Towards Understanding the Microepidemiology of Lymphatic Filariasis at the Community Level in Ghana
Abstract
1. Introduction
2. Materials and Methods
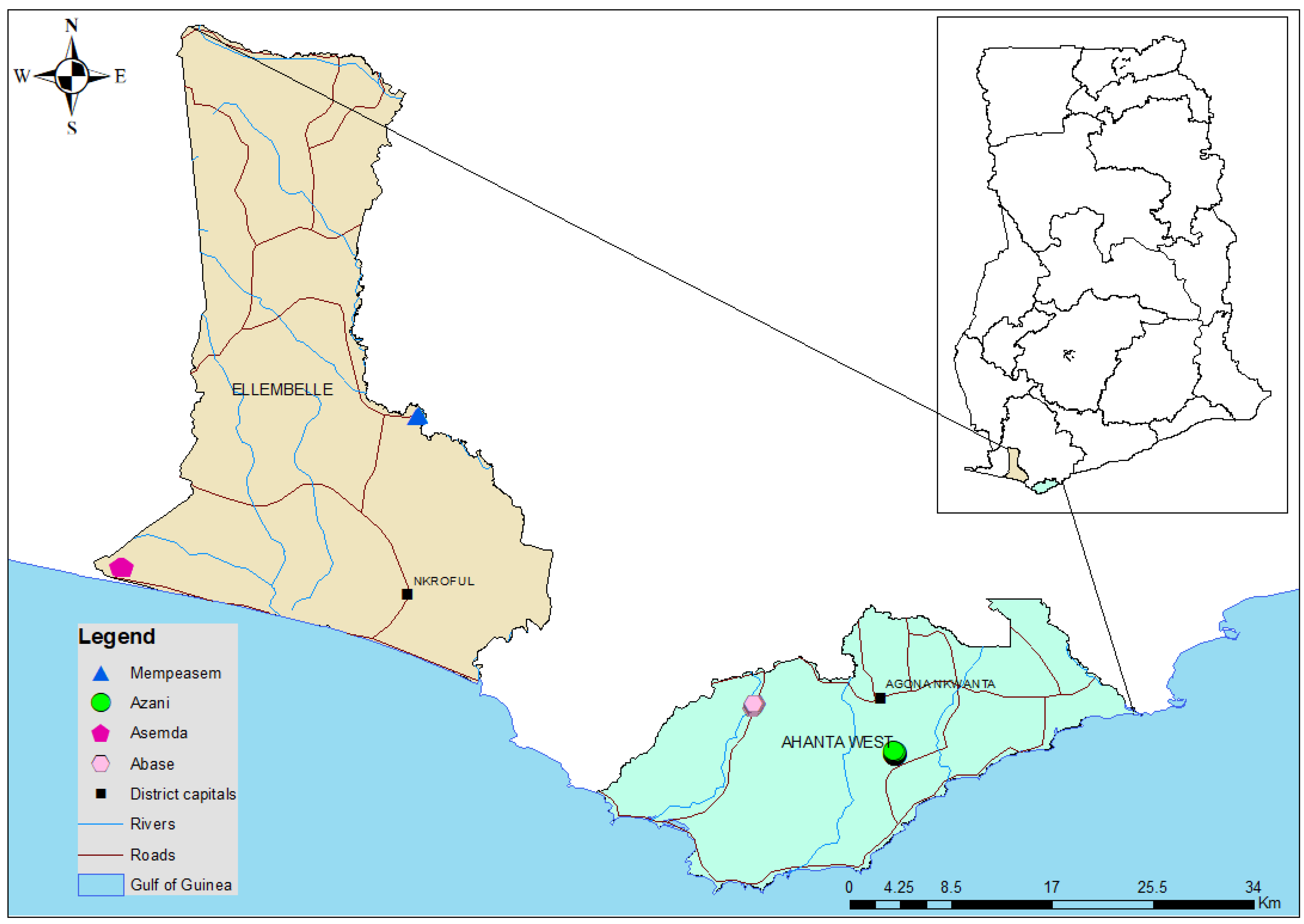
2.1. Study Sites
2.2. Study Design
- Adults 18 years of age and above
- Participants who have provided informed consent.
- Participants who are residents of communities being studied.
- Individuals who agree to blood collection and testing.
- Children under 18 years.
- Adults who refused consenting.
- Adults who refuse blood collection and testing.
- Adults who are sick or unwell at the time of the sampling.
2.3. Data Analysis
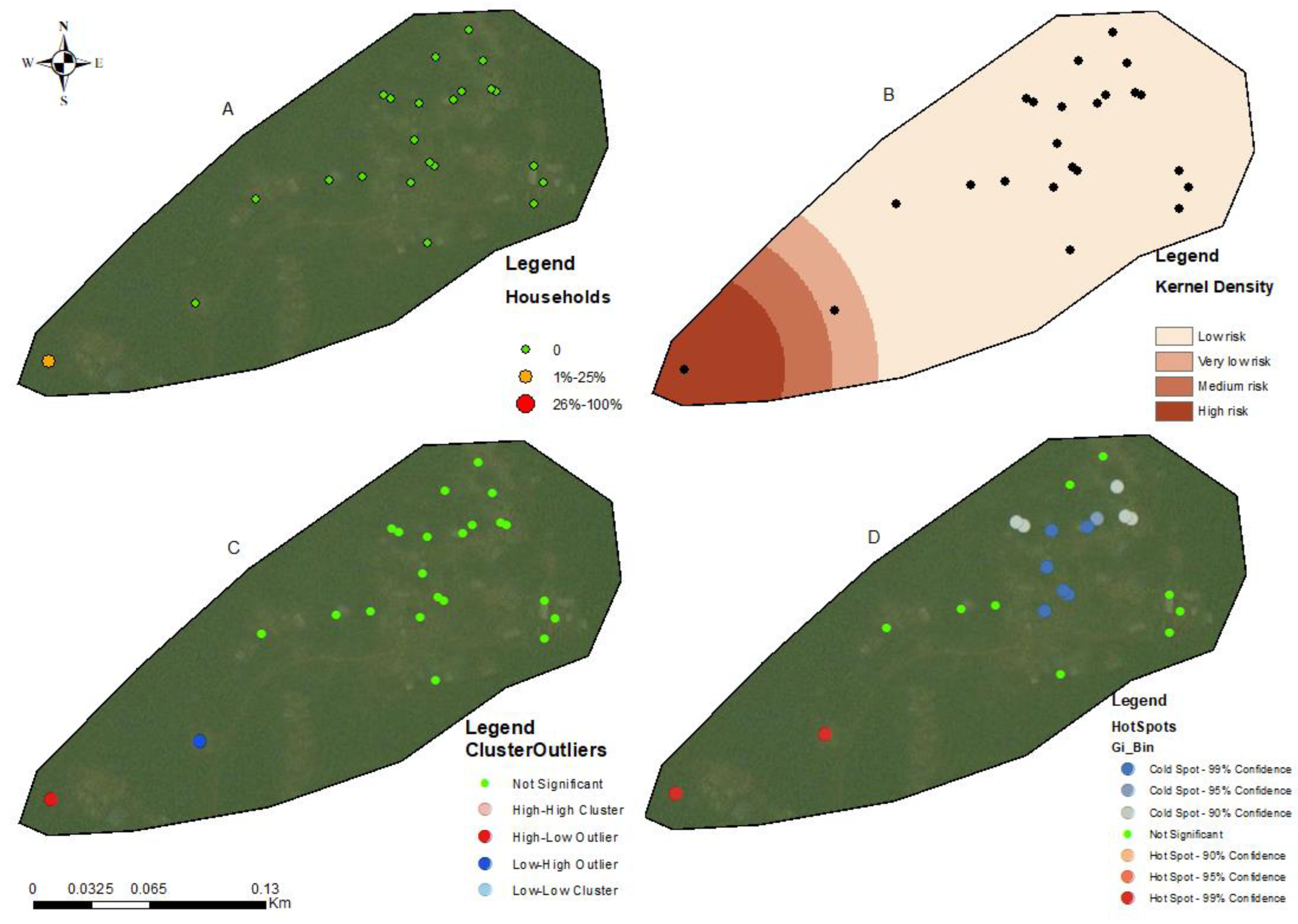
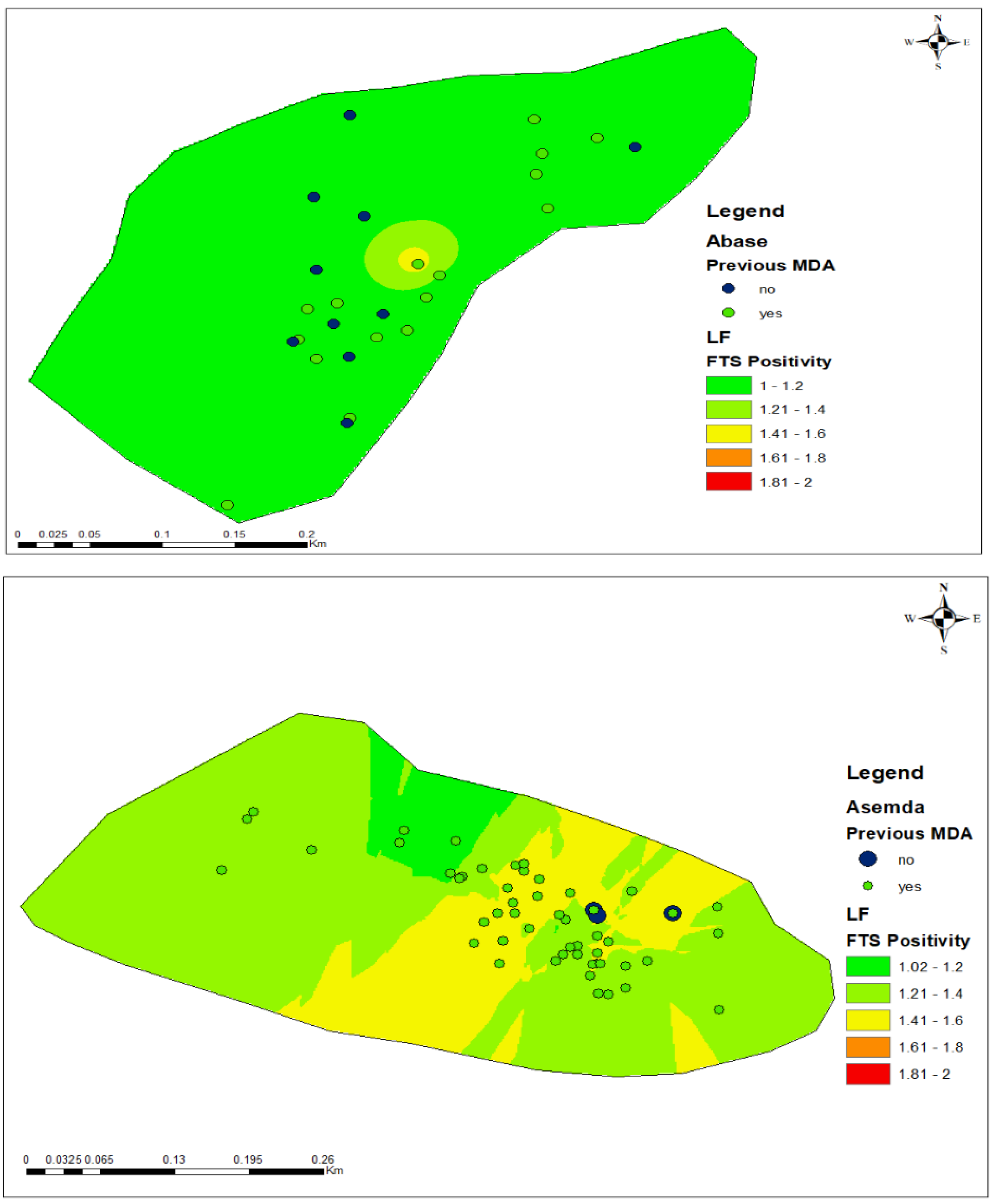
2.4. Spatial Mapping and Predictive Risk Maps
2.5. Spatial Autocorrelation Analysis
3. Results
3.1. Study Participants and Community Characteristics
3.2. Associations between Demographic Variables, MDA, and Infection
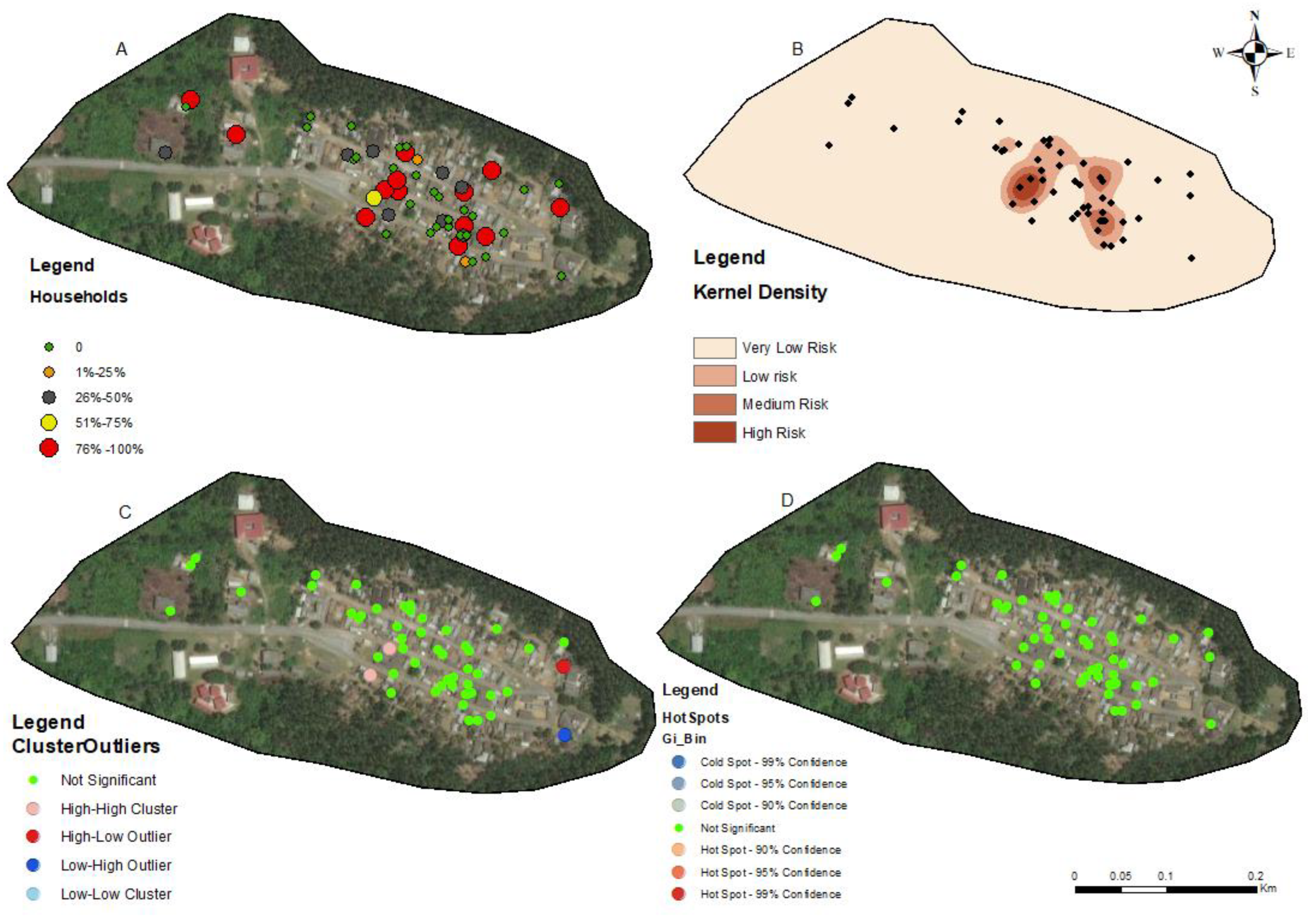
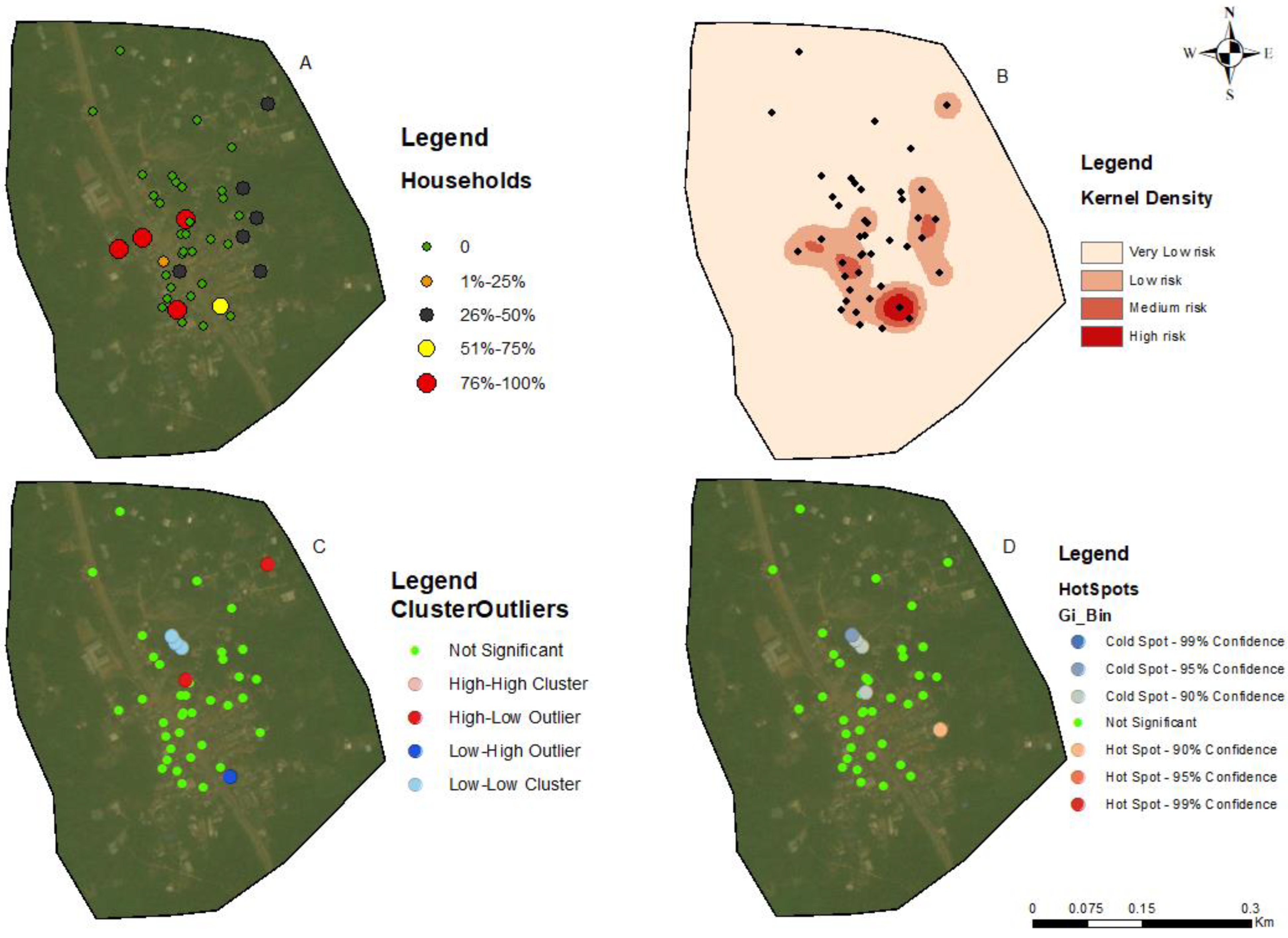
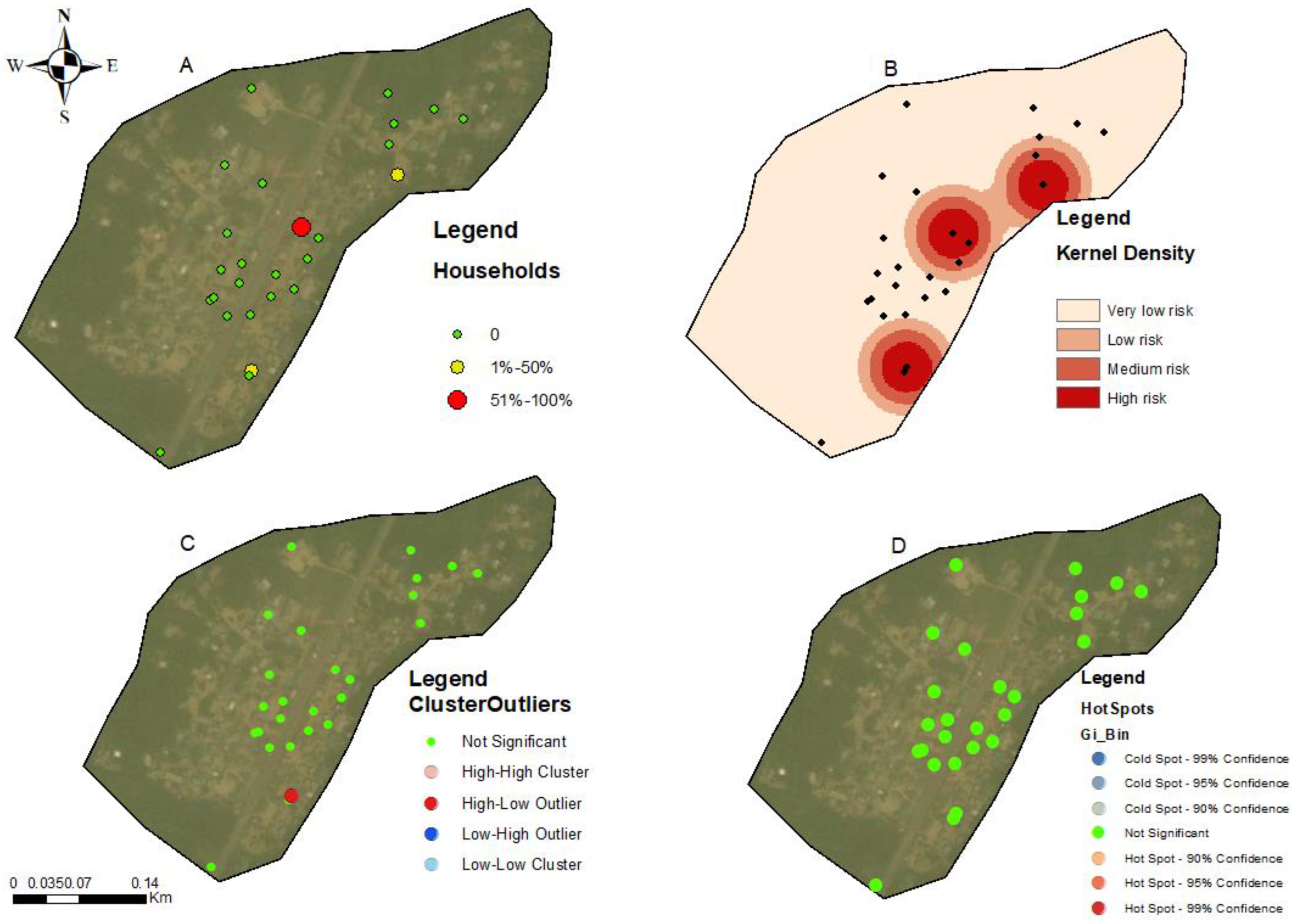
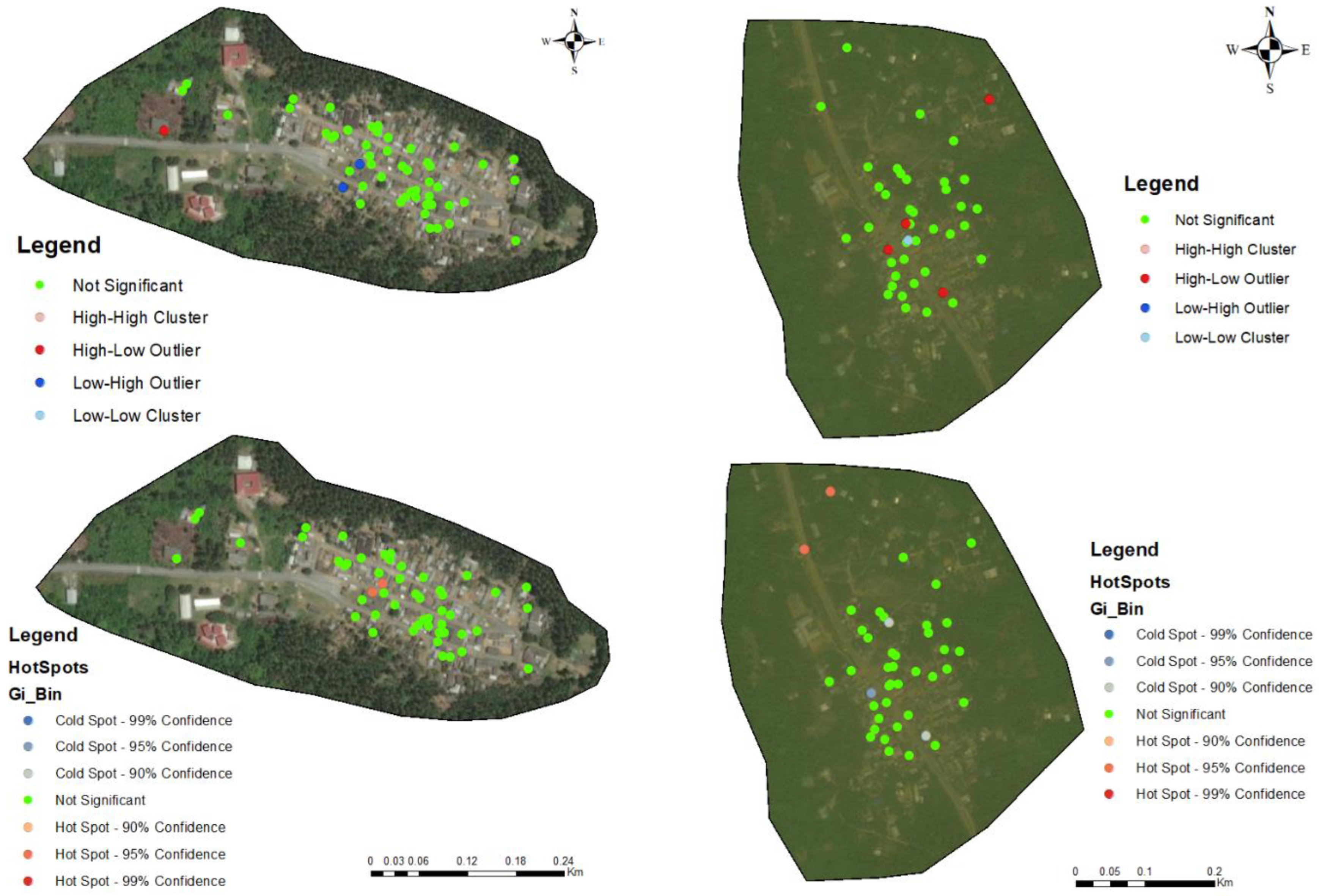
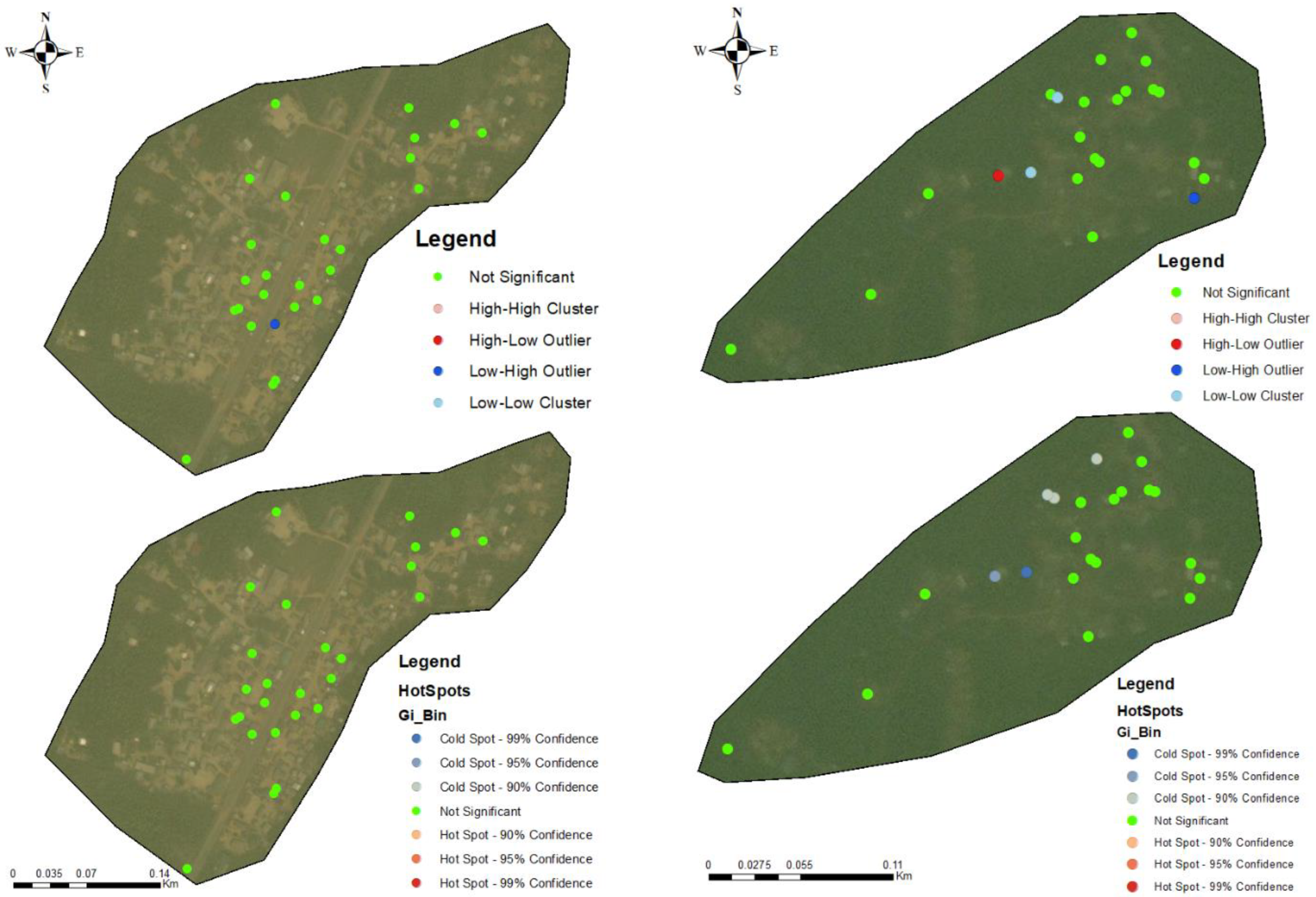
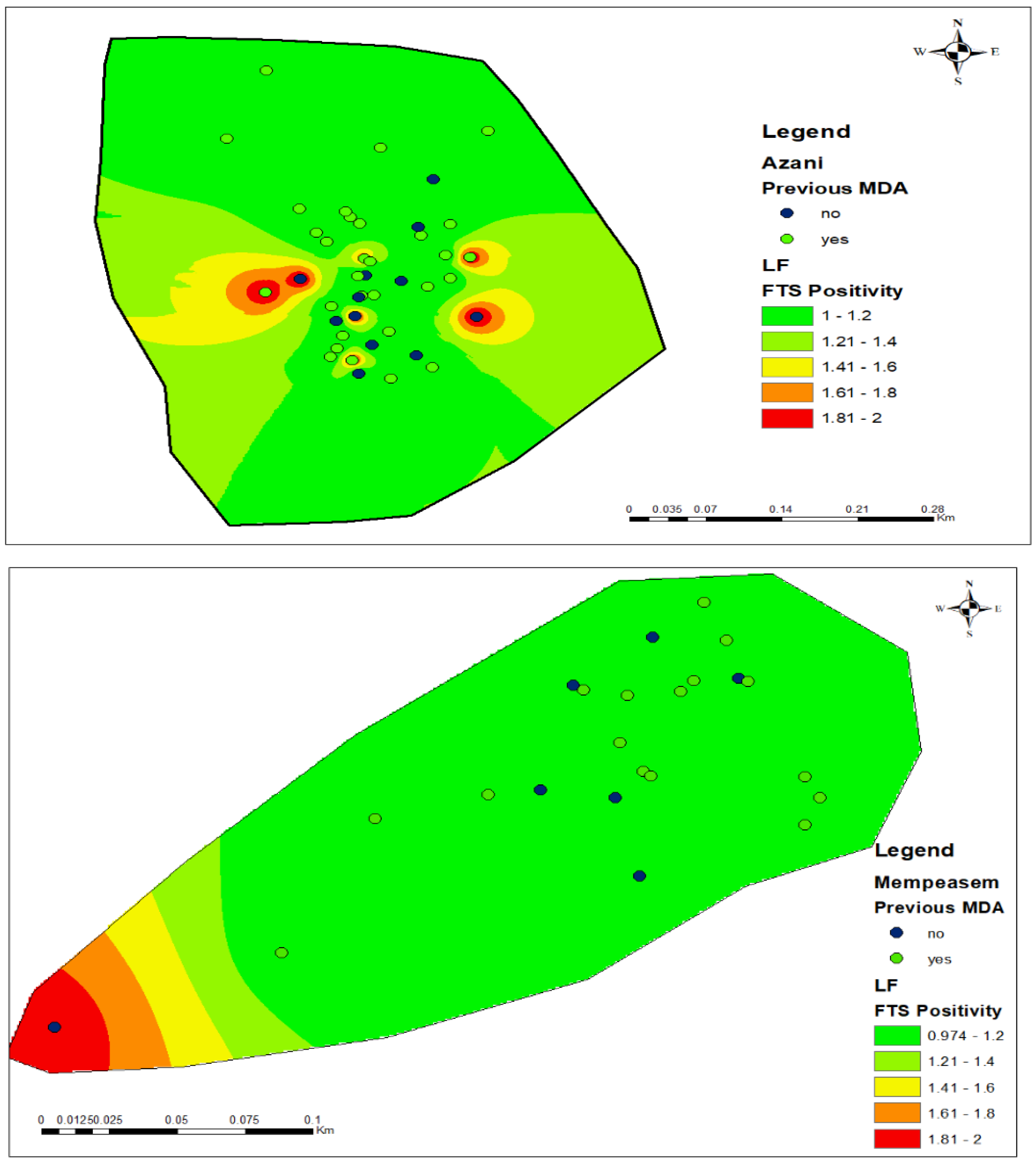
3.3. Hotspots for Community Infections
3.4. Spatial Autocorrelation
3.5. Ordinary Least Squares (OLS) and Geographic Weighted Regression Analysis (GWR)
3.6. FTS Positivity and Participation in Mass Drug Administration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Global Distribution of Lymphatic Filariasis, 2000-18: A Geospatial Analysis; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Cano, J.; Rebollo, M.P.; Golding, N.; Pullan, R.L.; Crellen, T.; Soler, A.; Hope, L.A.K.; Lindsay, S.W.; I Hay, S.; Bockarie, M.J.; et al. The global distribution and transmission limits of lymphatic filariasis: Past and present. Parasites Vectors 2014, 7, 466. [Google Scholar] [CrossRef]
- Gyapong, J.O.; Kumaraswami, V.; Biswas, G.; Ottesen, E.A. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin. Pharmacother. 2005, 6, 179–200. [Google Scholar] [CrossRef]
- Col, L.; Agrawal, V.K.; Cdr, W.; Sashindran, V.K. Lymphatic Filariasis in India: Problems, Challenges and New Initiatives. Med. J. Armed Forces India 2006, 62, 359–362. [Google Scholar]
- Ottesen, E.A.; Duke, B.O.L.; Karam, M.; Behbehani1, K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull. World Health Organ. 1997, 75, 491–503. [Google Scholar]
- Casulli, A. New global targets for ntds in the who roadmap 2021–2030. PLoS Neglected Trop. Dis. 2021, 15, e0009373. [Google Scholar] [CrossRef]
- Malecela, M.N.; Ducker, C. A road map for neglected tropical diseases 2021–2030. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 121–123. [Google Scholar] [CrossRef]
- Ottesen, E.A. Editorial: The Global Pl Programme to Eliminate Lymphatic Filariasis. Trop. Med. Int. Health 2000, 5, 591–594. [Google Scholar] [CrossRef]
- Srividya, A.; Subramanian, S.; Jambulingam, P.; Vijayakumar, B.; Dinesh Raja, J. Mapping and monitoring for a lymphatic filariasis elimination program: A systematic review. Res. Rep. Trop. Med. 2019, 10, 43–90. [Google Scholar] [CrossRef]
- de Souza, D.K.; Otchere, J.; Sumboh, J.G.; Asiedu, O.; Opare, J.; Asemanyi-Mensah, K.; Boakye, D.A.; Gass, K.M.; Long, E.F.; Ahorlu, C.S. Finding and eliminating the reservoirs: Engage and treat, and test and treat strategies for lymphatic filariasis programs to overcome endgame challenges. Front. Trop. Dis. 2022, 3, 953094. [Google Scholar] [CrossRef]
- Bockarie, M.J.; Taylor, M.J.; Gyapong, J.O. Current practices in the management of lymphatic filariasis. Expert Rev. Anti-Infect. Ther. 2009, 7, 595–605. [Google Scholar] [CrossRef]
- Manyeh, A.K.; Ibisomi, L.; Ramaswamy, R.; Baiden, F.; Chirwa, T. Exploring factors affecting quality implementation of lymphatic filariasis mass drug administration in bole and central gonja districts in northern ghana. PLoS Neglected Trop. Dis. 2020, 14, e0007009. [Google Scholar] [CrossRef] [PubMed]
- Mensah, D.A.; Debrah, L.B.; Gyamfi, P.A.; Rahamani, A.A.; Opoku, V.S.; Boateng, J.; Obeng, P.; Osei-Mensah, J.; Kroidl, I.; Klarmann-Schulz, U.; et al. Occurrence of Lymphatic Filariasis infection after 15 years of mass drug administration in two hotspot districts in the Upper East Region of Ghana. PLoS Neglected Trop. Dis. 2022, 16, e0010129. [Google Scholar] [CrossRef] [PubMed]
- Silumbwe, A.; Zulu, J.M.; Halwindi, H.; Jacobs, C.; Zgambo, J.; Dambe, R.; Chola, M.; Chongwe, G.; Michelo, C. A systematic review of factors that shape implementation of mass drug administration for lymphatic filariasis in sub-Saharan Africa. BMC Public Health 2017, 17, 484. [Google Scholar] [CrossRef] [PubMed]
- Biritwum, N.-K.; de Souza, D.K.; Marfo, B.; Odoom, S.; Alomatu, B.; Asiedu, O.; Yeboah, A.; Hervie, T.E.; Mensah, E.O.; Yikpotey, P.; et al. Fifteen years of programme implementation for the elimination of Lymphatic Filariasis in Ghana: Impact of MDA on immunoparasitological indicators. PLoS Neglected Trop. Dis. 2017, 11, e0005280. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.C. The Role of Spatial Statistics in the Control and Elimination of Neglected Tropical Diseases in Sub-Saharan Africa: A Focus on Human African Trypanosomiasis, Schistosomiasis and Lymphatic Filariasis. Adv. Parasitol. 2017, 97, 187–241. [Google Scholar] [PubMed]
- Robertson, C.; Nelson, T.A. An Overview of Spatial Analysis of Emerging Infectious Diseases. Prof. Geogr. 2014, 66, 579–588. [Google Scholar] [CrossRef]
- Eneanya, O.A.; Fronterre, C.; Anagbogu, I.; Okoronkwo, C.; Garske, T.; Cano, J.; Donnelly, C.A. Mapping the baseline prevalence of lymphatic filariasis across Nigeria. Parasites Vectors 2019, 12, 440. [Google Scholar] [CrossRef]
- Joseph, H.; Moloney, J.; Maiava, F.; McClintock, S.; Lammie, P.; Melrose, W. First evidence of spatial clustering of lymphatic filariasis in an Aedes polynesiensis endemic area. Acta Trop. 2011, 120, S39–S47. [Google Scholar] [CrossRef] [PubMed]
- de Souza, D.K.; Gass, K.; Otchere, J.; Htet, Y.M.; Asiedu, O.; Marfo, B.; Biritwum, N.K.; Boakye, D.A.; Ahorlu, C.S. Review of MDA registers for lymphatic filariasis: Findings, and potential uses in addressing the endgame elimination challenges. PLoS Neglected Trop. Dis. 2020, 14, e0008306. [Google Scholar] [CrossRef]
- Kwarteng, E.V.S.; Andam-Akorful, S.A.; Kwarteng, A.; Asare, D.C.B.; Quaye-Ballard, J.A.; Osei, F.B.; Duker, A.A. Spatial variation in lymphatic filariasis risk factors of hotspot zones in Ghana. BMC Public Health 2021, 21, 230. [Google Scholar] [CrossRef]
- Amekudzi, L.K.; Yamba, E.I.; Preko, K.; Asare, E.O.; Aryee, J.; Baidu, M.; Codjoe, S.N.A. Variabilities in rainfall onset, cessation and length of rainy season for the various agro-ecological zones of Ghana. Climate 2015, 3, 416–434. [Google Scholar] [CrossRef]
- Pantelias, A.; King, J.D.; Lammie, P.; Weil, G.J. Development and Introduction of the Filariasis Test Strip: A New Diagnostic Test for the Global Program to Eliminate Lymphatic Filariasis. Am. J. Trop. Med. Hyg. 2022, 106, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Touloupou, P.; Retkute, R.; Hollingsworth, T.D.; Spencer, S.E.F. Statistical methods for linking geostatistical maps and transmission models: Application to lymphatic filariasis in East Africa. Spat. Spatio Temporal Epidemiol. 2022, 41, 100391. [Google Scholar] [CrossRef] [PubMed]
- Sabesan, S.; Raju, H.K.K.; Srividya, A.N.; Das, P.K. Delimitation of lymphatic filariasis transmission risk areas: A geo-environmental approach. Filaria J. 2006, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Ray, E.L.; Sakrejda, K.; Lauer, S.A.; Johansson, M.A.; Reich, N.G. Infectious disease prediction with kernel conditional density estimation. Stat. Med. 2017, 36, 4908–4929. [Google Scholar] [CrossRef]
- Lin, C.H.; Wen, T.H. How Spatial Epidemiology Helps Understand Infectious Human Disease Transmission. Trop. Med. Infect. Dis. 2022, 7, 164. [Google Scholar] [CrossRef]
- Bhunia, G.S.; Roy, S.; Shit, P.K. Spatio-temporal analysis of COVID-19 in India—A geostatistical approach. Spat. Inf. Res. 2021, 29, 661–672. [Google Scholar] [CrossRef]
- Tripathi, B.; Roy, N.; Dhingra, N. Introduction of Triple-Drug Therapy for Accelerating Lymphatic Filariasis Elimination in India: Lessons Learned. Am. J. Trop. Med. Hyg. 2022, 106, 29–38. [Google Scholar] [CrossRef]
- Shaw, C.; McLure, A.; Graves, P.M.; Lau, C.L.; Glass, K. Lymphatic filariasis endgame strategies: Using GEOFIL to model mass drug administration and targeted surveillance and treatment strategies in American Samoa. PLoS Neglected Trop. Dis. 2023, 17, e0011347. [Google Scholar] [CrossRef]
- Senkwe, M.N.; Berta, K.K.; Logora, S.M.Y.; Sube, J.; Bidali, A.; Abe, A.; Onyeze, A.; Pita, J.; Rumunu, J.; Maleghemi, S.; et al. Prevalence and factors associated with transmission of lymphatic filariasis in South Sudan: A cross-sectional quantitative study. Pan Afr. Med. J. 2022, 42, 61–67. [Google Scholar]
- Kwarteng, E.V.S.; Osei, F.B.; Andam-Akorful, S.A.; Kwarteng, A.; Asare, D.-C.B.M.; Quaye-Ballard, J.A.; Duker, A.A. Mapping Spatial Variation and Impact of the National MDA Program on Lymphatic Filariasis Elimination in Ghana: An Initial Study. Front. Trop. Dis. 2022, 3, 811909. [Google Scholar] [CrossRef]
- Medeiros, Z.; Bonfim, C.; Brandão, E.; Netto, M.J.E.; Vasconcellos, L.; Ribeiro, L.; Portugal, J. Using kernel density estimates to investigate lymphatic filariasis in northeast brazil. Pathog. Glob. Health 2012, 106, 113–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gyapong, J.O.; Kyelem, D.; Kleinschmidt, I.; Agbo, K.; Ahouandogbo, F.; Gaba, J.; Owusu-Banahene, G.; Sanou, S.; Sodahlon, Y.K.; Biswas, G.; et al. The use of spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Ann. Trop. Med. Parasitol. 2002, 96, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Surjati, E.; Wiwoho, B.S. Transmission elimination of lymphatic filariasis using spatial autocorrelation. J. Phys. Conf. Ser. 2021, 1869, 012106. [Google Scholar] [CrossRef]
- Slater, H.; Michael, E. Mapping, Bayesian Geostatistical Analysis and Spatial Prediction of Lymphatic Filariasis Prevalence in Africa. PLoS ONE 2013, 8, e71574. [Google Scholar] [CrossRef]
- Slater, H.; Michael, E. Predicting the current and future potential distributions of lymphatic filariasis in africa using maximum entropy ecological niche modelling. PLoS ONE 2012, 7, e32202. [Google Scholar] [CrossRef] [PubMed]
- Eneanya, O.A.; Cano, J.; Dorigatti, I.; Anagbogu, I.; Okoronkwo, C.; Garske, T.; Donnelly, C.A. Environmental suitability for lymphatic filariasis in Nigeria 01 Mathematical Sciences 0104 Statistics. Parasites Vectors 2018, 11, 513. [Google Scholar] [CrossRef]
- Astuti, E.P.; Hendri, J.; Ipa, M.; Ruliansyah, A.; Garjito, T.A. Vector Surveillance for Lymphatic Filariasis After Mass Drug Administration in an Endemic Area: A Case Study in Bekasi. J. Kesehat. Lingkung. 2023, 15, 134–142. [Google Scholar] [CrossRef]
- Slater, H.C. Spatial Epidemiology and the Integrated Control of Malaria and Lymphatic Filariasis in Africa; School of Public Health, Imperial College London: London, UK, 2012. [Google Scholar]
- Nyandwi, E.; Veldkamp, T.; Amer, S.; Ruberanziza, E.; Rujeni, N.; Umulisa, I. Using Routinely Collected Health Records to Identify the Fine-Resolution Spatial Patterns of Soil-Transmitted Helminth Infections in Rwanda. Trop. Med. Infect. Dis. 2022, 7, 202. [Google Scholar] [CrossRef]
- Muhd Nor, K.; Che Dom, N.; Abdullah, S.; Precha, N. Spatial and Temporal Analysis of Dengue Cases in Peninsular Malaysia: A five-year analysis from 2016 to 2020. Environ. Behav. Proc. J. 2022, 7, 275–282. [Google Scholar] [CrossRef]
- Fornace, K.M.; Senyonjo, L.; Martin, D.L.; Gwyn, S.; Schmidt, E.; Agyemang, D.; Marfo, B.; Addy, J.; Mensah, E.; Solomon, A.W.; et al. Characterising spatial patterns of neglected tropical disease transmission using integrated sero-surveillance in Northern Ghana. PLoS Neglected Trop. Dis. 2022, 16, e0010227. [Google Scholar] [CrossRef]
- Tesema, G.A.; Tessema, Z.T.; Heritier, S.; Stirling, R.G.; Earnest, A. A Systematic Review of Joint Spatial and Spatiotemporal Models in Health Research. Int. J. Environ. Res. Public Health 2023, 20, 5295. [Google Scholar] [CrossRef]
- Bockarie, M.J.; Pedersen, E.M.; White, G.B.; Michael, E. Role of vector control in the global program to eliminate lymphatic filariasis. Annu. Rev. Entomol. 2009, 54, 469–487. [Google Scholar] [CrossRef]
- Gyapong, J.O.; Owusu, I.O.; da-Costa Vroom, F.B.; Mensah, E.O.; Gyapong, M. Elimination of lymphatic filariasis: Current perspectives on mass drug administration. Res. Rep. Trop. Med. 2018, 9, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ackley, C.; Elsheikh, M.; Zaman, S. Scoping review of neglected tropical disease interventions and health promotion: A framework for successful ntd interventions as evidenced by the literature. PLoS Neglected Trop. Dis. 2021, 15, e0009278. [Google Scholar] [CrossRef]
- Leonardo, L.; Bergquist, R.; Li, S.Z.; Lv, S.; Khieu, V.; Sayasone, S.; Xu, J.; Olveda, R.; Utzinger, J.; Sripa, B.; et al. Multi-disciplinary integration of networking through the RNAS+: Research on other target diseases. Adv. Parasitol. 2019, 105, 95–110. [Google Scholar]
- Sun, Y.; Hu, X.; Xie, J. Spatial inequalities of COVID-19 mortality rate in relation to socioeconomic and environmental factors across England. Sci. Total Environ. 2021, 758, 143595. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Karim, M.J.; Uddin, S.; Jahan, S.; Asm, S.M.; Forbes, S.P.; Hooper, A.; Taylor, M.J.; Kelly-Hope, L.A. Socio-economic and environmental factors associated with high lymphatic filariasis morbidity prevalence distribution in Bangladesh. PLoS Neglected Trop. Dis. 2023, 17, e0011457. [Google Scholar] [CrossRef]
- Gananalatha, E.; Edirisinghe, G. Socio-economic and Environmental Determinants of Filariasis in Matara District of Sri Lanka. Am. Sci. Res. J. Eng. 2017, 32, 105–118. [Google Scholar]
| Total | Positive FTS | Negative FTS | |||
|---|---|---|---|---|---|
| N = 252 (%) | N = 49 (%) | N = 203 (%) | Statistic | p-Value | |
| Community | Chi = 22.11 | <0.001 | |||
| Azani | 74 (29.37) | 15 (20.27) | 59 (79.73) | ||
| Abase | 46 (18.25) | 3 (6.52) | 43 (93.48) | ||
| Asemda | 87 (34.52) | 29 (33.33) | 58 (66.67) | ||
| Mempeasem | 45 (17.86) | 2 (4.44) | 43 (95.56) | ||
| District | Chi2 = 2.88 | 0.089 | |||
| Ahanta West | 120 (47.62) | 18 (15.00) | 102 (85.00) | ||
| Ellembelle | 132 (52.38) | 31 (23.48) | 101 (76.52) | ||
| Sex | Chi2 = 8.05 | 0.005 | |||
| Female | 148 (58.73) | 20 (13.51) | 128 (86.49) | ||
| Male | 104 (41.27) | 29 (27.88) | 75 (72.12) | ||
| Age | Chi2 = 5.73 | 0.572 | |||
| ≤20 | 13 (5.16) | 2 (15.38) | 11 (84.62) | ||
| 21–30 | 75 (29.76) | 12 (16.00) | 63 (84.62) | ||
| 31–40 | 40 (15.87) | 8 (20.00) | 32 (80.00) | ||
| 41–50 | 49 (19.44) | 8 (16.33) | 41 (83.67) | ||
| 51–60 | 34 (13.49) | 8 (23.53) | 26 (76.47) | ||
| 61–70 | 29 (11.51) | 6 (20.69) | 23 (79.31) | ||
| 71–80 | 6 (2.38) | 2 (33.33) | 4 (66.67) | ||
| 80> | 6 (2.38) | 3 (50.00) | 3 (50.00) | ||
| Participation in previous MDA | Chi2 = 2.61 | 0.106 | |||
| Yes | 200 (79.37) | 43 (21.50) | 157 (78.50) | ||
| No | 52 (20.63) | 6 (11.54) | 46 (88.46) |
| N | Positive (%) | Negative (%) | Chi2 | p-Value | ||
|---|---|---|---|---|---|---|
| Community | ||||||
| Abase | 46 | 3 (6.5) | 43 (93.5) | 1.22 | 0.27 | |
| Males | 17 | 2 (11.8) | 15 (88.2) | |||
| Females | 29 | 1 (3.5) | 28 (96.6) | |||
| Asemda | 87 | 29 (33.3) | 58 (66.7) | 5.9 | 0.015 | |
| Males | 41 | 19 (46.3) | 22 (53.7) | |||
| Females | 46 | 10 (21.7) | 36 (78.3) | |||
| Azani | 74 | 15 (20.3) | 59 (79.7) | 1.61 | 0.205 | |
| Males | 20 | 6 (30.0) | 14 (70.0) | |||
| Females | 54 | 9 (16.7) | 45 (83.3) | |||
| Mempeasem | 45 | 2 (4.4) | 43 (95.6) | 1.53 | 0.216 | |
| Males | 26 | 2 (7.7) | 24 (92.3) | |||
| Females | 19 | 0 (0.0) | 19 (100) | |||
| Age | ||||||
| ≤20 | 13 | 2 (15.4) | 11 (84.6) | 2.03 | 0.155 | |
| Males | 7 | 2 (28.6) | 5 (71.4) | |||
| Females | 6 | 0 (0.0) | 6 (100) | |||
| 21–30 | 75 | 12 (16.0) | 63 (84.0) | 3.53 | 0.06 | |
| Males | 26 | 7 (27.0) | 19 (73.0) | |||
| Females | 49 | 5 (10.2) | 44 (89.8) | |||
| 31–40 | 40 | 8 (20.0) | 32 (80.0) | 3.63 | 0.057 | |
| Males | 18 | 6 (33.3) | 12 (66.7) | |||
| Females | 22 | 2 (9.1) | 20 (90.9) | |||
| 41–50 | 49 | 8 (16.3) | 41 (83.7) | 0.04 | 0.84 | |
| Males | 20 | 3 (15.0) | 17 (85.0) | |||
| Females | 29 | 5 (17.2) | 24 (82.8) | |||
| 51–60 | 34 | 8 (23.5) | 26 (76.5) | 0.15 | 0.702 | |
| Males | 15 | 4 (26.7) | 11 (73.3) | |||
| Females | 19 | 4 (21.1) | 15 (78.9) | |||
| 61–70 | 29 | 6 (20.7) | 23 (79.3) | 2.65 | 0.103 | |
| Males | 11 | 4 (36.4) | 7 (63.6) | |||
| Females | 18 | 2 (11.1) | 16 (88.9) | |||
| 71–80 | 6 | 2 (33.3) | 4 (66.7) | 1.5 | 0.221 | |
| Males | 2 | 0 (0.0) | 2 (100) | |||
| Females | 4 | 2 (50.0) | 2 (50.0) | |||
| 80> | 6 | 3 (50.0) | 3 (50.0) | 1.2 | 0.273 | |
| Males | 5 | 3 (60.0) | 2 (40.0) | |||
| Females | 1 | 0 (0.00) | 1 (100) | |||
| Participation in previous MDA | ||||||
| Yes | 200 | 43 (21.50) | 157 (78.5) | 10.07 | 0.002 | |
| Males | 79 | 26 (32.9) | 53 (67.1) | |||
| Females | 121 | 17 (14.1) | 104 (85.9) | |||
| No | 52 | 6 (11.5) | 46 (88.5) | 0.01 | 0.92 | |
| Males | 25 | 3 (12.0) | 22 (88.0) | |||
| Females | 27 | 3 (11.1) | 24 (88.9) |
| Spatial Parameters | Asemda | Abase | Azani | Mempeasem |
|---|---|---|---|---|
| Moran’s index: | 0.007037 | −0.084898 | −0.019971 | 0.043047 |
| Expected index: | −0.019608 | −0.04 | −0.02439 | −0.045455 |
| Variance: | 0.002905 | 0.009348 | 0.003744 | 0.002159 |
| z-score: | 0.233218 | −0.464386 | 0.072227 | 1.9049 |
| p-value: | 0.815592 | 0.642371 | 0.942421 | 0.056793 |
| Observed general G | 0.010687 | 0.003277 | 0.005307 | nan |
| Expected general G | 0.011232 | 0.010113 | 0.008678 | 0.019148 |
| Variance: | 0.000005 | 0.000151 | 0.000011 | inf |
| z-score: | −0.233428 | −0.555806 | −1.017551 | nan |
| p-value: | 0.815429 | 0.578343 | 0.308891 | nan |
| Spatial Parameters | Asemda | Abase | Azani | Mempeasem |
|---|---|---|---|---|
| Moran’s index: | −0.048013 | −0.088814 | −0.060009 | −0.052879 |
| Expected index: | −0.019608 | −0.04 | −0.02439 | −0.045455 |
| Variance: | 0.002918 | 0.010323 | 0.004088 | 0.009874 |
| z-score: | −0.525878 | −0.480445 | −0.557114 | −0.074718 |
| p-value: | 0.598973 | 0.630911 | 0.577449 | 0.940439 |
| Observed General G | 0.011734 | 0.011612 | 0.005906 | 0.015728 |
| Expected General G | 0.011232 | 0.010113 | 0.008678 | 0.019148 |
| Variance: | 0.000001 | 0.000003 | 0.000002 | 0.000009 |
| z-score: | 0.417736 | 0.815016 | −2.197311 | −1.156848 |
| p-value: | 0.67614 | 0.415063 | 0.027998 | 0.247335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumboh, J.G.; Laryea, N.A.; Otchere, J.; Ahorlu, C.S.; de Souza, D.K. Towards Understanding the Microepidemiology of Lymphatic Filariasis at the Community Level in Ghana. Trop. Med. Infect. Dis. 2024, 9, 107. https://doi.org/10.3390/tropicalmed9050107
Sumboh JG, Laryea NA, Otchere J, Ahorlu CS, de Souza DK. Towards Understanding the Microepidemiology of Lymphatic Filariasis at the Community Level in Ghana. Tropical Medicine and Infectious Disease. 2024; 9(5):107. https://doi.org/10.3390/tropicalmed9050107
Chicago/Turabian StyleSumboh, Jeffrey Gabriel, Nii A. Laryea, Joseph Otchere, Collins S. Ahorlu, and Dziedzom K. de Souza. 2024. "Towards Understanding the Microepidemiology of Lymphatic Filariasis at the Community Level in Ghana" Tropical Medicine and Infectious Disease 9, no. 5: 107. https://doi.org/10.3390/tropicalmed9050107
APA StyleSumboh, J. G., Laryea, N. A., Otchere, J., Ahorlu, C. S., & de Souza, D. K. (2024). Towards Understanding the Microepidemiology of Lymphatic Filariasis at the Community Level in Ghana. Tropical Medicine and Infectious Disease, 9(5), 107. https://doi.org/10.3390/tropicalmed9050107







