Prevalence of Human and Animal Fasciolosis in Butajira and Gilgel Gibe Health Demographic Surveillance System Sites in Ethiopia
Abstract
1. Introduction
2. Methods
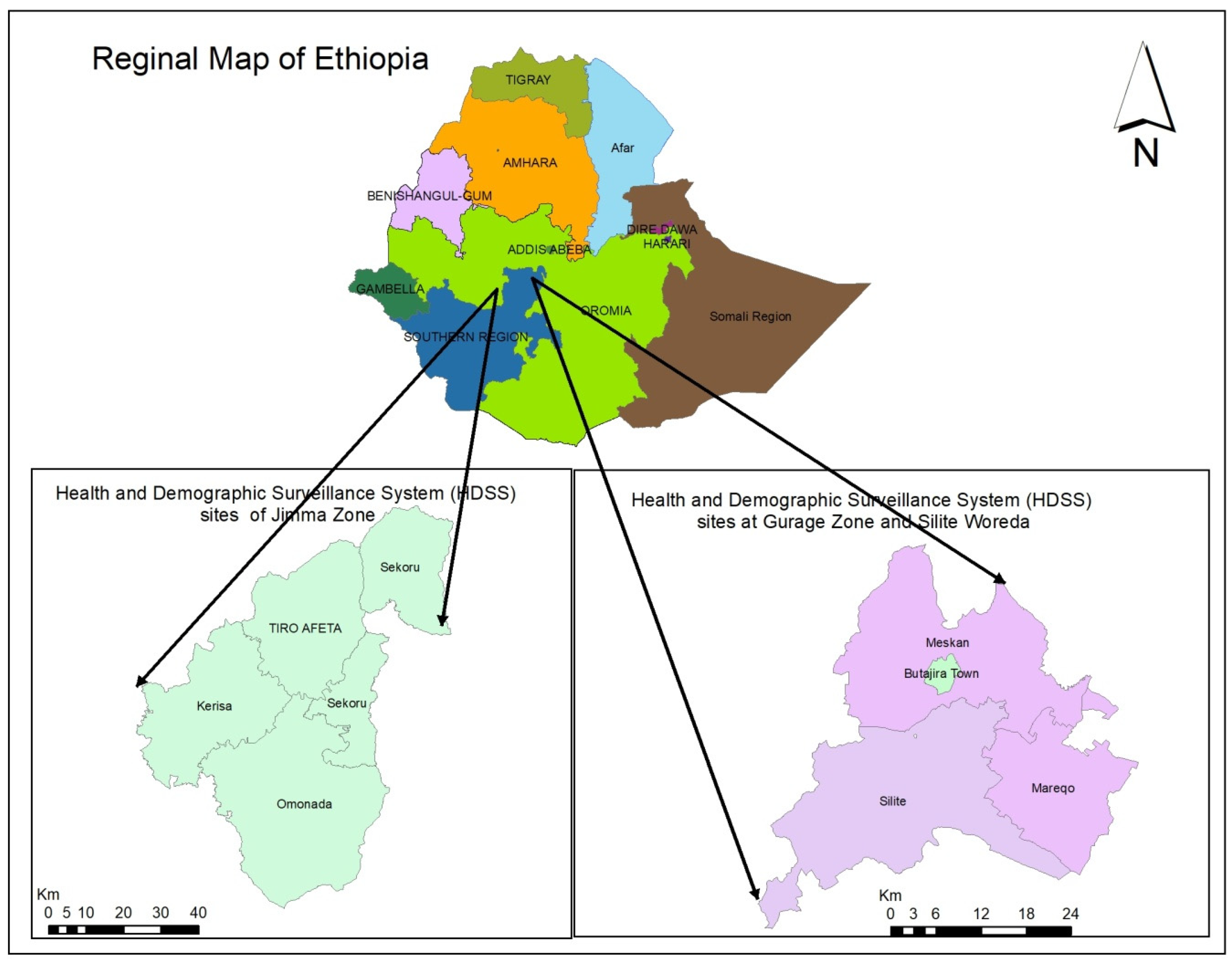
2.1. Study Sites
2.2. Study Population
2.3. Sample Size and Sampling Design
2.4. Data Collection
2.5. Laboratory Analysis
2.6. Data Management and Data Analysis
2.7. Ethical Considerations
3. Results
3.1. Sociodemographic Characteristics
3.2. Prevalence of Fasciolosis
3.3. Environmental Characteristics of Households
3.4. Knowledge, Attitudes and Practices Related to Fasciolosis in Respondents
3.5. Livestock Husbandry
3.6. Factors Associated with Fasciolosis in Animals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arbabi, M.; Nezami, E.; Hooshyar, H.; Delavari, M. Epidemiology and economic loss of fasciolosis and dicrocoeliosis in Arak. Iran.-Vet. World 2018, 11, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Magaji, A.A.; Ibrahim, K.; Salihu, M.D.; Saulawa, M.A.; Mohammed, A.A.; Musawa, A.I. Prevalence of Fascioliasis in Cattle Slaughtered in Sokoto Metropolitan Abattoir, Sokoto, Nigeria. Adv. Epidemiol. 2014, 2014, 247258. [Google Scholar] [CrossRef]
- Nyindo, M.; Lukambagire, A.H. Fascioliasis: An Ongoing Zoonotic Trematode Infection. BioMed. Res. Int. 2015, 2015, 786195. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinski, K.; O’Neill, S.M.; Donnelly, S.; Dalton, J.P. A prospective view of animal and human Fasciolosis. Parasite Immunol. 2016, 38, 558–568. [Google Scholar] [CrossRef]
- Carmona, C.; Tort, J.F. Fasciolosis in South America: Epidemiology and control challenges. J. Helminthol. 2017, 91, 99–109. [Google Scholar] [CrossRef]
- Fürst, T.; Keiser, J.; Utzinger, J. Global burden of human food-borne trematodiasis: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 210–221. [Google Scholar] [CrossRef]
- Fürst, T.; Duthaler, U.; Sripa, B.; Utzinger, J.; Keiser, J. Trematode Infections—Infectious Disease Clinics. Infect. Dis. Clin. N. Am. 2012, 26, 399–419. [Google Scholar] [CrossRef]
- Caravedo, M.A.; Cabada, M.M. Human Fascioliasis: Current Epidemiological Status and Strategies for Diagnosis, Treatment, and Control. Res. Rep. Trop. Med. 2020, 11, 149–158. [Google Scholar] [CrossRef]
- Chai, J.Y.; Jung, B.K. General overview of the current status of human foodborne trematodiasis. Parasitology 2022, 149, 1262–1285. [Google Scholar] [CrossRef]
- Mas-Coma, M.S.; Esteban, J.G.; Bargues, M.D. Epidemiology of human fascioliasis: A review and proposed new classification. Bull World Health Organ. 1999, 77, 340–346. [Google Scholar]
- Wamae, L.W.; Hammond, J.A.; Harrison, L.J.; Onyango-Abuje, J.A. Comparison of production losses caused by chronic Fasciola gigantica infection in yearling Friesian and Boran cattle. Trop. Anim. Health Prod. 1998, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Alemneh, T.; Ayelign, M. Study on Prevalence and Economic Importance of Bovine Fasciolosis in Three Districts of North-East Amhara Region, Ethiopia. J. Infect. Non. Infect. Dis. 2017, 3, 24. [Google Scholar]
- Yilma, J.M.; Malone, J.B. A geographic information system forecast model for strategic control of fasciolosis in Ethiopia. Vet. Parasitol. 1998, 78, 103–127. [Google Scholar] [CrossRef]
- Zewde, A.; Bayu, Y.; Wondimu, A. Prevalence of bovine fasciolosis and its economic loss due to liver condemnation at Wolaita Sodo Municipal Abattair, Ethiopia. Vet. Med. Int. 2019, 2019, 9572373. [Google Scholar] [CrossRef]
- Ergena, T. Prevalence of Bovine Fasciolosis and Associated Financial Loss due to liver Condemnation at Jimma Municipal Abattoir, Jimma, Ethiopia. Int. J. Adv. Res. Biol. Sci. 2019, 6, 132–139. [Google Scholar]
- Ayele, Y.; Wondmnew, F.; Tarekegn, Y. The Prevalence of Bovine and Ovine Fasciolosis and the Associated Economic Loss Due to Liver Condemnation in and around Debire Birhan, Ethiopia. SOJ Immunol. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Ngategize, P.K.; Bekele, T.; Tilahun, G. Financial losses caused by ovine fasciolosis in the Ethiopian highlands. Trop. Anim. Health Prod. 1993, 25, 155–161. [Google Scholar] [CrossRef]
- Bekana, T.; Berhe, N.; Eguale, T.; Aemero, M.; Medhin, G.; Tulu, B.; Ghiwot, Y.; Liang, S.; Hu, W. Prevalence and factors associated with intestinal schistosomiasis and human fascioliasis among school children in Amhara Regional State, Ethiopia. Trop. Med. Health. 2021, 49, 1–11. [Google Scholar] [CrossRef]
- Bayu, B.; Asnake, S.; Woretaw, A.; Ali, J.; Gedefaw, M.; Fente, T.; Getachew, A.; Tsegaye, S.; Dagne, T.; Yitayew, G. Cases of Human Fascioliasis in north-west Ethiopia. Ethiop. J. Health Dev. 2005, 19, 237–240. [Google Scholar] [CrossRef]
- Fentie, T.; Erqou, S.; Gedefaw, M.; Desta, A. Epidemiology of human fascioliasis and intestinal parasitosis among schoolchildren in Lake Tana Basin, northwest Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 480–486. [Google Scholar] [CrossRef]
- Mazeri, S.; Sargison, N.; Kelly, R.F.; Bronsvoort BM: Handel, I. Evaluation of the Performance of Five Diagnostic Tests for Fasciola hepatica Infection in Naturally Infected Cattle Using a Bayesian No Gold Standard Approach. PLoS ONE 2016, 11, e0161621. [Google Scholar] [CrossRef] [PubMed]
- Shafi, W.; Shafi, W. Prevalence of bovine fasciolosis in and around Bedelle. Int. J. Vet. Sci. Res. 2021, 7, 13–23. [Google Scholar]
- Charlier, J.; De Meulemeester, L.; Claerebout, E.; Williams, D.; Vercruysse, J. Qualitative and quantitative evaluation of coprological and serological techniques for the diagnosis of fasciolosis in cattle. Vet. Parasitol. 2008, 153, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Rapsch, C.; Schweizer, G.; Grimm, F.; Kohler, L.; Bauer, C.; Deplazes, P.; Braun, U.; Torgerson, P. Estimating the true prevalence of Fasciola hepatica in cattle slaughtered in Switzerland in the absence of an absolute diagnostic test. Int. J. Parasitol. 2006, 36, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, B.; Khabisi, S.A. Immunodiagnosis of Human Fascioliasis: An Update of Concepts and Performances of the Serological Assays. J. Clin. Diagn. Res. 2017, 11, 5–10. [Google Scholar] [CrossRef]
- Castro, E.; Freyre, A.; Hernández, Z. Serological responses of cattle after treatment and during natural re-infection with Fasciola hepatica, as measured with a dot-ELISA system. Vet. Parasitol. 2000, 90, 201–208. [Google Scholar] [CrossRef]
- Salimi-Bejestani, M.R.; McGarry, J.W.; Felstead, S.; Ortiz, P.; Akca, A.; Williams, D.J.L. Development of an antibody-detection ELISA for Fasciola hepatica and its evaluation against a commercially available test. Res. Vet. Sci. 2005, 78, 177–181. [Google Scholar] [CrossRef]
- Gonzales Santana, B.; Dalton, J.P.; Vasquez Camargo, F.; Parkinson, M.; Ndao, M. The Diagnosis of Human Fascioliasis by Enzyme-Linked Immunosorbent Assay (ELISA) Using Recombinant Cathepsin L Protease. PLoS Negl. Trop. Dis. 2013, 7, 1–9. [Google Scholar] [CrossRef]
- Valero, M.A.; Periago, M.V.; Pérez-Crespo, I.; Angles, R.; Villegas, F.; Aguirre, C.; Strauss, W.; Espinoza, J.R.; Herrera, P.; Terashima, A.; et al. Field Evaluation of a Coproantigen Detection Test for Fascioliasis Diagnosis and Surveillance in Human Hyperendemic Areas of Andean Countries. PLoS Neglected Trop. Dis. 2012, 6, e1812. [Google Scholar] [CrossRef]
- Mezo, M.; González-Warleta, M.; Carro, C.; Ubeira, F.M. An ultrasensitive capture ELISA for detection of Fasciola hepatica coproantigens in sheep and cattle using a new monoclonal antibody (MM3). J. Parasitol. 2004, 90, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Creo, A.; Díaz, P.; López, C.; Béjar, J.P.; Martínez-Sernández, V.; Panadero, R.; Díez-Baños, P.; Ubeira, F.M.; Morrondo, P. Fasciola hepatica in goats from north-western Spain: Risk factor analysis using a capture ELISA. Vet. J. 2016, 208, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Buchon, P.; Funatsu, I.R.; Angles, R.; Mas-Bargues, C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Donkey Fascioliasis Within a One Health Control Action: Transmission Capacity, Field Epidemiology, and Reservoir Role in a Human Hyperendemic Area. Front. Vet. Sci. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Rinaldi, L.; Gonzalez, S.; Guerrero, J.; Aguilera, L.C.; Musella, V.; Genchi, C.; Cringoli, G. A One-Health integrated approach to control fascioliasis in the Cajamarca valley of Peru. Geospat. Health. 2012, 6, S67–S73. [Google Scholar] [CrossRef]
- Aschale, A.; Adane, M.; Getachew, M.; Faris, K.; Gebretsadik, D.; Sisay, T.; Dewau, R.; Chanie, M.G.; Muche, A.; Zerga, A.A.; et al. Water, sanitation, and hygiene conditions and prevalence of intestinal parasitosis among primary school children in Dessie City, Ethiopia. Seale H, editor. PLoS ONE 2021, 16, e0245463. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, E.; Alda, P.; Vázquez, A.; Hurtrez-Boussès, S.; Vittecoq, M. Impact of Human Activities on Fasciolosis Transmission. Trends Parasitol. 2018, 34, 891–903. [Google Scholar] [CrossRef]
- Broussard, J.D. Optimal fecal assessment. Clin. Tech. Small Anim. Pract. 2003, 18, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Bio-X Diagnostics. ELISA Kit for Antigenic Diagnosis of Fasciola hepatica Indirect Sandwich Test for Faeces Diagnostic Test for Cattle and Sheep Double Wells. 2023. Available online: https://www.biox.com/en/bio-k-201-monoscreen-agelisa-fasciola-hepatica-indirect-sandwich-double-wells-p-257/ (accessed on 20 March 2023).
- Ubeira, F.M.; Mezo, M.; Más-Coma, S.; Paniagua, E.; Periago, M.V.; González-Warleta, M.; Cortizo, S.; Muiño, L.; Pérez-Crespo, I.; Llovo, J.; et al. MM3-ELISA detection of Fasciola hepatica coproantigens in preserved human stool samples. Am. J. Trop. Med. Hyg. 2009, 81, 156–162. [Google Scholar] [CrossRef]
- Hassan, M.M.; Moustafa, N.E.; Mahmoud, L.A.; Abbaza, B.E.; Hegab, M.H. Prevalence of Fasciola infection among school children in Sharkia Governorate, Egypt. J. Egypt Soc. Parasitol. 1995, 25, 543–549. [Google Scholar]
- Khanjari, A.; Bahonar, A.; Fallah, S.; Bagheri, M.; Alizadeh, A.; Fallah, M.; Khanjari, Z. Prevalence of fasciolosis and dicrocoeliosis in slaughtered sheep and goats in Amol Abattoir, Mazandaran, northern Iran. Asian Pac. J. Trop. Dis. 2014, 4, 120–124. [Google Scholar] [CrossRef]
- Relf, V.; Good, B.; Hanrahan, J.P.; McCarthy, E.; Forbes, A.B.; deWaal, T. Temporal studies on Fasciola hepatica in Galba truncatula in the west of Ireland. Vet. Parasitol. 2011, 175, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Ibrahim, N.; Tafese, W.; Deneke, Y. Prevalence of Bovine Fasciolosis in Municipal Abattoir of Haramaya, Ethiopia. Food Sci. Qual. Manag. 2016, 48, 1–7. [Google Scholar]
- Berhe, G.; Berhane, K.; Tadesse, G. Prevalence and economic significance of fasciolosis in cattle in Mekelle Area of Ethiopia. Trop. Anim. Health Prod. 2009, 41, 1503–1504. [Google Scholar] [CrossRef]
- Ibrahim, N.; Wasihun, P.; Tolosa, T. Prevalence of bovine fasciolosis and economic importance due to liver condemnation at Kombolcha industrial abattoir, Ethiopia. Internet. J. Vet. Med. 2009, 8, 1–8. [Google Scholar]
- Gemechu, B.; Mamo, E. A Preliminary Survey of Bovine Fascioliasis in Ethiopia. Ethiop. J. Agric. Sci. 1979. Available online: https://agris.fao.org/agris-search/search.do?recordID=ET2010000002 (accessed on 20 August 2022).
- Oljira, W.; Mideksa, B.; Mekonnen, G.; Kebebew, G.; Jorga, E. Fasciolosis in sheep and goats slaughtered at abattoirs in Central Ethiopia and associated financial losses. Food Waterborne Parasitol. 2022, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kantzoura, V.; Kouam, M.K.; Demiris, N.; Feidas, H.; Theodoropoulos, G. Risk factors and geospatial modelling for the presence of Fasciola hepatica infection in sheep and goat farms in the Greek temperate Mediterranean environment. Parasitology 2011, 138, 926–938. [Google Scholar] [CrossRef]
- Abdulhakim, Y.; Addis, M. An Abattoir Study on the Prevalence of Fasciolosis in Cattle, Sheep and Goats in Debre Zeit Town, Ethiopia. Glob. Vet. 2012, 8, 308–314. [Google Scholar]
- Mahato, S.N.; Harrison, L.J. Control of fasciolosis in stall-fed buffaloes by managing the feeding of rice straw. Trop. Anim. Health Prod. 2005, 37, 285–291. [Google Scholar] [CrossRef]
- Ahmad-Najib, M.; Wan-Nor-Amilah, W.A.W.; Kin, W.W.; Arizam, M.F.; Noor-Izani, N.J. Prevalence and Risk Factors of Bovine Fascioliasis in Kelantan, Malaysia: A Cross-Sectional Study. Trop. Life Sci. Res. 2021, 32, 1–14. [Google Scholar] [CrossRef]
- John, B.C.; Davies, D.R.; Williams, D.J.L.; Hodgkinson, J.E. A review of our current understanding of parasite survival in silage and stored forages, with a focus on Fasciola hepatica metacercariae. Grass Forage Sci. 2019, 74, 211–217. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nguyen, D.T.; Van Nguyen, T.; Huynh, V.V.; Le, D.Q.; Fukuda, Y.; Nakai, Y. Prevalence of Fasciola in cattle and of its intermediate host Lymnaea snails in central Vietnam. Trop. Anim. Health Prod. 2012, 44, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Kurnianto, H.; Ramanoon, S.; Abdul Aziz, N.A.; Indarjulianto, S. Prevalence, risk factors, and infection intensity of fasciolosis in dairy cattle in Boyolali, Indonesia. Vet. World 2022, 15, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | HDSS Site | Significance Level (Butajira versus Gilgel Gibe HDSS Site) | |||
|---|---|---|---|---|---|
| Butajira n = 194 (%) | Gilgel Gibe n = 195 (%) | Both Sites n = 389 (%) | |||
| Sex | Male | 90 (46.4) | 91 (46.7) | 181 (46.5) | 0.96 † |
| Female | 104 (53.6) | 104 (53.3) | 208 (53.5) | ||
| Age (years) | 18–30 | 38 (19.6) | 66 (33.8) | 104 (26.7) | 0.004 † |
| 31–45 | 118 (60.8) | 89 (45.6) | 207 (53.2) | ||
| 46–55 | 20 (10.3) | 27 (13.8) | 47 (12.1) | ||
| >55 | 18 (9.3) | 13 (6.7) | 31 (8.0) | ||
| Educational status | Can’t read or write | 99 (51.0) | 119 (61.0) | 218 (56.0) | 0.14 † |
| Primary Education | 83 (42.8) | 67 (34.4) | 150 (38.6) | ||
| Secondary education & above | 12 (6.2) | 9 (4.6) | 21 (5.4) | ||
| Family size | ≤5 people | 35 (18.0) | 28 (14.4) | 63 (16.2) | 0.32 † |
| >5 people | 159 (82.0) | 167 (85.6) | 326 (83.8) | ||
| Residence | Urban | 1 (0.5) | - | 1 (0.3) | N |
| Rural | 193 (99.5) | 195 (100) | 388 (99.7) | ||
| Residence duration in the area | ≤5 years | 2 (1.0) | 9 (4.6) | 11 (2.8) | 0.03 † |
| >5 years | 192 (99.0) | 186 (95.4) | 378 (97.2) | ||
| Occupation | Farmer | 95 (49.0) | 110 (56.4) | 205 (52.7) | 0.11 † |
| Housewife | 92 (47.4) | 83 (42.6) | 175 (45.0) | ||
| Government & private employee | 7 (3.6) | 2(1.0) | 9 (2.3) | ||
| 1 (0.5) | 2 (1.0) | 3 (0.8) | |||
| Annual household income | <5000 ETB (~$96 USD) | 75 (38.7) | 5 (2.6) | 80 (20.6) | <0.001 † |
| 5000–ETB (~$96–189 USD) | 60 (30.9) | 30 (15.3) | 90 (23.1) | ||
| >ETB (~$189 USD) | 59 (30.4) | 160 (82.1) | 219 (56.3) | ||
| Species | HDSS Sites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Butajira | Gilgel Gibe | Both Sites | ||||||||
| No. Sampled | Faecal Antigen Test Results | n (%) | No. Sampled | Faecal Antigen Test Results | n (%) | Significance Level (Butajira versus Gilgel Gibe HDSS Sites) | No. Sampled | Faecal Antigen Test Results | n (%) | |
| Children | 182 | Positive | 1 (0.5) | 195 | Positive | 2 (1.0) | 0.60 † | 377 | Positive | 3 (0.8) |
| Negative | 181 (99.5) | Negative | 193 (99.0) | Negative | 374 (99.2) | |||||
| Cattle | 194 | Positive | 16 (8.2) | 195 | Positive | 97 (49.7) | <0.001 † | 389 | Positive | 113 (29.0) |
| Negative | 178 (91.8) | Negative | 98 (50.3) | Negative | 276 (71.0) | |||||
| Sheep | 141 | Positive | 13 (9.2) | 195 | Positive | 85 (43.6) | <0.001 † | 336 | Positive | 98 (29.2) |
| Negative | 128 (90.8) | Negative | 110 (56.4) | Negative | 238 (70.8) | |||||
| Goat | 50 | Positive | 3 (6.0) | - | Positive | - | N | 50 | Positive | 3 (6.0) |
| Negative | 47 (94.0) | Negative | - | Negative | 47 (94.0) | |||||
| Variable | HDSS Site | p-Value | ||
|---|---|---|---|---|
| Butajira n (%) | Gilgel Gibe n (%) | |||
| Source of water for drinking | n = 194 | n = 195 | ||
| Protected water source | 154 (79.4) | 139 (71.3) | 0.06 † | |
| Unprotected water source | 40 (21.6) | 56 (28.7) | ||
| Sharing water source with livestock | n = 194 | n = 195 | ||
| Yes | 53 (27.3) | 32 (16.4) | 0.09 † | |
| No | 141 (72.7) | 163 (83.6) | ||
| Do you grow vegetables? | n = 194 | n = 195 | ||
| Yes | 89 (45.9) | 37 (19.0) | <0.001 † | |
| No | 105 (54.1) | 158 (81.0) | ||
| Source of water for vegetable growth | n = 89 | n = 37 | ||
| Surface water | 53 (59.6) | 34 (91.9) | <0.001 † | |
| Groundwater | 36 (40.4) | 3 (8.1) | ||
| Type of water for washing vegetable | n = 89 | n = 37 | ||
| Treated water | 24 (27.0) | - | N | |
| Untreated water | 65 (73.0) | 37 (100) | ||
| Do you treat drinking water that comes from sources other than piped water? | n = 194 | n = 195 | ||
| Yes | 13 (6.7) | 12 (6.2) | 0.83 † | |
| No | 181 (93.3) | 183 (93.8) | ||
| Water treatment methods | n = 13 | n = 12 | ||
| Chlorination | - | 1 (8.3) | N | |
| Boiling | 10 (76.9) | 9 (75.1) | ||
| Filtering | 3(23.1) | 1 (8.3) | ||
| Other | - | 1 (8.3) | ||
| Household latrine availability | n = 194 | n = 195 | ||
| Yes | 168 (86.6) | 174 (89.2) | 0.43 † | |
| No | 26 (13.4) | 21 (10.8) | ||
| Type of latrine | n = 168 | n = 174 | ||
| Traditional latrine | 168 (100%) | 174 (100%) | N | |
| Is there a hand-washing facility with soap around the latrine? | n = 168 | n = 174 | ||
| Yes, with soap | 3 (1.8) | 101 (58.0) | 0.14 † | |
| Yes, without soap | 88 (52.4) | - | ||
| No facility | 77 (45.8) | 73 (42.0) | ||
| If no toilet, what do you use? | n = 26 | n = 21 | ||
| Open field | 19 (73.1) | 21 (100) | N | |
| Share with neighborhood | 7 (26.9) | - | ||
| Reason for not having a latrine | n = 26 | n = 21 | ||
| Lack of space | 1 (3.8) | - | N | |
| The water table is high | 1 (3.8) | - | ||
| Soil type is not appropriate | 7 (27.0) | - | ||
| Shortage of resources | 16 (61.6) | 21 (100) | ||
| Lack of awareness | 1 (3.8) | |||
| Variable | HDSS Site | p-Value | ||
|---|---|---|---|---|
| Butajira n (%) | Gilgel Gibe n (%) | |||
| Do you know a disease called liver fluke? | ||||
| Yes | 165 (85.1) | 56 (28.7) | <0.001 † | |
| No | 29 (14.9) | 139 (71.3) | ||
| Do you know the cause of fasciolosis? | ||||
| Yes | 28 (14.4) | 25 (12.8) | 0.64 † | |
| No | 166 (85.6) | 170 (87.2) | ||
| What causes fasciolosis? | n = 28 | n = 25 | ||
| Bacterial infection | 3 (10.7) | 1 (4.0) | N | |
| Snails | - | 1 (4.0) | ||
| Parasite worm | 16 (57.1) | 11 (44.0) | ||
| Don’t known/Not sure | 9 (32.2) | 12 (48.0) | ||
| Can human be infected with Fasciola spp.? | ||||
| Yes | 162 (83.5) | 80 (41.0) | <0.001 † | |
| No | 32 (16.5) | 115 (59.0) | ||
| What are the transmission routes of fasciolosis in humans? | ||||
| Eating improperly washed vegetable | 18 (9.3) | 1 (0.5) | N | |
| Eating raw vegetables | 21 (10.8) | 1 (0.5) | ||
| Drinking impure water | 27 (13.9) | - | ||
| Dirty kitchen utensils | 6 (3.1) | 1 (0.5) | ||
| Using contaminated water to irrigate crops | 1 (0.5) | 1 (0.5) | ||
| Eating raw meat/liver | 71 (36.6) | 71 (36.2) | ||
| I don’t know | 95 (48.9) | 124 (63.6) | ||
| In your opinion can fasciolosis be prevented? | n = 165 | n = 56 | ||
| Yes | 88 (53.3) | 43 (76.8) | <0.001 † | |
| No | 77 (46.7) | 13 (23.2) | ||
| Do you think that human fasciolosis can be treated? | n = 165 | n = 56 | ||
| Yes | 130 (78.8) | 35 (62.5) | <0.001 † | |
| No | 15 (9.1) | 6 (10.7) | ||
| Not known | 20 (12.1) | 15 (26.8) | ||
| What should you do if you are infected with Fasciola? | ||||
| Self-treatment | 1 (0.5) | 1 (0.5) | N | |
| Go traditional healer | 179 (91.8) | - | ||
| Go to health institution | 102 (52.6) | 539 (91.8) | ||
| Others | 15 (7.7) | 45 (7.7) | ||
| Do you eat raw vegetables? | ||||
| Yes | 46 (23.7) | 148 (75.9) | <0.001 † | |
| No | 148 (76.3) | 47 (24.1) | ||
| Kind of vegetable consumed raw | n = 46 | n = 148 | ||
| Tomato | 26 (56.5) | 76 (51.4) | <0.001 † | |
| Lettuce | 16 (34.8) | 71 (47.9) | ||
| Swiss chard | 4 (8.7) | 1 (0.7) | ||
| If you notice in your meal any vegetables watered from surface water? | ||||
| Not eat | 57 (29.4) | 185 (94.9) | N | |
| Eat when well cooked | 136 (70.1) | 4 (2.1) | ||
| Eat when treated carefully | - | 4 (2.1) | ||
| Still eat raw | 1 (0.5) | 2 (1.0) | ||
| How often do you eat raw vegetables? | n = 46 | n = 148 | ||
| Every day | - | 3 (2.0) | N | |
| Once a week | 33 (71.7) | 101 (68.2) | ||
| Once a month | 10 (21.7) | 32 (21.6) | ||
| Once a year | 2 (4.4) | 3 (2.0) | ||
| Never | 1 (2.2) | 9 (6.2) | ||
| Variable | HDSS Site, n (%) | p-Value | ||
|---|---|---|---|---|
| Butajira n = 194 | Gilgel Gibe n = 195 | |||
| Is there a separate shelter for ruminants | ||||
| Yes | 151 (77.8) | 148 (75.9) | 0.65 † | |
| No | 43 (22.2) | 47 (24.1) | ||
| Which form of animal husbandry do you practice? | ||||
| Cut and carry | 91 (46.9) | - | N | |
| Grazing | 103 (53.1) | 195 (100) | ||
| How often do you clean the cattle shelter? | ||||
| Every day | 192 (99.0) | 176 (90.3) | 0.001 † | |
| Every other day | 1 (0.5) | 7 (3.6) | ||
| Weekly | 1 (0.5) | 12 (6.2) | ||
| What kind of water do you use to clean the shelter | ||||
| Treated | 34 (17.5) | - | N | |
| Untreated | 160 (82.5) | 195 (100) | ||
| Have you ever dewormed your cattle? | ||||
| Yes | 187 (96.4) | 160 (82.1) | <0.001 † | |
| No | 7 (3.6) | 35 (17.9) | ||
| How often do you deworm | n = 187 | n = 160 | ||
| Once a year | 40 (21.4) | 31 (19.4) | <0.001 † | |
| Twice a year | 57 (30.5) | 97 (60.6) | ||
| More than twice a year | 90 (48.1) | 32 (20.0) | ||
| Do you use animal dung to fertilizer pastures | ||||
| Yes | 123 (63.4) | 133 (68.2) | 0.32 † | |
| No | 71 (36.6) | 62 (31.8) | ||
| Variable | Frequency | Fasciolosis Status | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | OR (95% CI) | p-Value | AOR (95% CI) | p-Value | |||
| Is there a separate shelter for ruminants | ||||||||
| Yes (reference) | 595 | 161 | 434 | 1 | 1 | |||
| No | 180 | 53 | 127 | 1.1 (0.78–1.62) | 0.5 | 1.0 (0.66–1.45) | 0.9 | |
| Type of feed | ||||||||
| Cut and carry (reference) | 179 | 13 | 166 | 1 | 1 | |||
| Grazing | 596 | 201 | 395 | 6.5 (3.6–11.7) | 0.001 | 7.2 (3.91–13.17) | 0.001 * | |
| How often do you clean the cattle shelter? | ||||||||
| Every day (reference) | 733 | 196 | 537 | 1 | 1 | |||
| Every other day | 16 | 7 | 9 | 2.1 (0.78–5.8) | 0.13 | 1.3 (0.46–3.69) | 0.68 | |
| Weekly | 26 | 11 | 15 | 2.0 (0.90–4.4) | 0.08 | 1.4 (0.60–3.06) | 0.46 | |
| Have you ever dewormed your cattle? | ||||||||
| Yes (reference) | 691 | 180 | 511 | 1 | 1 | |||
| No | 84 | 34 | 50 | 1.9 (1.3–3.1) | 0.006 | 2.2 (1.25–3.78) | 0.001 * | |
| Do you use animal dung to fertilize pastures? | ||||||||
| Yes | 509 | 138 | 371 | 0.9 (0.66–1.29) | 0.6 | 1.7 (1.16–2.56) | 0.007 * | |
| No (reference) | 266 | 76 | 190 | 1 | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abaya, S.W.; Mereta, S.T.; Tulu, F.D.; Mekonnen, Z.; Ayana, M.; Girma, M.; Vineer, H.R.; Mor, S.M.; Caminade, C.; Graham-Brown, J. Prevalence of Human and Animal Fasciolosis in Butajira and Gilgel Gibe Health Demographic Surveillance System Sites in Ethiopia. Trop. Med. Infect. Dis. 2023, 8, 208. https://doi.org/10.3390/tropicalmed8040208
Abaya SW, Mereta ST, Tulu FD, Mekonnen Z, Ayana M, Girma M, Vineer HR, Mor SM, Caminade C, Graham-Brown J. Prevalence of Human and Animal Fasciolosis in Butajira and Gilgel Gibe Health Demographic Surveillance System Sites in Ethiopia. Tropical Medicine and Infectious Disease. 2023; 8(4):208. https://doi.org/10.3390/tropicalmed8040208
Chicago/Turabian StyleAbaya, Samson Wakuma, Seid Tiku Mereta, Fikirte Demissie Tulu, Zeleke Mekonnen, Mio Ayana, Musse Girma, Hannah Rose Vineer, Siobhan M. Mor, Cyril Caminade, and John Graham-Brown. 2023. "Prevalence of Human and Animal Fasciolosis in Butajira and Gilgel Gibe Health Demographic Surveillance System Sites in Ethiopia" Tropical Medicine and Infectious Disease 8, no. 4: 208. https://doi.org/10.3390/tropicalmed8040208
APA StyleAbaya, S. W., Mereta, S. T., Tulu, F. D., Mekonnen, Z., Ayana, M., Girma, M., Vineer, H. R., Mor, S. M., Caminade, C., & Graham-Brown, J. (2023). Prevalence of Human and Animal Fasciolosis in Butajira and Gilgel Gibe Health Demographic Surveillance System Sites in Ethiopia. Tropical Medicine and Infectious Disease, 8(4), 208. https://doi.org/10.3390/tropicalmed8040208






