Infectious Diseases and Secondary Antibody Deficiency in Patients from a Mesoregion of São Paulo State, Brazil
Abstract
1. Introduction
2. Methods
2.1. Regional Characteristics
2.2. Demographic, Clinical, and Laboratory Characteristics of the Participants
2.3. Immunoglobulin Replacement
2.4. Inclusion and Exclusion Criteria
2.5. Statistical Analysis
2.6. Study Approval
3. Results
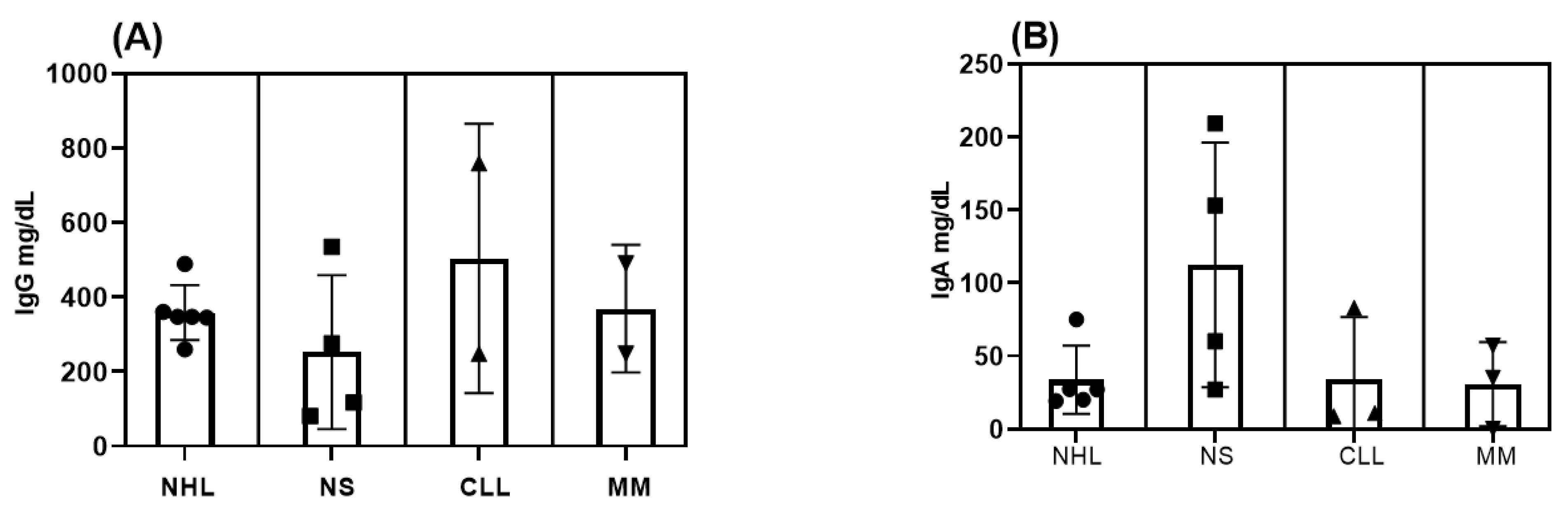
3.1. Demographics, Clinical, and Laboratory Characteristics of the Participants at Baseline
3.2. Impact of Infections on Patients with Secondary Antibody Deficiency after Treatment with Immunosuppressants
3.3. Infections in Different Sites in the Context of Gender, Age, and SAD Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuano, K.S.; Seth, N.; Chinen, J. Secondary immunodeficiencies: An overview. Ann. Allergy Asthma Immunol. 2021, 127, 617–626. [Google Scholar] [CrossRef]
- Sánchez-Ramón, S.; Bermúdez, A.; González-Granado, L.I.; Rodríguez-Gallego, C.; Sastre, A.; Soler-Palacín, P.; ID-Signal Onco-Haematology Group. Primary and Secondary Immunodeficiency Diseases in Oncohaematology: Warning Signs, Diagnosis, and Management. Front. Immunol. 2019, 10, 586. [Google Scholar] [CrossRef]
- Kaplan, B.; Bonagura, V.R. Secondary Hypogammaglobulinemia: An Increasingly Recognized Complication of Treatment with Immunomodulators and After Solid Organ Transplantation. Immunol. Allergy Clin. N. Am. 2019, 39, 31–47. [Google Scholar] [CrossRef]
- Axelrod, H.; Adams, M. Biologic Agents and Secondary Immune Deficiency. Immunol. Allergy Clin. N. Am. 2021, 41, 639–652. [Google Scholar] [CrossRef]
- Crickx, E.; Weill, J.C.; Reynaud, C.A.; Mahévas, M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: Successes, failures and future perspectives. Kidney Int. 2020, 97, 885–893. [Google Scholar] [CrossRef]
- de Souza, K.J.; Ferro, R.S.; Prestes-Carneiro, L.E.; Carrilho, P.A.M.; Vasconcelos, D.M. Infectious diseases and immunological markers associated with patients with non-Hodgkin lymphoma treated with rituximab. Immunopharmacol. Immunotoxicol. 2018, 40, 13–17. [Google Scholar] [CrossRef]
- Stabler, S.; Giovannelli, J.; Launay, D.; Cotteau-Leroy, A.; Heusele, M.; Lefèvre, G.; Terriou, L.; Lambert, M.; Dubucquoi, S.; Hachulla, E.; et al. Serious Infectious Events and Immunoglobulin Replacement Therapy in Patients With Autoimmune Disease Receiving Rituximab: A Retrospective Cohort Study. Clin. Infect. Dis. 2021, 72, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Cannon, L.; Pan, A.; Kovalick, L.; Sarkissian, A.; Wu, E.Y. Secondary immunodeficiencies and infectious considerations of biologic immunomodulatory therapies. Ann. Allergy Asthma Immunol. 2023, 130, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Jolles, S.; Smith, B.D.; Vinh, D.C.; Mallick, R.; Espinoza, G.; DeKoven, M.; Divino, V. Risk factors for severe infections in secondary immunodeficiency: A retrospective US administrative claims study in patients with hematological malignancies. Leuk. Lymphoma 2022, 63, 64–73. [Google Scholar] [CrossRef]
- Jolles, S.; Michallet, M.; Agostini, C.; Albert, M.H.; Edgar, D.; Ria, R.; Trentin, L.; Lévy, V. Treating secondary antibody deficiency in patients with haematological malignancy: European expert consensus. Eur. J. Haematol. 2021, 106, 439–449. [Google Scholar] [CrossRef]
- Boton Pereira, D.H.; Primo, L.S.; Pelizari, G.; Flores, E.; de Moraes-Vasconcelos, D.; Condino-Neto, A.; Prestes-Carneiro, L.E. Primary Immunodeficiencies in a Mesoregion of São Paulo, Brazil: Epidemiologic, Clinical, and Geospatial Approach. Front. Immunol. 2020, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Lee, K.Y.; Hwang, J.Y.; Koh, D.K.; Lee, J.S. Antibody status in children with steroid-sensitive nephrotic syndrome. Yonsei Med. J. 2010, 51, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Ben-Batalla, I.; Vargas-Delgado, M.E.; Meier, L.; Loges, S. Sexual dimorphism in solid and hematological malignancies. Semin. Immunopathol. 2019, 41, 251–263. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, International Agency for Research on Cancer, Global Cancer Observatory. Available online: https://gco.iarc.fr/en (accessed on 23 January 2023).
- Houpert, R.; Almont, T.; Belahreche, R.; Faro, M.; Okouango, J.; Vestris, M.; Macni, J.; Pierre-Louis, O.; Montabord, C.; Beaubrun-Renard, M.; et al. A population-based analysis of hematological malignancies from a French-West-Indies cancer registry’s data (2009–2018). BMC Cancer 2023, 23, 1197. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde, Instituto Nacional de Câncer Incidência de Câncer no Brasil. Available online: https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/estimativa-2023.pdf (accessed on 10 February 2023).
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Jones, R.B.; Smith, R.M.; Alberici, F.; Kumaratne, D.S.; Burns, S.; Jayne, D.R. Rituximab-associated hypogammaglobulinemia: Incidence, predictors and outcomes in patients with multi-system autoimmune disease. J. Autoimmun. 2015, 57, 60–65. [Google Scholar] [CrossRef]
- Barmettler, S.; Ong, M.S.; Farmer, J.R.; Choi, H.; Walter, J. Association of Immunoglobulin Levels, Infectious Risk, and Mortality With Rituximab and Hypogammaglobulinemia. JAMA Netw. Open 2018, 1, e184169. [Google Scholar] [CrossRef]
- Ottaviano, G.; Sgrulletti, M.; Moschese, V. Secondary rituximab-associated versus primary immunodeficiencies: The enigmatic border. Eur. J. Immunol. 2022, 52, 1572–1580. [Google Scholar] [CrossRef]
- Wudhikarn, K.; Palomba, M.L.; Pennisi, M.; Garcia-Recio, M.; Flynn, J.R.; Devlin, S.M.; Afuye, A.; Silverberg, M.L.; Maloy, M.A.; Shah, G.L.; et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020, 10, 79. [Google Scholar] [CrossRef]
- Horn, C.; Sprute, R.; Kretschmer, A.C.; Do, C.; Cornely, O.A.; Jung, N.; Lehmann, C.; Fischer, J. Sex in infectious diseases-How sex differences influence the immune response to infections. Inn. Med. 2023, 64, 752–757. [Google Scholar]
- Glynn, J.R.; Moss, P.A.H. Systematic analysis of infectious disease outcomes by age shows lowest severity in school-age children. Sci. Data 2020, 7, 329. [Google Scholar] [CrossRef] [PubMed]


| No. | IgG | IgA | γ-Globulins | CD19 | CD3+ | CD4+ | CD8+ | Path | Target | Drug |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 360 | 27 | 0.30 | 0 | 590 | 273 | 311 | NHL | Anti-CD-20 | RTX |
| 2 | 489 | 75 | 0.43 | 584 | 1129 | 467 | 577 | NHL | Anti-CD-20 | RTX |
| 3 | 260 | 27.2 | 0.23 | 25 | 1877 | 482 | 1337 | NHL | Anti-CD-20 | RTX |
| 4 | 345 | 19.2 | 0.32 | 0 | 5025 | 1044 | 3704 | NHL | Anti-CD-20 | RTX |
| 5 | 347 | 20 | 0.32 | 148 | 878 | 371 | 392 | NHL | BTK | Ibrut |
| 6 | 347 | 2210 | 0.20 | 00 | 1297 | 324 | 877 | NHL | BTK | Ibrut |
| 7 | 117 | 153 | 0.22 | 141 | 406 | 251 | 100 | NS | Anti-CD-20 | Pred |
| 8 | 320 | NA | NA | NA | NA | NA | NA | NS | Anti-CD-20 | Pred |
| 9 | 535 | 209 | 0.55 | 26 | 1188 | 343 | 808 | NS | IMPDH | Pred; myc |
| 10 | 276 | 27 | 0.24 | 109 | 1102 | 650 | 421 | NS | Anti-CD-20 | Pred |
| 11 | 81 | 60 | 0.15 | 181 | 4374 | 2011 | 2052 | NS | Anti-CD-20 | Pred |
| 12 | 248 | 10.9 | 0.24 | 246 | 2923 | 435 | 2408 | CLL | Anti-CD-20 | RTX |
| 13 | 654 | 8.73 | 0.70 | 45 | 855 | 334 | 470 | CLL | Anti-CD-20 | RTX |
| 14 | 759 | 83 | 0.97 | 310 | NA | NA | NA | CLL | Anti-CD-20 | RTX |
| 15 | 415 | NA | NA | NA | NA | NA | NA | CLL | Anti-CD-20 | RTX; ibrut |
| 16 | 490 | 35 | 0.50 | NA | NA | NA | NA | MM | DNA | Cycloph; tali |
| 17 | 248 | 57 | 0.57 | 7 | 643 | 278 | 329 | MM | Anti-CD-20 | RTX |
| 18 | 282 | 0 | 0.25 | 0 | 863 | 345 | 499 | MM | BCMA/CD3 | Teclistamab |
| 19 | 76 | 33 | 0.11 | 218 | 2309 | 1600 | 772 | PD | 0 | No drugs |
| 20 | 460 | 39.7 | 0.35 | 0 | 2425 | 1367 | 941 | ITP | Anti-CD-20 | RTX |
| Participants (n= 20) | n (%) | Mean ± SD | 95% Confidence Interval | |
|---|---|---|---|---|
| Age (years) | <18 | 7 (35) | 10.80 ± 7.29 | 4.05–17.54 |
| 18–60 | 7 (35) | 49.29 ± 12.74 | 37.51–61.07 | |
| ≥60 | 6 (30) | 70.83 ± 8.25 | 62.17–79.50 | |
| Gender | Female | 8 (40) | ||
| Male | 12 (60) | |||
| Race | European | 10 (50) | ||
| South American | 6 (30) | |||
| African | 2 (10) | |||
| Asian | 2 (10) | |||
| SID type | Non-Hodgkin’s lymphoma | 6 (30) | ||
| Nephrotic syndrome | 5 (25) | |||
| Chronic lymphocyte leukemia | 4 (25) | |||
| Multiple myeloma | 3 (15) | |||
| Protein-losing enteropathy | 1 (5) | |||
| Immune thrombocytopenia | 1 (5) | |||
| Origin of the service | Public | 10 (50) | ||
| Private | 10 (50) | |||
| IgG replacement | Endovenous | 12 (60) | ||
| Subcutaneous | 4 (25) | |||
| Not indicated | 4 (25) |
| Patient Number | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Infection sites | ||||||||||||||||||||
| Upper and lower airway infections and otitis | ||||||||||||||||||||
| Tonsilitis | _ | _ | _ | _ | + | +++ | _ | _ | _ | _ | _ | _ | _ | ++ | ++ | ++ | _ | + | _ | + |
| Sinusitis | ++++ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | + | _ | _ | _ | _ |
| Otitis | _ | _ | _ | _ | _ | _ | _ | _ | _ | + | _ | _ | _ | _ | _ | _ | _ | _ | ||
| Pneumonia | +++ | ++ | _ | ++++ | _ | _ | _ | _ | _ | ++++ | _ | _ | _ | _ | _ | _ | _ | +++ | +++ | _ |
| Skin diseases and soft tissues | ||||||||||||||||||||
| Herpes simplex | ++ | _ | _ | _ | +++ | _ | _ | _ | _ | _ | _ | +++ | + | _ | _ | _ | _ | _ | _ | _ |
| Herpes zoster | _ | _ | _ | _ | _ | _ | _ | _ | _ | ++ | _ | + | + | _ | +++ | _ | _ | _ | _ | |
| Skin mycosis | _ | _ | _ | _ | _ | _ | _ | _ | ++++ | _ | _ | _ | _ | _ | +++ | _ | _ | _ | _ | |
| Head and neck | ||||||||||||||||||||
| Tongue lesion | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | ++ | _ | _ | _ | _ | _ | _ | _ | _ |
| Gastrointestinal | ||||||||||||||||||||
| Gastroenteritis | + | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | + | _ | _ | +++ | _ | _ | _ | ++++ | + |
| Urinary tract | ||||||||||||||||||||
| UTI | +++ | ++ | _ | _ | _ | _ | _ | _ | _ | ++++ | _ | _ | _ | _ | _ | _ | _ | _ | ++ | _ |
| Sepsis | ||||||||||||||||||||
| Sepsis | + | _ | _ | + | _ | _ | _ | _ | + | ++ | _ | _ | _ | _ | _ | _ | _ | ++ | +++ | _ |
| Dengue and COVID-19 | ||||||||||||||||||||
| Dengue | + | + | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |||
| COVID-19 | + | + | ++ | + | + | ++ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| Severity of infections | ||||||||||||||||||||
| Mild | _ | _ | + | + | _ | _ | _ | _ | _ | _ | + | + | + | + | + | |||||
| Moderate | _ | + | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| Life-threatening | + | _ | _ | + | _ | + | _ | _ | + | + | _ | _ | _ | _ | + | _ | _ | + | + | _ |
| Fatal | + | _ | - | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | + | _ | _ | _ | + | _ |
| Patient No. | Basal IgG | IgG | Basal IgA | IgA | Basal γ-glo | γ-glob |
|---|---|---|---|---|---|---|
| 1 | 753 | 360 | 41 | 27 | NA | 0.30 |
| 2 | 540 | 489 | 73 | 75 | 0.70 | 0.43 |
| 3 | 480 | 260 | 35 | 27.2 | NA | 0.23 |
| 4 | 360 | 345 | 23 | 19.2 | 0.50 | 0.32 |
| 5 | 447 | 347 | 27.1 | 20 | NA | 0.32 |
| 6 | 410 | 347 | 2430 | 2210 | 31.10 | 0.20 |
| 7 | 125 | 117 | 170 | 153 | 0.25 | 0.22 |
| 8 | NA | 320 | NA | NA | NA | NA |
| 9 | 586 | 535 | 223 | 209 | 0.81 | 0.55 |
| 10 | 235 | 276 | 59.6 | 27 | 0.30 | 0.24 |
| 11 | 65 | 81 | 71 | 60 | 0.25 | 0.15 |
| 12 | 950 | 248 | <5.0 | 10.9 | NA | 0.24 |
| 13 | 906 | 654 | 11.9 | 8.73 | NA | 0.70 |
| 14 | NA | 759 | NA | 83 | NA | 0.97 |
| 15 | NA | 415 | NA | NA | NA | NA |
| 16 | NA | 490 | NA | 35 | NA | 0.50 |
| 17 | 516 | 248 | 2693 | 57 | 0.45 | 0.57 |
| 18 | 310 | 282 | <5.0 | 0 | 0.48 | 0.25 |
| 19 | 217 | 76 | 35 | 33 | 0.23 | 0.11 |
| 20 | 490 | 460 | 45 | 39.7 | 0.70 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prestes-Carneiro, L.E.; Carrilho, P.A.M.; Torelli, D.F.H.d.B.; Bressa, J.A.N.; Parizi, A.C.G.; Vieira, P.H.M.; Sa, F.M.C.; Ferreira, M.D. Infectious Diseases and Secondary Antibody Deficiency in Patients from a Mesoregion of São Paulo State, Brazil. Trop. Med. Infect. Dis. 2024, 9, 104. https://doi.org/10.3390/tropicalmed9050104
Prestes-Carneiro LE, Carrilho PAM, Torelli DFHdB, Bressa JAN, Parizi ACG, Vieira PHM, Sa FMC, Ferreira MD. Infectious Diseases and Secondary Antibody Deficiency in Patients from a Mesoregion of São Paulo State, Brazil. Tropical Medicine and Infectious Disease. 2024; 9(5):104. https://doi.org/10.3390/tropicalmed9050104
Chicago/Turabian StylePrestes-Carneiro, Luiz Euribel, Paula Andreia Martins Carrilho, Danielle Francisco Honorato de Barros Torelli, Jose Antonio Nascimento Bressa, Ana Carolina Gomes Parizi, Pedro Henrique Meireles Vieira, Fernanda Miranda Caliani Sa, and Mauricio Domingues Ferreira. 2024. "Infectious Diseases and Secondary Antibody Deficiency in Patients from a Mesoregion of São Paulo State, Brazil" Tropical Medicine and Infectious Disease 9, no. 5: 104. https://doi.org/10.3390/tropicalmed9050104
APA StylePrestes-Carneiro, L. E., Carrilho, P. A. M., Torelli, D. F. H. d. B., Bressa, J. A. N., Parizi, A. C. G., Vieira, P. H. M., Sa, F. M. C., & Ferreira, M. D. (2024). Infectious Diseases and Secondary Antibody Deficiency in Patients from a Mesoregion of São Paulo State, Brazil. Tropical Medicine and Infectious Disease, 9(5), 104. https://doi.org/10.3390/tropicalmed9050104







