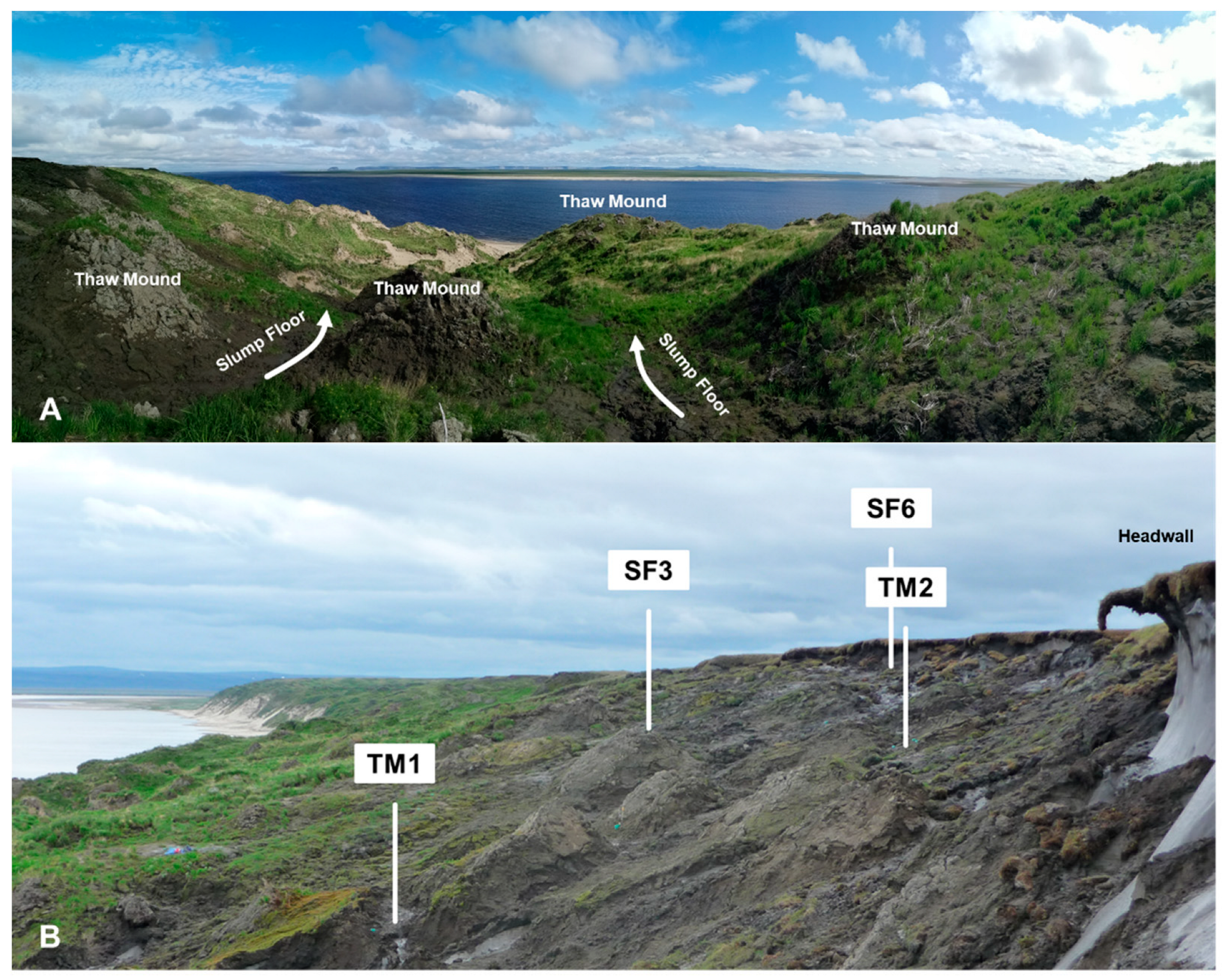
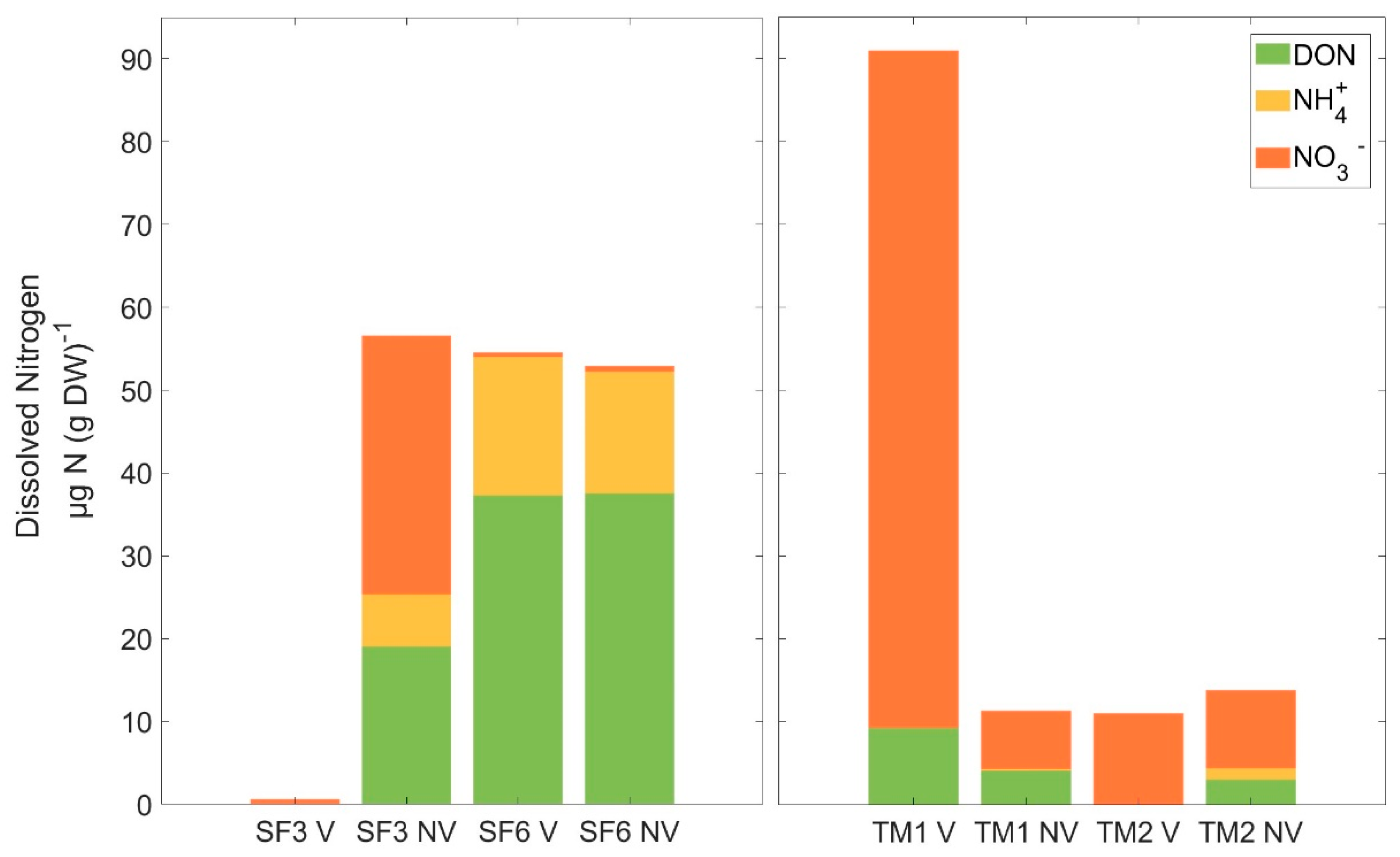



| Site | AL Depth (cm) | n | WC (%) | WFPS (%) | pH | TC (%) | C/N | δ15N Bulk (‰) | WEOC (µg C (g DW)−1) | P (µg P (g DW)−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| SF3 V | 30 | 3 | 28 ± 2 | 67 ± 3 | 6.1 ± 0.2 | 6.5 ± 1.0 | 15.3 ±1.7 | 1.76 ± 0.07 | 2083 ± 307 | 7.7 ± 0.3 |
| SF3 NV | 100 | 3 | 25 ± 2 | 63 ± 5 | 5.0 ± 0.2 | 5.2 ± 0.9 | 19.2 ± 0.3 | 2.31 ± 0.09 | 1325 ± 175 | 7.0 ± 0.4 |
| SF6 V | 30 | 5 | 25 ± 1 | 66 ± 1 | 6.2 ± 0.1 * | 4.7 ± 0.3 | 17.4 ± 0.1 | 2.02 ± 0.12 | 1671 ± 36 ** | 12.5 ± 0.8 * |
| SF6 NV | 30 | 2 | 21 ± 2 | 52 ± 5 | 5.6 ± 0.1 | 3.4 ± 0.7 | 18.1 ± 0.3 | 2.10 ± 0.11 | 1302 ± 139 ** | 18.6 ± 1.0 |
| TM1 V | 30 | 3 | 24 ± 2 | 60 ± 3 | 7.9 ± 0.0 | 4.4 ± 0.2 | 12.8 ± 0.1 | 1.95 ± 0.08 | 1702 ± 15 | 22.9 ± 5.6 |
| TM1 NV | 70 | 3 | 29 ± 0 | 74 ± 1 | 7.8 ± 0.0 | 3.9 ± 0.1 | 12.9 ± 0.1 | 1.52 ± 0.03 | 1919 ± 104 | 18.2 ± 0.4 |
| TM2 V | 30 | 3 | 19 ± 0 | 49 ± 1 | 8.1 ± 0.1 | 2.8 ± 0.1 | 14.2 ± 0.3 | 1.65 ± 0.16 | 1218 ± 53 | 11.8 ± 0.9 |
| TM2 NV | 110 | 3 | 17 ± 0 | 45 ± 0 | 8.2 ± 0.0 | 2.0 ± 0.1 | 14.4 ± 0.2 | 2.60 ± 0.04 | 753 ± 25 | 9.0 ± 0.3 |
| Parameter | Between TM and SF Sites | Between TM V and TM NV Sites | Between SFV and SF NV Sites | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sign. | TM | SF | Sign. | TM V | TM NV | Sign. | SF V | SF NV | |
| WC | n.s. | n.s. | n.s. | ||||||
| WFPS | n.s | n.s. | |||||||
| pH | *** | a | n.s. | *** | a | ||||
| TC | *** | a | n.s | ||||||
| WEOC | n.s. | n.s | ** | a | |||||
| C/N | *** | a | n.s | ** | a | ||||
| P | n.s. | n.s | n.s. | ||||||
| δ15N bulk | n.s. | n.s. | ** | a | |||||
| DON | *** | a | n.s. | ||||||
| NH4+ | *** | a | n.s. | ||||||
| NO3− | *** | a | n.s. | (**) | a | ||||
| Net ammonification | *** | a | n.s. | n.s. | |||||
| Net nitrification | n.s. | n.s. | n.s. | ||||||
| N2O production | n.s. | n.s. | (**) | a | |||||
| CO2 production | *** | a | n.s | *** | a | ||||
| CH4 production | ** | a | n.s | ** | a | ||||
| CO2 equivalents | n.s. | n.s. | n.s. | ||||||
| Site | n | Net Ammonification (ng N (g DW)−1 day−1) | Net Nitrification (ng N (g DW)−1 day−1) |
|---|---|---|---|
| SF3 V | 3 | 23.25 ± 8.74 | 484.39 ± 289.91 |
| SF3 NV | 3 | 54.39 ± 74.98 | 1088.40 ± 539.50 |
| SF6 V | 5 | 58.57 ± 39.10 | 999.04 ± 595.15 |
| SF6 NV | 2 | 69.51 ± 5.26 | 0.00 ± 0.00 |
| TM1 V | 3 | 7.60 ± 0.32 | 1437.41 ± 539.73 |
| TM1 NV | 3 | 5.10 ± 3.64 | 383.11 ± 85.47 |
| TM2 V | 3 | 5.01 ± 1.97 | 197.13 ± 2.48 |
| TM2 NV | 3 | 1.97 ± 1.16 | 278.16 ± 58.26 |
| Site | n | N2O In–Situ (mg N2O-N m−2 Day−1) | Anaerobic CO2 (µg CO2-C (g DW)−1 Day−1) | Anaerobic CH4 (ng CH4-C (g DW)−1 Day−1) |
|---|---|---|---|---|
| SF3 V | 3 | n.d. | 6.00 ± 2.57 | 14.90 ± 13.80 |
| SF3 NV | 3 | 2.03 ± 1.39 | 0.99 ± 0.33 | 0.00 ± 0.00 |
| SF6 V | 5 | n.d. | 7.07 ± 1.76 | 65.46 ± 6.83 |
| SF6 NV | 2 * | 0.01 ± 0.02 | 2.67 ± 1.30 | 16.97 ± 13.17 |
| TM1 V | 3 | n.d. | 0.89 ± 0.28 | 7.80 ± 11.02 |
| TM1 NV | 3 | 0.68 ± 0.23 | 2.34 ± 0.84 | 6.22 ± 8.58 |
| TM2 V | 3 | n.d. | 0.24 ± 0.04 | 0.57 ± 0.81 |
| TM2 NV | 3 | 0.10 ± 0.04 | 0.52 ± 0.13 | 0.11 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegner, R.; Fiencke, C.; Knoblauch, C.; Sauerland, L.; Beer, C. Rapid Permafrost Thaw Removes Nitrogen Limitation and Rises the Potential for N2O Emissions. Nitrogen 2022, 3, 608-627. https://doi.org/10.3390/nitrogen3040040
Wegner R, Fiencke C, Knoblauch C, Sauerland L, Beer C. Rapid Permafrost Thaw Removes Nitrogen Limitation and Rises the Potential for N2O Emissions. Nitrogen. 2022; 3(4):608-627. https://doi.org/10.3390/nitrogen3040040
Chicago/Turabian StyleWegner, Rica, Claudia Fiencke, Christian Knoblauch, Lewis Sauerland, and Christian Beer. 2022. "Rapid Permafrost Thaw Removes Nitrogen Limitation and Rises the Potential for N2O Emissions" Nitrogen 3, no. 4: 608-627. https://doi.org/10.3390/nitrogen3040040
APA StyleWegner, R., Fiencke, C., Knoblauch, C., Sauerland, L., & Beer, C. (2022). Rapid Permafrost Thaw Removes Nitrogen Limitation and Rises the Potential for N2O Emissions. Nitrogen, 3(4), 608-627. https://doi.org/10.3390/nitrogen3040040








