Differences in Cytokine Expression at Baseline and in Response to Mineral Stimulation by Peripheral Blood Mononuclear Cells from Podoconiosis Cases and Healthy Control Individuals
Abstract
1. Introduction
2. Results
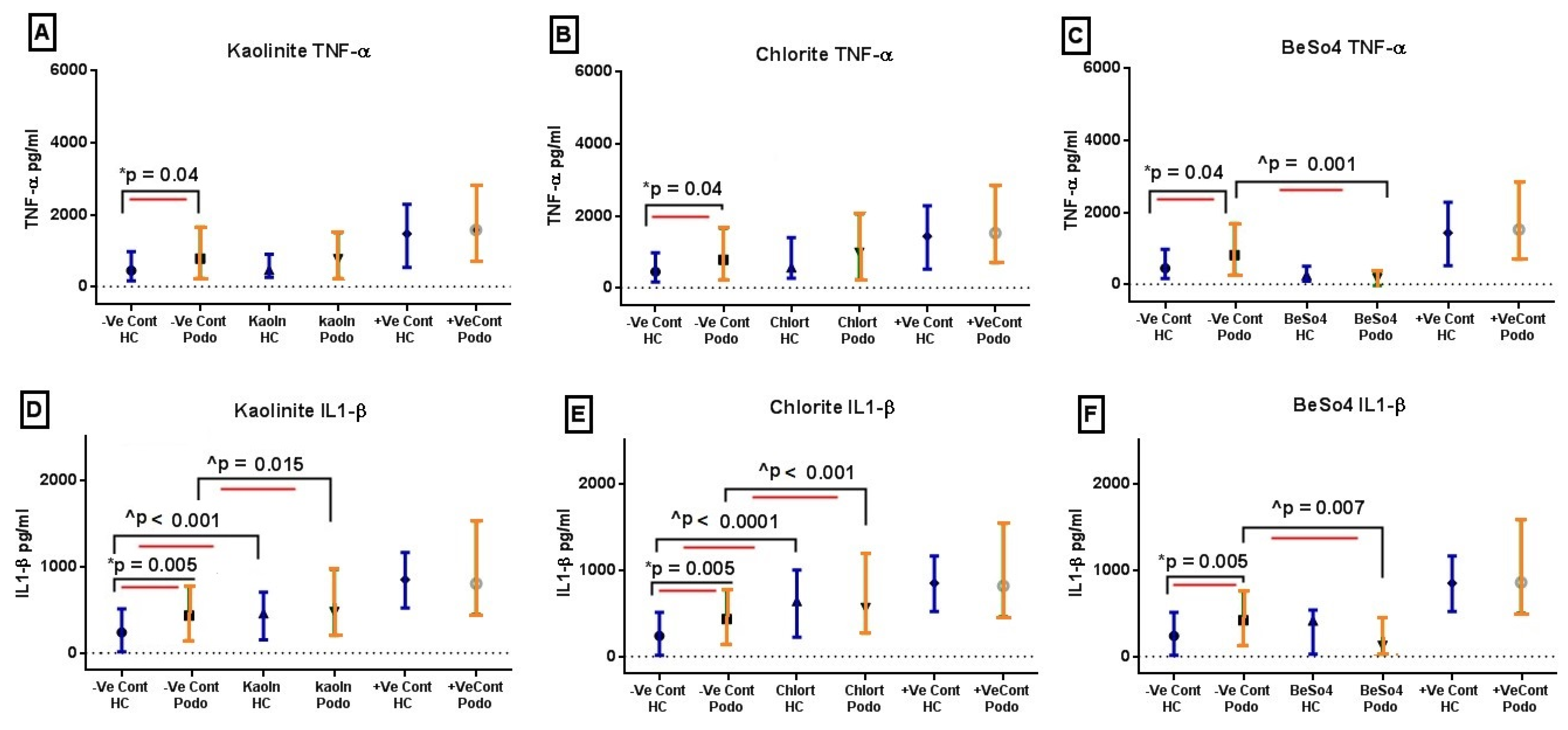
2.1. TNF-α and IL-1β Levels Following 24 h In Vitro Mineral Stimulation
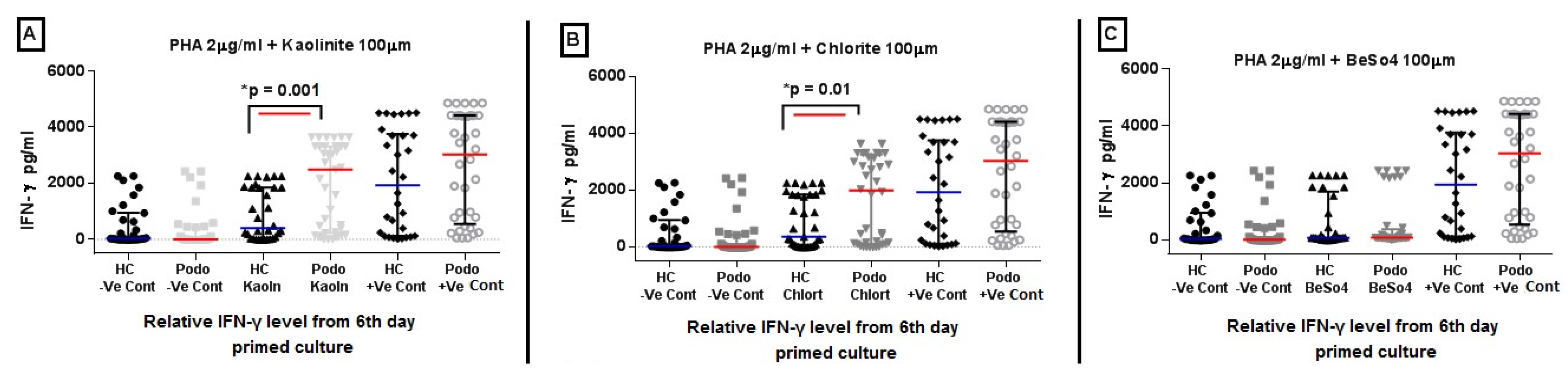
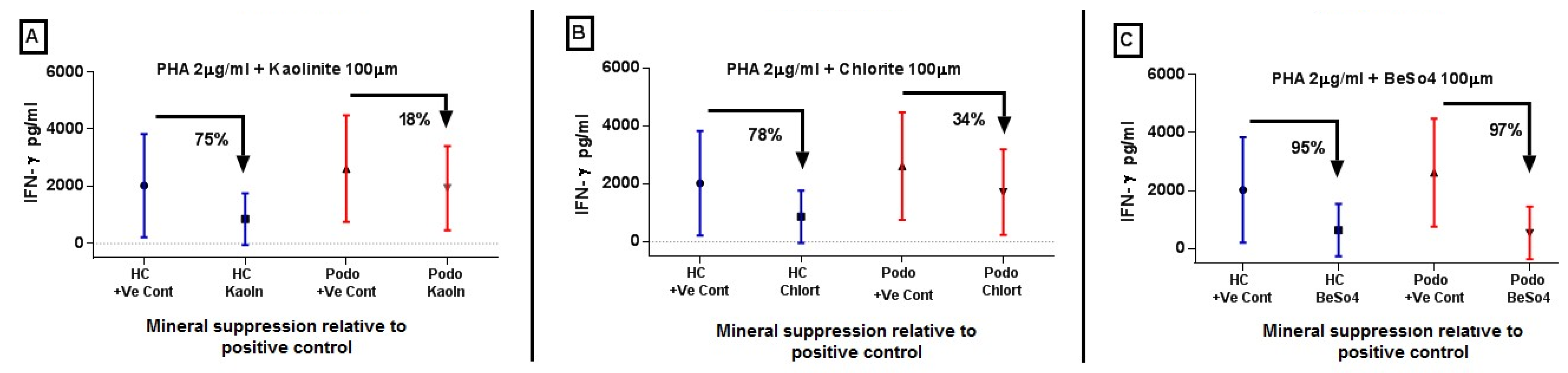
2.2. IFN-γ Levels in Response to Mineral Stimulation Following PHA Priming
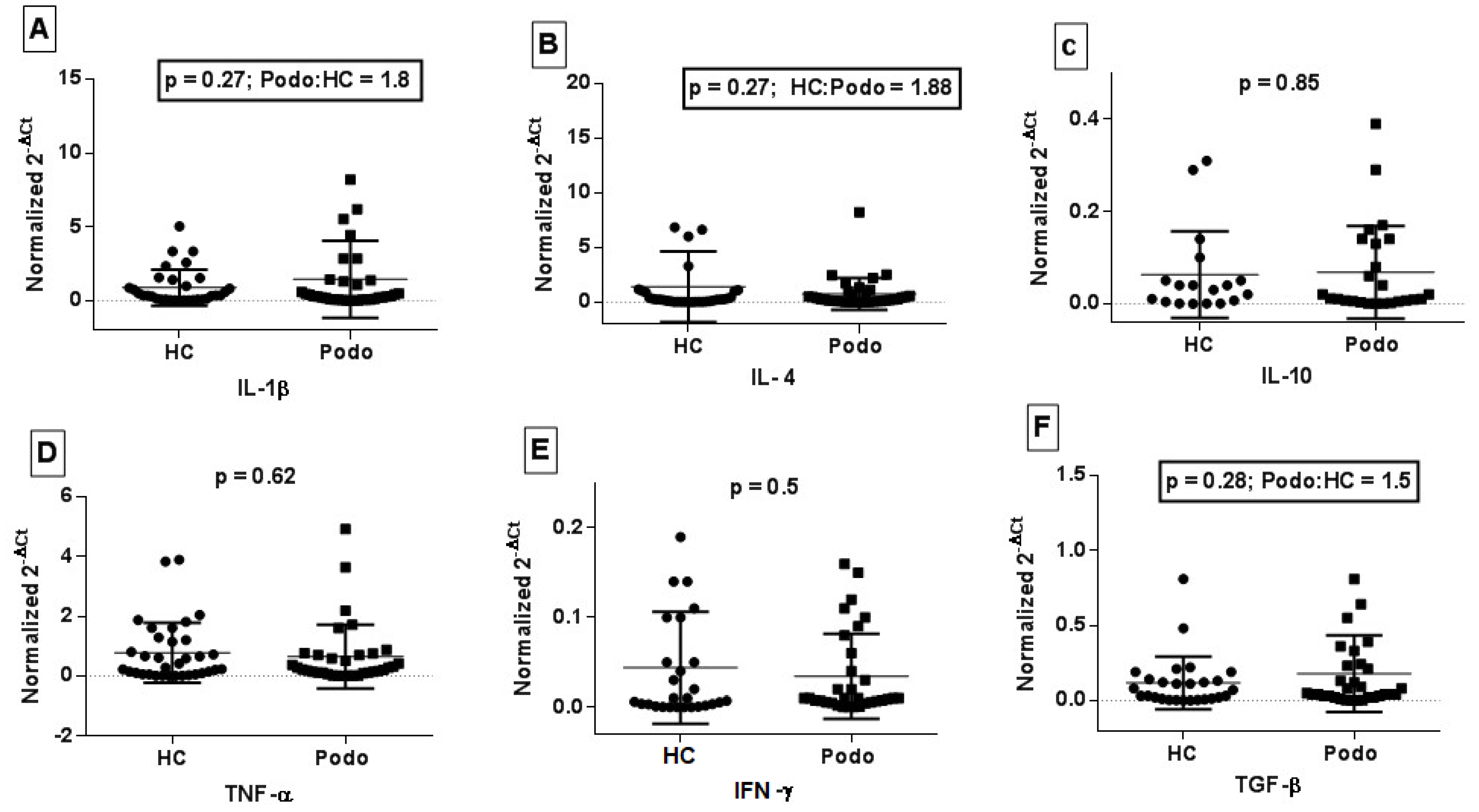
2.3. Cytokine Gene Expression in Peripheral Blood from Podoconiosis Patients and Healthy Control Subjects
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Study Area and Study Participants
5.2. Sample Collection and PBMC Isolation
5.3. Cell Culture for In Vitro Mineral Stimulation
5.4. ELISA Assay
5.5. RNA Extraction and cDNA Synthesis
5.6. Real Time Quantitative PCR
5.7. Gene Expression and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, E. The association of endemic elephantiasis of the lower legs in East Africa with soil derived from volcanic rocks. Trans. R. Soc. Trop. Med. Hyg. 1976, 70, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Price, E. Non-filarial elephantiasis--confirmed as a geochemical disease, and renamed podoconiosis. Ethiop. Med. J. 1988, 26, 151–153. [Google Scholar] [PubMed]
- Price, E.; Henderson, W. Endemic elephantiasis of the lower legs in the United Cameroon Republic. Trop. Geogr. Med. 1981, 33, 23–29. [Google Scholar]
- Deribe, K.; Brooker, S.J.; Pullan, R.L.; Sime, H.; Gebretsadik, A.; Assefa, A.; Kebede, A.; Hailu, A.; Rebollo, M.P.; Shafi, O.; et al. Epidemiology and individual, household and geographical risk factors of podoconiosis in Ethiopia: Results from the first nationwide mapping. Am. J. Trop. Med. Hyg. 2015, 92, 148–158. [Google Scholar] [CrossRef]
- Molla, Y.B.; Wardrop, N.A.; Le Blond, J.S.; Baxter, P.; Newport, M.J.; Atkinson, P.M.; Davey, G. Modelling environmental factors correlated with podoconiosis: A geospatial study of non-filarial elephantiasis. Int. J. Health Geogr. 2014, 13, 24. [Google Scholar] [CrossRef]
- Gislam, H.; Brolly, M.; Burnside, N.; Davey, G.; Deribe, K.; Wanji, S.; Suh, C.E.; Kemp, S.J.; Watts, M.J.; Le Blond, J.S. (Eds.) Links between Soil Composition and Podoconiosis Occurrence and Prevalence in Cameroon; Sharing Geoscience Online; EGU2020: Vienna, Austria, 2020. [Google Scholar]
- Wanji, S.; Tendongfor, N.; Esum, M.; Che, J.; Mand, S.; Tanga Mbi, C.; Enyong, P.; Hoerauf, A. Elephantiasis of non-filarial origin (podoconiosis) in the highlands of north–western Cameroon. Ann. Trop. Med. Parasitol. 2008, 102, 529–540. [Google Scholar] [CrossRef]
- Blundell, G.; Henderson, W.; Price, E. Soil particles in the tissues of the foot in endemic elephantiasis of the lower legs. Ann. Trop. Med. Parasitol. 1989, 83, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Price, E.; Henderson, W. Silica and silicates in femoral lymph nodes of barefooted people in Ethiopia with special reference to elephantiasis of the lower legs. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Price, E.; Henderson, W. The elemental content of lymphatic tissues of barefooted people in Ethiopia, with reference to endemic elephantiasis of the lower legs. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 132–136. [Google Scholar] [CrossRef]
- Price, E.; Plant, D. The significance of particle size of soils as a risk factor in the etiology of podoconiosis. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 885–886. [Google Scholar] [CrossRef]
- Frommel, D.; Ayranci, B.; Pfeifer, H.; Sanchez, A.; Frommel, A.; Mengistu, G. Podoconiosis in the Ethiopian Rift Valley. Role Beryllium Zirconium. Trop. Geogr. Med. 1993, 45, 165–167. [Google Scholar] [PubMed]
- Price, E. The relationship between endemic elephantiasis of the lower legs and the local soils and climate. Trop. Geogr. Med. 1974, 26, 225–230. [Google Scholar] [PubMed]
- Wendemagegn, E.; Tirumalae, R.; Böer-Auer, A. Histopathological and immunohistochemical features of nodular podoconiosis. J. Cutan. Pathol. 2015, 42, 173–181. [Google Scholar]
- Tekola, F.; Adeyemo, A.; Finan, C.; Hailu, E.; Sinnott, P.; Burlinson, N.D.; Aseffa, A.; Rotimi, C.N.; Newport, M.J.; Davey, G. HLA class II locus and susceptibility to podoconiosis. N. Engl. J. Med. 2012, 366, 1200–1208. [Google Scholar] [CrossRef]
- Gebresilase, T.; Finan, C.; Suveges, D.; Tessema, T.S.; Aseffa, A.; Davey, G.; Hatzikotoulas, K.; Zeggini, E.; Newport, M.J.; Tekola-Ayele, F. Replication of HLA class II locus association with susceptibility to podoconiosis in three Ethiopian ethnic groups. Sci. Rep. 2021, 11, 3285. [Google Scholar] [CrossRef]
- Matsuno, H.; Yudoh, K.; Katayama, R.; Nakazawa, F.; Uzuki, M.; Sawai, T.; Yonezawa, T.; Saeki, Y.; Panayi, G.S.; Pitzalis, C.; et al. The role of TNF-α in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): A study using a human RA/SCID mouse chimera. Rheumatology 2002, 41, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lis, K.; Kuzawinska, O.; Balkowiec-Iskra, E. Tumor necrosis factor inhibitors—State of knowledge. Arch. Med. Sci. 2014, 10, 1175–1185. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G.; Isenberg, D.A.; Mauri, C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef]
- Sands, B.E.; Kaplan, G.G. The role of TNFα in ulcerative colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Ehses, J.A.; Donath, M.Y.; Mandrup-Poulsen, T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care 2009, 32, 1663–1668. [Google Scholar] [CrossRef]
- Aksentijevich, I.; Masters, S.L.; Ferguson, P.J.; Dancey, P.; Frenkel, J.; van Royen-Kerkhoff, A.; Laxer, R.; Tedgård, U.; Cowen, E.W.; Pham, T.-H.; et al. An autoinflammatory disease with deficiency of the interleukin-1–receptor antagonist. N. Engl. J. Med. 2009, 360, 2426–2437. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Borrero-Wolff, D.; Ritter, M.; Arndts, K.; Wiszniewsky, A.; Debrah, L.B.; Debrah, A.Y.; Osei-Mensah, J.; Chachage, M.; Hoerauf, A.; et al. Distinct immune profiles of exhausted effector and memory CD8+ T cells in individuals with filarial lymphedema. Front. Cell Infect. Microbiol. 2021, 11, 680832. [Google Scholar] [CrossRef] [PubMed]
- Dillingh, M.R.; van Poelgeest, E.P.; Malone, K.E.; Kemper, E.M.; Stroes, E.S.; Moerland, M.; Burggraaf, J. Characterization of inflammation and immune cell modulation induced by low-dose LPS administration to healthy volunteers. J. Inflamm. 2014, 11, 28. [Google Scholar]
- Franchi, L.; Eigenbrod, T.; Núñez, G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 2009, 183, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Negash, M.; Chanyalew, M.; Girma, T.; Alemu, F.; Alcantara, D.; Towler, B.; Davey, G.; Boyton, R.J.; Altmann, D.M.; Howe, R.; et al. Evidence for immune activation in pathogenesis of the HLA class II associated disease, podoconiosis. Nat. Commun. 2024, 15, 2020. [Google Scholar] [CrossRef]
- Holian, A.; Uthman, M.O.; Goltsova, T.; Brown, S.D.; Hamilton, R.F., Jr. Asbestos and silica-induced changes in human alveolar macrophage phenotype. Env. Health Perspect. 1997, 105 (Suppl. 5), 1139–1142. [Google Scholar]
- Hamilton, R.F.; Marshall, G.D.; Holian, A. Silica and PM1648 modify human alveolar macrophage antigen-presenting cell activity in vitro. J. Env. Pathol. Toxicol. Oncol. 2001, 20 (Suppl. 1), 75–84. [Google Scholar] [CrossRef]
- Baik, J.E.; Park, H.J.; Kataru, R.P.; Savetsky, I.L.; Ly, C.L.; Shin, J.; Encarnacion, E.M.; Cavali, M.R.; Klang, M.G.; Riedel, E.; et al. TGF-β1 mediates pathologic changes of secondary lymphedema by promoting fibrosis and inflammation. Clin. Transl. Med. 2022, 12, e758. [Google Scholar] [CrossRef]
- Wynn, T. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Avraham, T.; Daluvoy, S.; Zampell, J.; Yan, A.; Haviv, Y.S.; Rockson, S.G.; Mehrara, B.J. Blockade of transforming growth factor-β1 accelerates lymphatic regeneration during wound repair. Am. J. Phatol. 2010, 177, 3202–3214. [Google Scholar] [CrossRef]
- Lombardi, G.; Germain, C.; Uren, J.; Fiorillo, M.T.; du Bois, R.M.; Jones-Williams, W.; Saltini, C.; Sorrentino, R.; Lechler, R. HLA-DP allele-specific T cell responses to beryllium account for DP-associated susceptibility to chronic beryllium disease. J. Immunol. 2001, 166, 3549–3555. [Google Scholar] [CrossRef] [PubMed]
- Central-Statistical-Authority. Central Statistical Authority Abstract of Federal Democratic Republic of Ethiopia; Central-Statistical-Authority: Addis Ababa, Ethiopia, 2011. [Google Scholar]
- Tekola, F.; Ayele, Z.; Mariam, D.H.; Fuller, C.; Davey, G. Development and testing of a de novo clinical staging system for podoconiosis (endemic non-filarial elephantiasis). Trop. Med. Int. Health 2008, 13, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

| Variable | Podoconiosis (%), n = 56 | Healthy Control (%) n = 44 |
|---|---|---|
| Sex: Male Female | 32 (57) | 26 (59) |
| 24 (43) | 18 (31) | |
| Age (years): Mean (SD) | 45 (10.3) | 37 (9.3) |
| Occupation: Farmer Student | 56 (100) | 40 (91) 4 (9) |
| Marital status: Married Divorced Single | 47 (84) 7 (12.5) 2 (3.5) | 35 (79.5) 4 (9) 5 (11.5) |
| Disease stage: Stage 2 Stage 3 | 52 (92.8) 4 (7.2) | |
| Duration of disease in years: mean (sd) | 17 (9.2) | |
| Site involved: Both legs Left leg Right leg | 50 (89.3) 4 (7.2) 2 (3.5) |
| Cytokine | Day 1 | Day 2 | Day 3 | Day 6 |
|---|---|---|---|---|
| TNF-α and IL-1β | Minerals added to PBMC | SN harvested for ELISA | -- | -- |
| IFN-γ | PHA added to prime PBMCs | Minerals added to PBMCs | IL-2 added | SN harvested for ELISA |
| S.No. | Primer Name | Sequence | Tm | %GC |
|---|---|---|---|---|
| 1 | HuPO-Fw | GCTTCCTGGAGGGTGTCC | 60.5 | 67 |
| HuPO-Rv | GGACTCGTTTGTACCCGTTG | 59.4 | 55 | |
| 2 | IL-4-Fw | GGCAGTTCTACAGCCACCAT | 59.4 | 55 |
| IL-4-Rv | TGGTTGGCTTCCTTCACAGG | 59.4 | 55 | |
| 3 | IFN-γ-Fw | GGCTTTTCAGCTCTGCATCC | 59.4 | 55 |
| IFN-γ Rv | TCTGTCACTCTCCTCTTTCCA | 58 | 48 | |
| 4 | IL-10 Fw | TGAGAACCAAGACCCAGACA | 57 | 50 |
| IL-10 Rv | TCATGGCTTTGTAGATGCCT | 55 | 45 | |
| 5 | TNF-α Fw | AGCCCATGTTGTAGCAAACC | 57 | 50 |
| TNF-α Rv | GCTGGTTATCTCTCAGCTCCA | 60 | 52 | |
| 6 | IL-1 β Fw | AGCCCAGCCAACTCAATTC | 57 | 53 |
| IL-1 β Rv | CATGGAGAACACCACTTGTTGC | 60 | 50 | |
| 7 | TGF-β-Fw | GGACCAGTGGGGAACACTAC | 61 | 60 |
| TGF-β-Rv | TAAAGCAGGTTCCTGGTGGG | 59 | 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negash, M.; Girma, T.; Chanyalew, M.; Alemayehu, D.H.; Alcantara, D.; Davey, G.; Boyton, R.J.; Altmann, D.M.; Newport, M.J.; Howe, R. Differences in Cytokine Expression at Baseline and in Response to Mineral Stimulation by Peripheral Blood Mononuclear Cells from Podoconiosis Cases and Healthy Control Individuals. Trop. Med. Infect. Dis. 2024, 9, 252. https://doi.org/10.3390/tropicalmed9110252
Negash M, Girma T, Chanyalew M, Alemayehu DH, Alcantara D, Davey G, Boyton RJ, Altmann DM, Newport MJ, Howe R. Differences in Cytokine Expression at Baseline and in Response to Mineral Stimulation by Peripheral Blood Mononuclear Cells from Podoconiosis Cases and Healthy Control Individuals. Tropical Medicine and Infectious Disease. 2024; 9(11):252. https://doi.org/10.3390/tropicalmed9110252
Chicago/Turabian StyleNegash, Mikias, Tigist Girma, Menberework Chanyalew, Dawit H. Alemayehu, Diana Alcantara, Gail Davey, Rosemary J. Boyton, Daniel M. Altmann, Melanie J. Newport, and Rawleigh Howe. 2024. "Differences in Cytokine Expression at Baseline and in Response to Mineral Stimulation by Peripheral Blood Mononuclear Cells from Podoconiosis Cases and Healthy Control Individuals" Tropical Medicine and Infectious Disease 9, no. 11: 252. https://doi.org/10.3390/tropicalmed9110252
APA StyleNegash, M., Girma, T., Chanyalew, M., Alemayehu, D. H., Alcantara, D., Davey, G., Boyton, R. J., Altmann, D. M., Newport, M. J., & Howe, R. (2024). Differences in Cytokine Expression at Baseline and in Response to Mineral Stimulation by Peripheral Blood Mononuclear Cells from Podoconiosis Cases and Healthy Control Individuals. Tropical Medicine and Infectious Disease, 9(11), 252. https://doi.org/10.3390/tropicalmed9110252






