High Level of Knowledge about Tungiasis but Little Translation into Control Practices in Karamoja, Northeastern Uganda
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Study Population
2.2. Study Design
- Knowledge: Questions on cause, risk factors for infection, symptoms and signs, prevention and treatment of tungiasis in humans and animals.
- Practices: Questions on activities regarding the treatment and prevention of tungiasis in humans and animals.
- Attitudes: Questions with a focus on stigma and the impairment of life quality.
2.3. Data Collection and Analysis
2.4. Ethical Considerations
3. Results
3.1. Socio-Demographic Data and Tungiasis Status
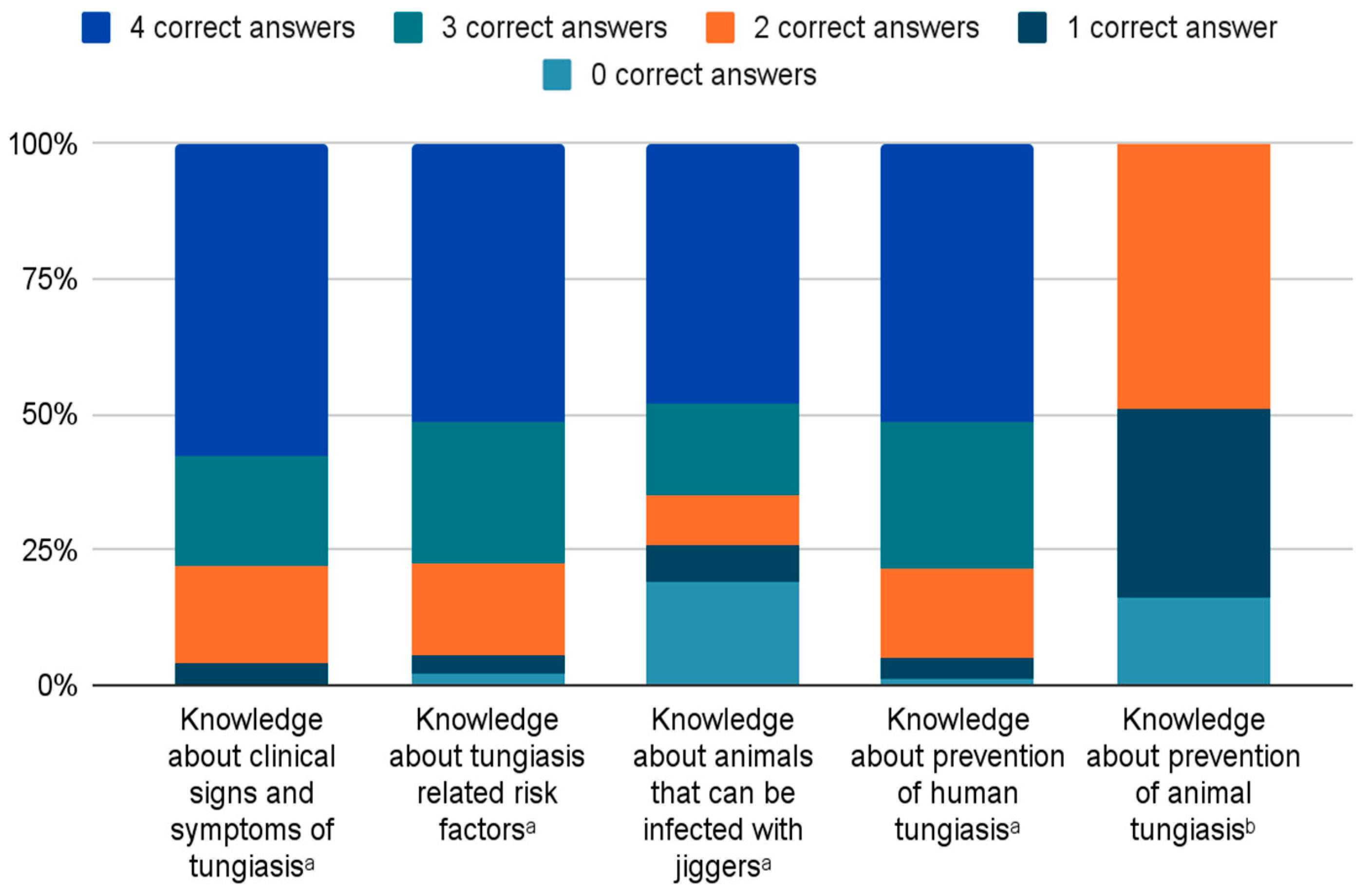
3.2. Knowledge about Cause of Tungiasis and Disease Manifestation
3.3. Knowledge about Risk Factors and Animal Tungiasis
3.4. Knowledge and Practices concerning the Prevention of Human Tungiasis
3.5. Knowledge and Practices concerning the Prevention of Animal Tungiasis
| Answer Options (Multiple Answers Possible) | Frequency of Answers | (%) |
|---|---|---|
| Spraying animals with insecticides | 890 | (67.0) |
| Keeping animal dwellings clean | 724 | (54.5) |
| I don’t know | 214 | (16.1) |
| Cementing the floor of animal dwellings | 171 | (12.9) |
| Others * | 28 | (2.1) |
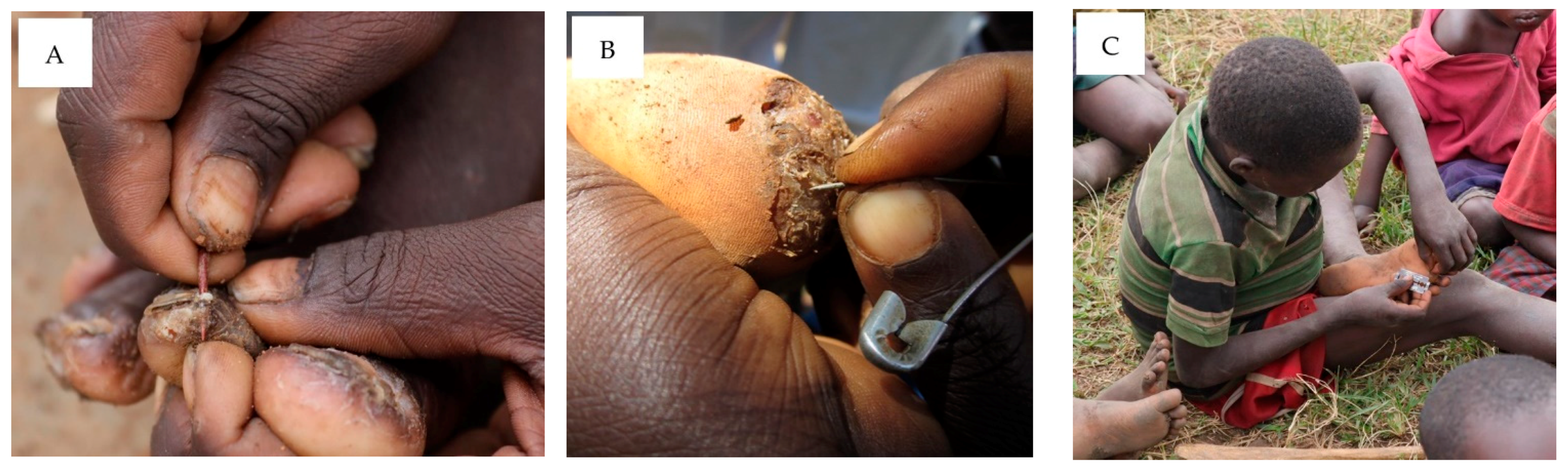
3.6. Knowledge and Practices concerning the Treatment of Human Tungiasis
| Answer Options (Multiple Answers Possible) | Frequency of Answers | (%) |
|---|---|---|
| Mechanical extraction of embedded sand fleas | 416 | (31.3) |
| Cleaning wounds | 251 | (18.9) |
| Visiting health facility | 235 | (17.7) |
| Topical application of dimeticone | 179 | (13.5) |
| Others * | 179 | (13.5) |
| Topical application of BBE | 126 | (9.5) |
| I don’t know | 114 | (8.6) |
3.7. Knowledge and Practices concerning the Treatment of Animal Tungiasis
| Answer Options (Multiple Answers Possible) | Frequency of Answers | (%) |
|---|---|---|
| Application of veterinary insecticide | 875 | (65.8) |
| I don’t know | 297 | (22.3) |
| Spraying of chemicals * | 116 | (8.7) |
| Topical application of dimeticone | 98 | (7.4) |
| Others ** | 69 | (5.2) |
| Mechanical extraction of embedded sand fleas | 19 | (1.4) |
3.8. Community Dialogue Meetings
4. Discussion
4.1. Knowledge about Human and Animal Tungiasis
4.2. Shoes as a Controversial Preventive Measure
4.3. Translation of Knowledge into Practice
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linardi, P.M.; de Avelar, D.M. Neosomes of Tungid Fleas on Wild and Domestic Animals. Parasitol. Res. 2014, 113, 3517–3533. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Costa, A.M.L.; Wilcke, T.; Mencke, N.; Feldmeier, H. The Animal Reservoir of Tunga Penetrans in Severely Affected Communities of North-East Brazil. Med. Vet. Entomol. 2004, 18, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, F.; Krücken, J.; Feldmeier, H.; Waiswa, C.; Mencke, N.; Sentongo, E.; von Samson-Himmelstjerna, G. Animal Reservoirs of Zoonotic Tungiasis in Endemic Rural Villages of Uganda. PLoS Negl. Trop. Dis. 2015, 9, e0004126. [Google Scholar] [CrossRef]
- Pilger, D.; Schwalfenberg, S.; Heukelbach, J.; Witt, L.; Mehlhorn, H.; Mencke, N.; Khakban, A.; Feldmeier, H. Investigations on the Biology, Epidemiology, Pathology, and Control of Tunga Penetrans in Brazil: VII. The Importance of Animal Reservoirs for Human Infestation. Parasitol. Res. 2008, 102, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ugbomoiko, U.S.; Ariza, L.; Heukelbach, J. Pigs Are the Most Important Animal Reservoir for Tunga Penetrans (jigger Flea) in Rural Nigeria. Trop. Doct. 2008, 38, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Eisele, M.; Heukelbach, J.; Van Marck, E.; Mehlhorn, H.; Meckes, O.; Franck, S.; Feldmeier, H. Investigations on the Biology, Epidemiology, Pathology and Control of Tunga Penetrans in Brazil: I. Natural History of Tungiasis in Man. Parasitol. Res. 2003, 90, 87–99. [Google Scholar] [CrossRef]
- Nyangacha, R.M.; Odongo, D.; Oyieke, F.; Ochwoto, M.; Korir, R.; Ngetich, R.K.; Nginya, G.; Makwaga, O.; Bii, C.; Mwitari, P.; et al. Secondary Bacterial Infections and Antibiotic Resistance among Tungiasis Patients in Western, Kenya. PLoS Negl. Trop. Dis. 2017, 11, e0005901. [Google Scholar] [CrossRef]
- Feldmeier, H.; Heukelbach, J.; Eisele, M.; Sousa, A.Q.; Barbosa, L.M.M.; Carvalho, C.B.M. Bacterial Superinfection in Human Tungiasis. Trop. Med. Int. Health 2002, 7, 559–564. [Google Scholar] [CrossRef]
- Dassoni, F.; Polloni, I.; Margwe, S.B.; Veraldi, S. Tungiasis in Northern Tanzania: A Clinical Report from Qameyu Village, Babati District, Manyara Region. J. Infect. Dev. Ctries. 2014, 8, 1456–1460. [Google Scholar] [CrossRef][Green Version]
- Mazigo, H.D.; Bahemana, E.; Dyegura, O.; Mnyone, L.L.; Kweka, E.J.; Zinga, M.; Konje, E.T.; Waihenya, R.; Heukelbach, J. Severe Tungiasis in Western Tanzania: Case Series. J. Public Health Afr. 2011, 2, e21. [Google Scholar] [CrossRef]
- Feldmeier, H.; Heukelbach, J.; Ugbomoiko, U.S.; Sentongo, E.; Mbabazi, P.; von Samson-Himmelstjerna, G.; Krantz, I.; The International Expert Group for Tungiasis. Tungiasis—A Neglected Disease with Many Challenges for Global Public Health. PLoS Negl. Trop. Dis. 2014, 8, e3133. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Wilcke, T.; Harms, G.; Feldmeier, H. Seasonal Variation of Tungiasis in an Endemic Community. Am. J. Trop. Med. Hyg. 2005, 72, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Abari, E.; D’Haese, J.; Calheiros, C.; Heukelbach, J.; Mencke, N.; Feldmeier, H.; Mehlhorn, H. Investigations on the Life Cycle and Morphology of Tunga Penetrans in Brazil. Parasitol. Res. 2007, 101 (Suppl. 2), S233–S242. [Google Scholar] [CrossRef] [PubMed]
- Linardi, P.M.; Calheiros, C.M.L.; Campelo-Junior, E.B.; Duarte, E.M.; Heukelbach, J.; Feldmeier, H. Occurrence of the off-Host Life Stages of Tunga Penetrans (Siphonaptera) in Various Environments in Brazil. Ann. Trop. Med. Parasitol. 2010, 104, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Muehlen, M.; Feldmeier, H.; Wilcke, T.; Winter, B.; Heukelbach, J. Identifying Risk Factors for Tungiasis and Heavy Infestation in a Resource-Poor Community in Northeast Brazil. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 371–380. [Google Scholar] [CrossRef]
- Ugbomoiko, U.S.; Ariza, L.; Ofoezie, I.E.; Heukelbach, J. Risk Factors for Tungiasis in Nigeria: Identification of Targets for Effective Intervention. PLoS Negl. Trop. Dis. 2007, 1, e87. [Google Scholar] [CrossRef]
- Wiese, S.; Elson, L.; Reichert, F.; Mambo, B.; Feldmeier, H. Prevalence, Intensity and Risk Factors of Tungiasis in Kilifi County, Kenya: I. Results from a Community-Based Study. PLoS Negl. Trop. Dis. 2017, 11, e0005925. [Google Scholar] [CrossRef]
- Elson, L.; Wright, K.; Swift, J.; Feldmeier, H. Control of Tungiasis in Absence of a Roadmap: Grassroots and Global Approaches. Trop. Med. Infect. Dis. 2017, 2, 33. [Google Scholar] [CrossRef]
- Thielecke, M.; Nordin, P.; Ngomi, N.; Feldmeier, H. Treatment of Tungiasis with Dimeticone: A Proof-of-Principle Study in Rural Kenya. PLoS Negl. Trop. Dis. 2014, 8, e3058. [Google Scholar] [CrossRef]
- Miller, H.; Trujillo-Trujillo, J.; Mutebi, F.; Feldmeier, H. Efficacy and Safety of Dimeticones in the Treatment of Epidermal Parasitic Skin Diseases with Special Emphasis on Tungiasis: An Evidence-Based Critical Review. Braz. J. Infect. Dis. 2020, 24, 170–177. [Google Scholar] [CrossRef]
- Winter, B.; Oliveira, F.A.; Wilcke, T.; Heukelbach, J.; Feldmeier, H. Tungiasis-Related Knowledge and Treatment Practices in Two Endemic Communities in Northeast Brazil. J. Infect. Dev. Ctries. 2009, 3, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Kiire, C.F. The Epidemiology and Prophylaxis of Hepatitis B in Sub-Saharan Africa: A View from Tropical and Subtropical Africa. Gut 1996, 38 (Suppl. 2), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Feldmeier, H.; Sentongo, E.; Krantz, I. Tungiasis (sand Flea Disease): A Parasitic Disease with Particular Challenges for Public Health. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gitau, A.K.; Oyieke, F.O.; Richard, W. Assessment of Tungiasis Management Knowledge in Kandara Sub County, Kenya. Available online: https://www.entomoljournal.com/archives/2021/vol9issue4/PartB/9-4-27-910.pdf (accessed on 22 June 2023).
- McNeilly, H.; Thielecke, M.; Mutebi, F.; Banalyaki, M.; Reichert, F.; Wiese, S.; Feldmeier, H. Tungiasis Stigma and Control Practices in a Hyperendemic Region in Northeastern Uganda. Trop. Med. Infect. Dis. 2023, 8, 206. [Google Scholar] [CrossRef]
- Mutebi, F.; McNeilly, H.; Thielecke, M.; Reichert, F.; Wiese, S.; Mukone, G.; Feldmeier, H. Prevalence and Infection Intensity of Human and Animal Tungiasis in Napak District, Karamoja, Northeastern Uganda. Trop. Med. Infect. Dis. 2023, 8, 111. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Available online: https://apps.who.int/iris/bitstream/handle/10665/338565/9789240026605-ara.pdf (accessed on 22 June 2023).
- Malecela, M.N.; Ducker, C. A Road Map for Neglected Tropical Diseases 2021–2030. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 121–123. [Google Scholar] [CrossRef]
- Cantey, P.T.; Rout, J.; Rao, G.; Williamson, J.; Fox, L.M. Increasing Compliance with Mass Drug Administration Programs for Lymphatic Filariasis in India through Education and Lymphedema Management Programs. PLoS Negl. Trop. Dis. 2010, 4, e728. [Google Scholar] [CrossRef]
- Njomo, D.W.; Amuyunzu-Nyamongo, M.; Mukoko, D.N.; Magambo, J.K.; Njenga, S.M. Social Mobilization and Compliance with Mass Treatment for Lymphatic Filariasis Elimination in Kenya. Afr. J. Health Sci. 2012, 20, 42–49. [Google Scholar]
- Karki, P.; Prabandari, Y.S.; Probandari, A.; Banjara, M.R. Feasibility of School-Based Health Education Intervention to Improve the Compliance to Mass Drug Administration for Lymphatic Filariasis in Lalitpur District, Nepal: A Mixed Methods among Students, Teachers and Health Program Manager. PLoS ONE 2018, 13, e0203547. [Google Scholar] [CrossRef]
- Nyang’inja, R.A.; Angwenyi, D.N.; Musyoka, C.M.; Orwa, T.O. Mathematical Modeling of the Effects of Public Health Education on Tungiasis—A Neglected Disease with Many Challenges in Endemic Communities. Adv. Differ. Equ. 2018, 2018, 426. [Google Scholar] [CrossRef]
- Simelane, S.M.; Dlamini, P.G.; Osaye, F.J.; Obaido, G.; Ogbukiri, B.; Aruleba, K.; Jones, C.M.; Chukwu, C.W.; Egbelowo, O.F. Modeling the Impact of Public Health Education on Tungiasis Dynamics with Saturated Treatment: Insight through the Caputo Fractional Derivative. Math. Biosci. Eng. 2023, 20, 7696–7720. [Google Scholar] [CrossRef]
- Muhanguzi, D.; Mugenyi, A.; Bigirwa, G.; Kamusiime, M.; Kitibwa, A.; Akurut, G.G.; Ochwo, S.; Amanyire, W.; Okech, S.G.; Hattendorf, J.; et al. African Animal Trypanosomiasis as a Constraint to Livestock Health and Production in Karamoja Region: A Detailed Qualitative and Quantitative Assessment. BMC Vet. Res. 2017, 13, 355. [Google Scholar] [CrossRef]
- Mayring, P.; Fenzl, T. Qualitative Inhaltsanalyse. In Handbuch Methoden der Empirischen Sozialforschung; Baur, N., Blasius, J., Eds.; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2019; pp. 633–648. ISBN 9783658213084. [Google Scholar]
- Kimani, B.; Nyagero, J.; Ikamari, L. Knowledge, Attitude and Practices on Jigger Infestation among Household Members Aged 18 to 60 Years: Case Study of a Rural Location in Kenya. Pan Afr. Med. J. 2012, 13 (Suppl. 1), 7. [Google Scholar]
- Mutebi, F.; Krücken, J.; von Samson-Himmelstjerna, G.; Waiswa, C.; Mencke, N.; Eneku, W.; Andrew, T.; Feldmeier, H. Animal and Human Tungiasis-Related Knowledge and Treatment Practices among Animal Keeping Households in Bugiri District, South-Eastern Uganda. Acta Trop. 2018, 177, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Van’t Noordende, A.T.; Lisam, S.; Ruthindartri, P.; Sadiq, A.; Singh, V.; Arifin, M.; van Brakel, W.H.; Korfage, I.J. Leprosy Perceptions and Knowledge in Endemic Districts in India and Indonesia: Differences and Commonalities. PLoS Negl. Trop. Dis. 2021, 15, e0009031. [Google Scholar] [CrossRef] [PubMed]
- Urgesa, K.; Bobosha, K.; Seyoum, B.; Geda, B.; Weldegebreal, F.; Mihret, A.; Howe, R.; Kaba, M.; Aseffa, A. Knowledge of and Attitude Toward Leprosy in a Leprosy Endemic District, Eastern Ethiopia: A Community-Based Study. Risk Manag. Healthc. Policy 2020, 13, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Bai, Y.; Li, X.; Zhang, Y. Scabies Knowledge Among Undergraduate Nursing Students in China: A Questionnaire Survey. Clin. Cosmet. Investig. Dermatol. 2022, 15, 133–138. [Google Scholar] [CrossRef]
- Alsaidan, M.S.; Alhaqbani, Y.J.; Alfaifi, A.M.; Alotaibi, F.G.; Alsomari, A.K.; Alzhrani, A.A.; Al-Ghamdi, S.H. Assessing Knowledge of Scabies among Physicians Working in Primary Health Care Setting. J. Fam. Med. Prim. Care 2020, 9, 5320–5326. [Google Scholar] [CrossRef]
- El-Mouhdi, K.; Chahlaoui, A.; Fekhaoui, M. The Cutaneous Leishmaniasis and the Sand Fly: Knowledge and Beliefs of the Population in Central Morocco (El Hajeb). Dermatol. Res. Pract. 2020, 2020, 1896210. [Google Scholar] [CrossRef]
- Garapati, P.; Pal, B.; Siddiqui, N.A.; Bimal, S.; Das, P.; Murti, K.; Pandey, K. Knowledge, Stigma, Health Seeking Behaviour and Its Determinants among Patients with Post Kalaazar Dermal Leishmaniasis, Bihar, India. PLoS ONE 2018, 13, e0203407. [Google Scholar] [CrossRef]
- Kamga, H.L.F.; Assob, N.J.C.; Nsagha, D.S.; Njunda, A.L.; Njimoh, D.L. A Community Survey on the Knowledge of Neglected Tropical Diseases in Cameroon. J. Mol. Genet. Med. 2012, 1, 131–140. [Google Scholar] [CrossRef]
- Sumo, L.; Ntonifor, N.H.; Lenou-Nanga, C.G.; Chenkumo-Kengmoni, N.; Amana-Bokagne, V.T.; Awah, C.G.; Niamsi-Emalio, Y.; Nana-Djeunga, H.C. An Integrated Approach to Assess Knowledge/perceptions and Attitudes/practices (KAP) Regarding Major Neglected Tropical Diseases Endemic in the Mbengwi Health District, North West Region, Cameroon. J. Epidemiol. Glob. Health 2021, 11, 426–434. [Google Scholar] [CrossRef]
- Olamiju, O.J.; Olamiju, F.O.; Adeniran, A.A.; Mba, I.C.; Ukwunna, C.C.; Okoronkwo, C.; Ekpo, U.F. Public Awareness and Knowledge of Neglected Tropical Diseases (NTDs) Control Activities in Abuja, Nigeria. PLoS Negl. Trop. Dis. 2014, 8, e3209. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.N.; Ozwara, H.S.; Gicheru, M.M. Epidemiology of Tunga Penetrans Infestation in Selected Areas in Kiharu Constituency, Murang’a County, Kenya. Trop. Dis. Travel Med. Vaccines 2015, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Njau, N.N.; Wanzala, P.; Mutugi, M.; Ariza, L.; Heukelbach, J. Tungiasis (jigger Infestation) in Rural Kenya, an Emerging Infectious Disease. Retrovirology 2012, 9, 37. [Google Scholar] [CrossRef]
- Tomczyk, S.; Deribe, K.; Brooker, S.J.; Clark, H.; Rafique, K.; Knopp, S.; Utzinger, J.; Davey, G. Association between Footwear Use and Neglected Tropical Diseases: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2014, 8, e3285. [Google Scholar] [CrossRef] [PubMed]
- Thielecke, M.; Raharimanga, V.; Rogier, C.; Stauss-Grabo, M.; Richard, V.; Feldmeier, H. Prevention of Tungiasis and Tungiasis-Associated Morbidity Using the Plant-Based Repellent Zanzarin: A Randomized, Controlled Field Study in Rural Madagascar. PLoS Negl. Trop. Dis. 2013, 7, e2426. [Google Scholar] [CrossRef]
- Saquib, Q.; Siddiqui, M.A.; Ansari, S.M.; Alwathnani, H.A.; Musarrat, J.; Al-Khedhairy, A.A. Cytotoxicity and Genotoxicity of Methomyl, Carbaryl, Metalaxyl, and Pendimethalin in Human Umbilical Vein Endothelial Cells. J. Appl. Toxicol. 2021, 41, 832–846. [Google Scholar] [CrossRef]
- Clyti, E.; Couppie, P.; Deligny, C.; Jouary, T.; Sainte-Marie, D.; Pradinaud, R. Effectiveness of 20% salicylated vaseline in the treatment of profuse tungiasis. Report of 8 cases in French Guiana. Bull. Soc. Pathol. Exot. 2003, 96, 412–414. [Google Scholar]
- Mitchell, C.J.; Stephany, P. Case Report: Infestation of Tunga Penetrans in Villages near Zomba Central Hospital. Malawi Med. J. 2013, 25, 88–89. [Google Scholar]
- Miller, H.; Ocampo, J.; Ayala, A.; Trujillo, J.; Feldmeier, H. Very Severe Tungiasis in Amerindians in the Amazon Lowland of Colombia: A Case Series. PLoS Negl. Trop. Dis. 2019, 13, e0007068. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Polman, K.; Allen, T. Neglected Tropical Diseases in Biosocial Perspective. J. Biosoc. Sci. 2016, 48 (Suppl. 1), S1–S15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brownson, R.C.; Kumanyika, S.K.; Kreuter, M.W.; Haire-Joshu, D. Implementation Science Should Give Higher Priority to Health Equity. Implement. Sci. 2021, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.; Kamara, K.; Umar, Z.A.; Chahine, T.; Daulaire, N.; Bossert, T. Applied Systems Thinking: A Viable Approach to Identify Leverage Points for Accelerating Progress towards Ending Neglected Tropical Diseases. Health Res. Policy Syst. 2020, 18, 56. [Google Scholar] [CrossRef]


| Answer Options (Multiple Answers Possible) | Number | (%) |
|---|---|---|
| No animals | 672 | (50.6) |
| Chickens | 372 | (28.0) |
| Goats | 357 | (26.9) |
| Cattle | 352 | (26.5) |
| Sheep | 289 | (21.7) |
| Cats | 152 | (11.4) |
| Other poultry * | 136 | (10.2) |
| Dogs | 124 | (9.3) |
| Pigs | 12 | (0.9) |
| Other animals ** | 12 | (0.9) |
| Answer Options (Multiple Answers Possible) | Frequency of Answers | (%) |
|---|---|---|
| Itching | 1287 | (96.8) |
| Pain | 1115 | (83.9) |
| Swelling | 718 | (54.0) |
| Loss of nails | 350 | (26.3) |
| Ulcers | 325 | (24.5) |
| Warm skin | 272 | (20.5) |
| Loss of toes | 240 | (18.1) |
| Deformed feet | 98 | (7.4) |
| Alteration of gait | 93 | (7.0) |
| Others * | 37 | (2.8) |
| Bacterial superinfection | 33 | (2.5) |
| I don’t know | 5 | (0.4) |
| Answer Options (Multiple Answers Possible) | Frequency of Answers | (%) |
|---|---|---|
| Dry/dusty/dirty floor | 1150 | (86.5) |
| Poor body hygiene | 943 | (71.0) |
| Poor housing | 793 | (59.7) |
| Close contact to animals | 553 | (41.6) |
| Dry weather | 362 | (27.2) |
| Sleeping on the floor | 196 | (14.7) |
| Overcrowded homes | 125 | (9.4) |
| No footwear | 100 | (7.5) |
| Waste on the compound | 90 | (6.8) |
| Others * | 28 | (2.1) |
| Presence of rats/mice | 26 | (2.0) |
| I don’t know | 22 | (1.7) |
| Open defecation | 11 | (0.8) |
| Answer Options (Multiple Answers Possible) | Frequency of Answers | (%) |
|---|---|---|
| Pigs | 649 | (48.8) |
| Dogs | 648 | (48.8) |
| Goats | 582 | (43.8) |
| Cattle | 519 | (39.1) |
| Cats | 501 | (37.7) |
| Sheep | 436 | (32.8) |
| Chicken | 307 | (23.1) |
| I don’t know | 259 | (19.5) |
| Other animals * | 21 | (1.6) |
| Donkeys | 13 | (1.0) |
| Answer Options (Multiple Answers Possible) | Frequency of Answers | (%) |
|---|---|---|
| Regular washing of the feet | 1202 | (90.4) |
| Keeping houses/compound clean | 1013 | (76.2) |
| Apply concrete/cow dung on the floor | 574 | (43.2) |
| Keep animals away from houses | 531 | (40.0) |
| Proper waste disposal | 475 | (35.7) |
| Spraying the house with insecticides | 447 | (33.6) |
| Wearing shoes | 143 | (10.8) |
| Others * | 25 | (1.9) |
| I don’t know | 15 | (1.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thielecke, M.; McNeilly, H.; Mutebi, F.; Banalyaki, M.B.; Arono, R.; Wiese, S.; Reichert, F.; Mukone, G.; Feldmeier, H. High Level of Knowledge about Tungiasis but Little Translation into Control Practices in Karamoja, Northeastern Uganda. Trop. Med. Infect. Dis. 2023, 8, 425. https://doi.org/10.3390/tropicalmed8090425
Thielecke M, McNeilly H, Mutebi F, Banalyaki MB, Arono R, Wiese S, Reichert F, Mukone G, Feldmeier H. High Level of Knowledge about Tungiasis but Little Translation into Control Practices in Karamoja, Northeastern Uganda. Tropical Medicine and Infectious Disease. 2023; 8(9):425. https://doi.org/10.3390/tropicalmed8090425
Chicago/Turabian StyleThielecke, Marlene, Hannah McNeilly, Francis Mutebi, Mike B. Banalyaki, Rebecca Arono, Susanne Wiese, Felix Reichert, George Mukone, and Hermann Feldmeier. 2023. "High Level of Knowledge about Tungiasis but Little Translation into Control Practices in Karamoja, Northeastern Uganda" Tropical Medicine and Infectious Disease 8, no. 9: 425. https://doi.org/10.3390/tropicalmed8090425
APA StyleThielecke, M., McNeilly, H., Mutebi, F., Banalyaki, M. B., Arono, R., Wiese, S., Reichert, F., Mukone, G., & Feldmeier, H. (2023). High Level of Knowledge about Tungiasis but Little Translation into Control Practices in Karamoja, Northeastern Uganda. Tropical Medicine and Infectious Disease, 8(9), 425. https://doi.org/10.3390/tropicalmed8090425






