Spatial Distribution of Off-Host Stages of Tunga penetrans in the Soil within the Home Range of Nine Infected Dogs in An Endemic Tourist Area in Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Area
2.3. Selection of Dogs and Home Range Calculation
2.3.1. Selection of Dogs
2.3.2. Distribution of GPS Collars
2.3.3. Necklace Data Collection
2.3.4. Living Areas
2.3.5. Comparison of Sand Collection Points and Dogs’ Home Range
2.4. Soil Analysis for the Presence of Tunga spp.
2.4.1. Demarcation of Soil Collection Points
2.4.2. Collection of Soil Samples
2.4.3. Investigation of the Presence of Tunga spp. in the Soil Samples
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldmeier, H.; Eisele, M.; Sabóia-moura, R.C.; Heukelbach, J. Severe Tungiasis in Underprivileged Communities: Case Series from Brazil. Emerg. Infect. Dis. 2003, 9, 949–955. [Google Scholar] [CrossRef]
- Pampiglione, S.; Fioravanti, M.L.; Gustinelli, A.; Onore, G.; Mantovani, B.; Luchetti, A.; Trentini, M. Sand flea (Tunga spp.) infections in humans and domestic animals: State of the art. Med. Vet. Entomol. 2009, 23, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Wilcke, T.; Heukelbach, J.; Moura, R.C.S.; Kerr-Pontes, R.L.S.; Feldmeier, H. High prevalence of tungiasis in a poor neighbourhood in Fortaleza, Northeast Brazil. Acta Trop. 2002, 83, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.V.; Dos Santos, F.Z.; Dos Santos, K.C.; De Jesus, A.V.; Guedes, P.E.B.; Da Paixão, S.A.; De Almeida, B.F.; Carlos, R.S.A. Clinical and macroscopic morphological features of canine tungiasis. Parasitol. Res. 2021, 120, 807–818. [Google Scholar] [CrossRef]
- Scofield, A.; Forlano, M.D.; Elisei, C.; Fernandes, K.R.; Massard, C.L. Infestação de Tunga penetrans (Siphonaptera: Tungidae) em cães da comunidade indígena Potiguara no município da Baía da Traição—PB. Rev. Bras. Parasitol. Vet. 2004, 13, 331. [Google Scholar]
- Lima, L.C.F.; Brugnerotto, M.; Galdioli, L.; Ferraz, C.P.; Garcia, R.C.M. Enfrentamento de surto de Tunga penetrans em comunidade rural, Campo Magro, PR. Rev. Clin. Vet. 2020, 145, 44–49. [Google Scholar]
- Rodrigues, D.F.; Daemon, E.; Rodrigues, F.S.F. Caracterização da população de ectoparasitos em cães de núcleos de expansão urbana em Juiz de Fora, Minas Gerais, Brasil. Rev. Bras. Parasitol. Vet. 2008, 17, 185–188. [Google Scholar] [CrossRef]
- Ariza, L.; Seidenschwang, M.; Buckendahl, J.; Gomide, M. Tungíase: Doença negligenciada causando patologia grave em uma favela de Fortaleza, Ceará. Rev. Soc. Bras. Med. Trop. 2007, 40, 63–67. [Google Scholar] [CrossRef]
- Heukelbach, J.; Costa, A.M.L.; Wilcke, T.; Mencke, N.; Feldmeier, H. The animal reservoir of Tunga penetrans in severely affected communities of north-east Brazil. Med. Vet. Entomol. 2004, 18, 329–335. [Google Scholar] [CrossRef]
- Linardi, P.M.; Calheiros, C.M.L.; Campelo-Junior, E.B.; Duarte, E.M.; Heukelbach, J.; Feldmeier, H. Occurrence of the off-host life stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann. Trop. Med. Parasitol. 2010, 104, 337–345. [Google Scholar] [CrossRef]
- Klimpel, S.; Mehlhorn, H.; Heukelbach, J.; Feldmeier, H.; Mencke, N. Field trial of the efficacy of a combination of imidacloprid and permethrin against Tunga penetrans (sand flea, jigger flea) in dogs in Brazil. Parasitol. Res. 2005, 97, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.V.; Heukelbach, J.; Assunção, M.S.; Fernandes, T.M.; Da Rocha, C.M.B.M.; Carlos, R.S.A. Canine tungiasis: High prevalence in a tourist region in Bahia state, Brazil. Prev. Vet. Med. 2017, 139, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.V.; Heukelbach, J.; Assunção, M.S.; Fernandes, T.M.; Da Rocha, C.M.B.M.; Carlos, R.S.A. Seasonal variation and persistence of tungiasis infestation in dogs in an endemic community, Bahia State (Brazil): Longitudinal study. Parasitol. Res. 2019, 118, 1711–1718. [Google Scholar] [CrossRef]
- Carvalho, R.W.; Almeida, A.B.; Barbosa-Silva, S.C.; Amorim, M.; Ribeiro, P.C.; Serra-Freire, N.M. The Patterns of Tungiasis in Araruama Township, State of Rio de Janeiro, Brazil. Mem. Inst. Osvaldo Cruz. 2003, 98, 31–36. [Google Scholar] [CrossRef]
- Ugbomoiko, U.S.; Ofoezie, I.E.; Heukelbach, J. Tungiasis: High prevalence, parasite load, and morbidity in a rural community in Lagos State, Nigeria. Int. J. Dermatol. 2007, 46, 475–481. [Google Scholar] [CrossRef]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Panoroma Ilhéus-Bahia. Available online: https://www.ibge.gov.br/cidades-e-estados/ba/ilheus.html (accessed on 8 November 2021).
- Eisele, M.; Heukelbach, J.; Marck, E.V.; Mehlhorn, H.; Franck, S.; Meckes, O.; Feldmeier, H. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: I. Natural history of tungiasis in man. Parasitol. Res. 2003, 90, 87–99. [Google Scholar] [CrossRef]
- Calabrese, J.M.; Fleming, C.H.; Gurarie, E. Ctmm: An R Package for Analyzing Animal Relocation Data as a Continuous-Time Stochastic Process. Methods Ecol. Evol. 2016, 7, 1124–1132. [Google Scholar] [CrossRef]
- Pfeiffer, D.U. Veterinary Epidemiology—An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2002; Volume 44, pp. 1–62. [Google Scholar]
- Monteiro, S.G. Parasitologia na Medicina Veterinária, 2nd ed.; Roca: São Paulo, Brazil, 2007. [Google Scholar]
- Feldmeier, H.; Heukelbach, J.; Eisele, M.; Sousa, A.Q.; Barbosa, L.M.M.; Carvalho, C.B.M. Bacterial superinfection in human tungiasis. Trop. Med. Int. Health 2002, 7, 559–564. [Google Scholar] [CrossRef]
- Veraldi, S.; Dassoni, F.; Çuca, E.; Nazzaro, G. Two cases of imported tungiasis with severe Staphylococcus aureus superinfection. Acta Derm. Venereol. 2014, 94, 463–464. [Google Scholar] [CrossRef]
- Brooker, S.; Utzinger, J. Integrated disease mapping in a polyparasitic world. Geospat. Health 2007, 1, 141–146. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J. Need for improved methods to collect and present spatial epidemiologic data for vectorborne diseases. Emerg. Infect. Dis. 2007, 13, 1816–1820. [Google Scholar] [CrossRef]
- Brooker, S.; Hotez, P.J.; Bundy, D.A.P. The global atlas of helminth infection: Mapping the way forward in neglected tropical disease control. PLoS Negl. Trop. Dis. 2010, 4, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Elson, L.; Wright, K.; Swift, J.; Feldmeier, H. Control of tungiasis in absence of a roadmap: Grassroots and global approaches. Trop. Med. Infect. Dis. 2017, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, G.; Genovese, G.; Veraldi, S. Clinical and histopathologic study of 39 patients with imported tungiasis. J. Cutan. Pathol. 2019, 46, 251–255. [Google Scholar] [CrossRef]
- Vaira, F.; Nazzaro, G.; Veraldi, S. Tungiasis “The greatest course that has ever afflicted Africa”. JAMA Dermatol. 2014, 150, 708. [Google Scholar] [PubMed]

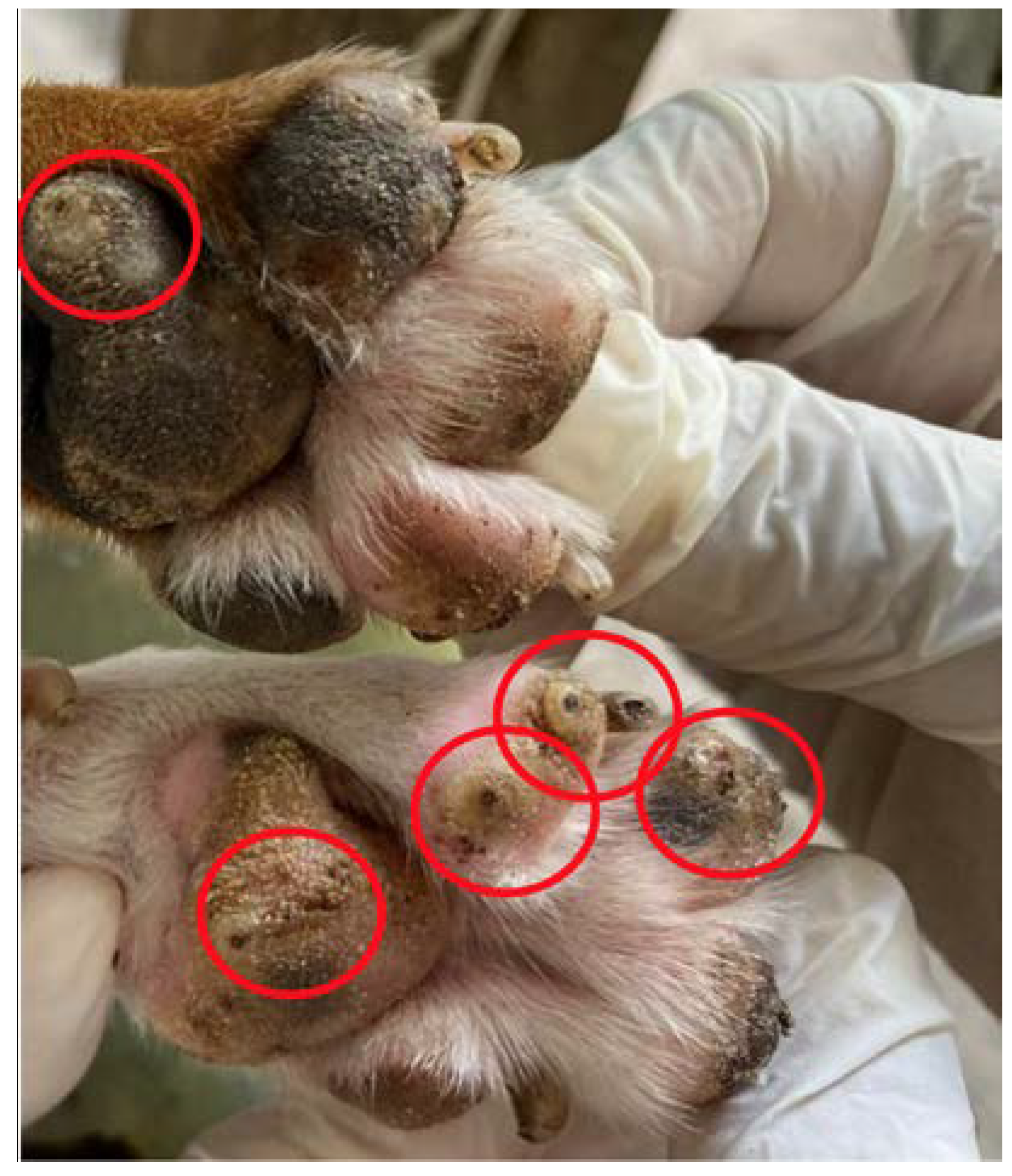

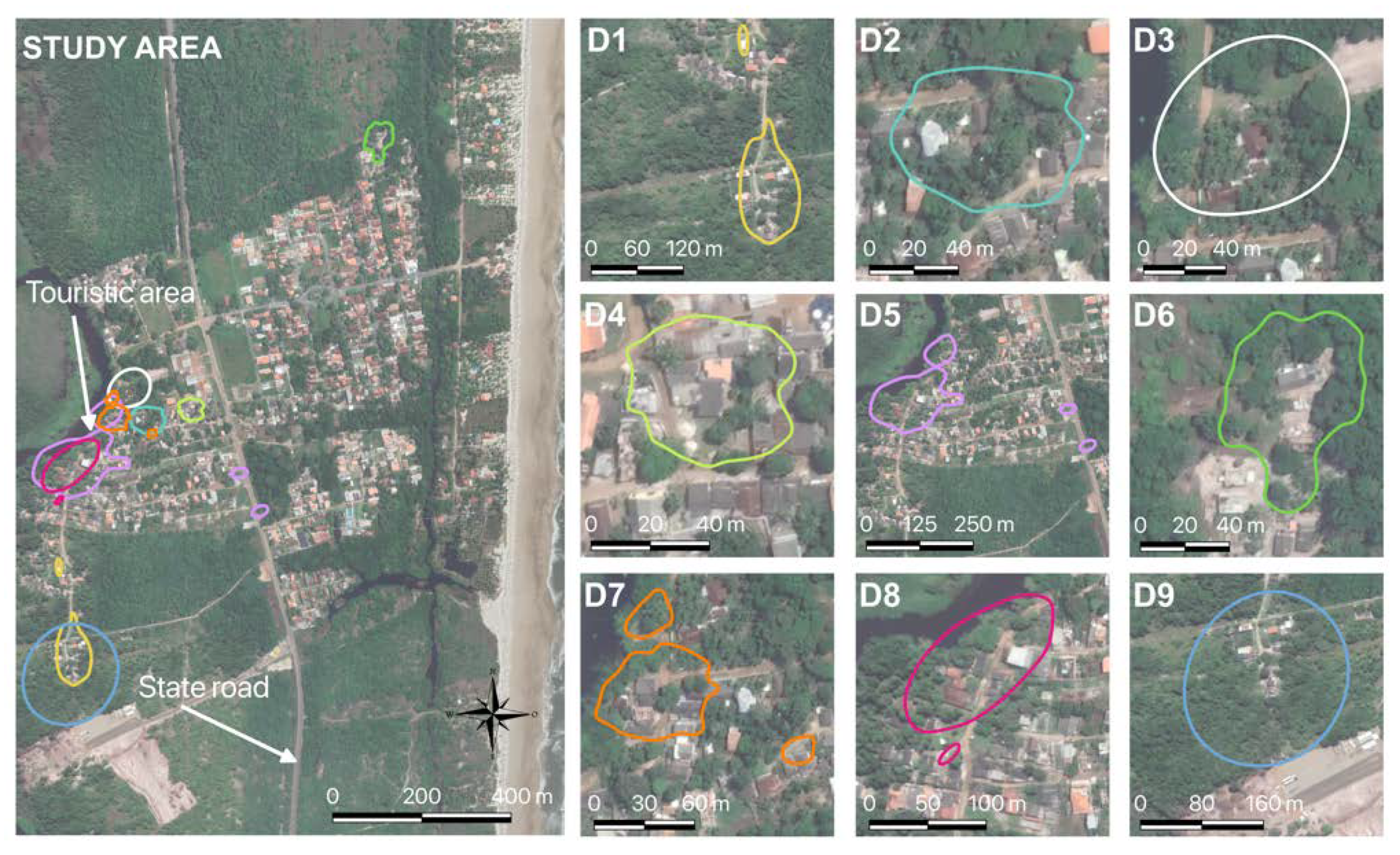
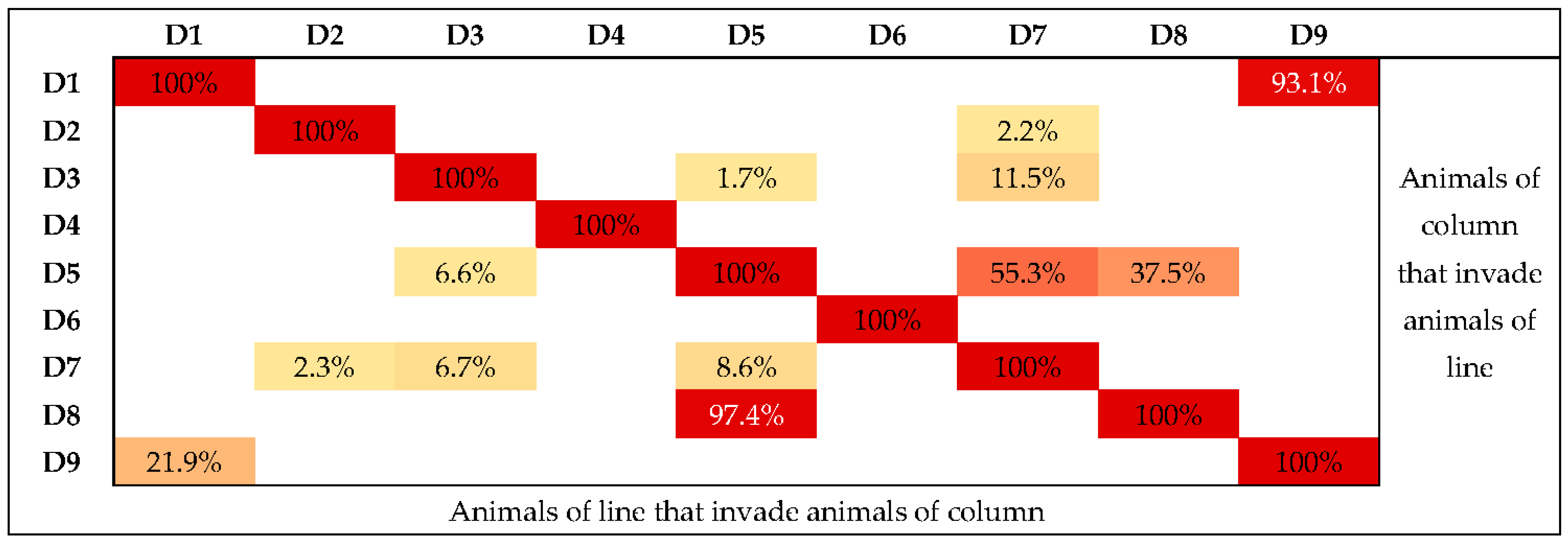
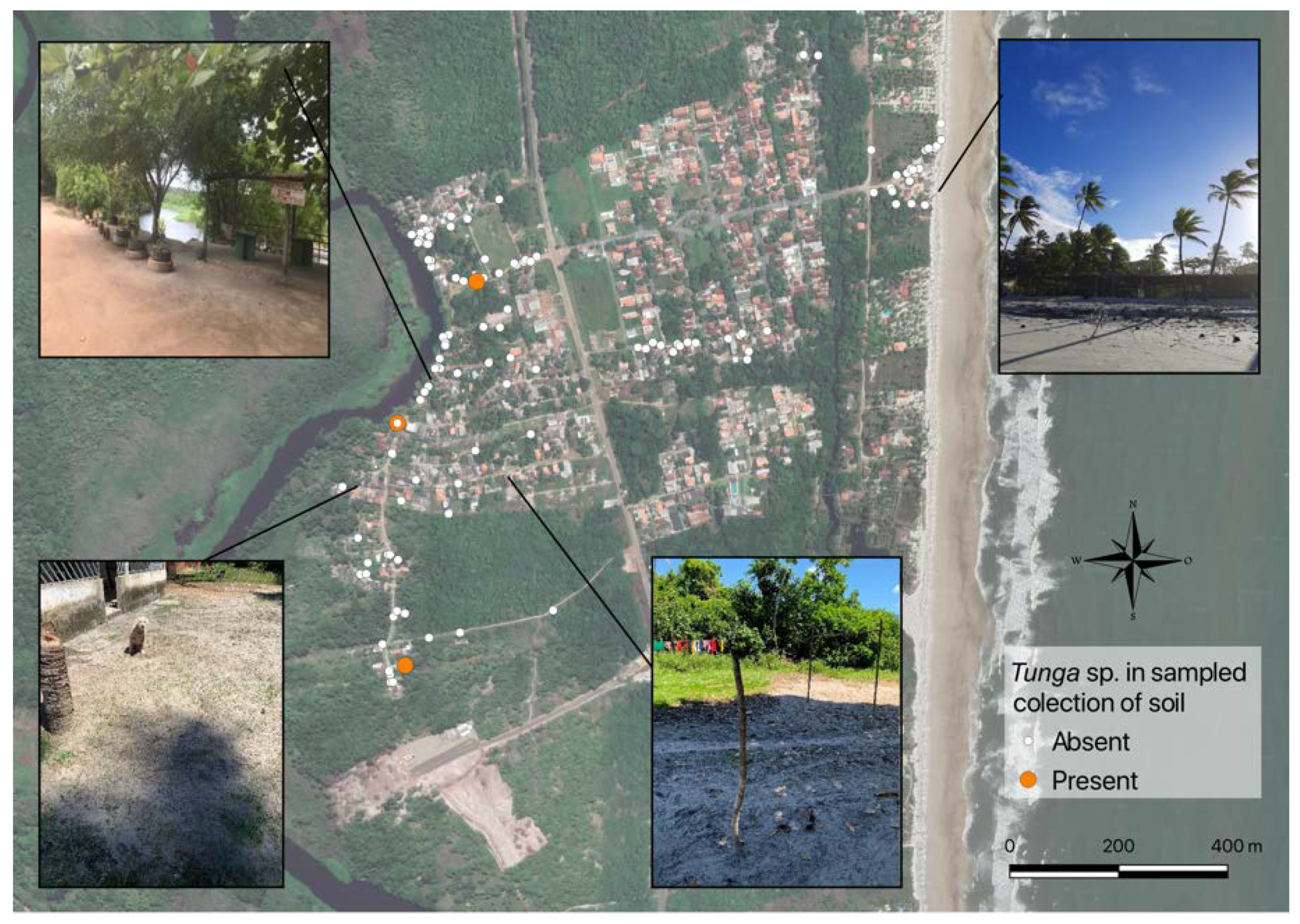

| Animal | I | II | III | IV | V |
|---|---|---|---|---|---|
| D1 | 0 | 1 | 4 | 0 | 0 |
| D2 | 0 | 4 | 5 | 1 | 0 |
| D3 | 0 | 0 | 99 | 10 | 19 |
| D4 | 0 | 4 | 14 | 1 | 3 |
| D5 | 7 | 4 | 22 | 1 | 0 |
| D6 | 0 | 10 | 8 | 4 | 3 |
| D7 | 0 | 2 | 73 | 2 | 10 |
| D8 | 18 | 1 | 179 | 1 | 0 |
| D9 | 0 | 16 | 5 | 2 | 5 |
| Dogs | Area (m2) | CI (95%) |
|---|---|---|
| D1 | 9127.1 | 8214.2–10,075.7 |
| D2 | 3767.3 | 3488.5–4056.5 |
| D3 | 6502.0 | 5958.2–7066.6 |
| D4 | 2310.9 | 2148.3–2479.8 |
| D5 | 24,603.2 | 22,156.7–27,172.6 |
| D6 | 3956.3 | 3637.2–4288.5 |
| D7 | 3817.9 | 3492.8–4155.6 |
| D8 | 9478.9 | 8726.8–10,250.5 |
| D9 | 38,807.7 | 32,574.1–45,553.8 |
| Total mean | 6502.0 | 5958.2–7066.6 |
| Animal | Peridomicile | Free Area | River | Beach | Positives/Total | Prevalence (%) | CI (95%) |
|---|---|---|---|---|---|---|---|
| D1 | 8 | 4 | 0 | 0 | 1/12 | 8.33 | 0–23.97 |
| D2 | 3 | 1 | 0 | 0 | 0/4 | 0 | 0 |
| D3 | 1 | 1 | 3 | 0 | 0/5 | 0 | 0 |
| D4 | 3 | 0 | 0 | 0 | 0/3 | 0 | 0 |
| D5 | 2 | 3 | 10 | 0 | 1/15 | 6.67 | 0–19.29% |
| D6 | 0 | 0 | 0 | 0 | 0/0 | 0 | 0 |
| D7 | 1 | 4 | 4 | 0 | 0/0 | 0 | 0 |
| D8 * | 1 | 0 | 0 | 4 | 1/5 | 20.0 | 0–55.06% |
| D9 * | 0 | 0 | 1 | 0 | ½ | 50.0 | 0–100% |
| Positive | Negative | Fisher Test | |||||
|---|---|---|---|---|---|---|---|
| Variable | Category | N | % | N | % | Total | |
| Organic material | Dry | 3 | 1 | 305 | 99 | 308 | |
| Food | 0 | 0 | 11 | 100 | 11 | nr | |
| Feces | 0 | 0 | 5 | 100 | 5 | ||
| None | 0 | 0 | 4 | 100 | 4 | ||
| Animal | Chicken | 0 | 0 | 57 | 100 | 57 | |
| Dog | 3 | 4.3 | 67 | 95.7 | 70 | ||
| Cat | 0 | 0 | 2 | 100 | 2 | nr | |
| Duck | 0 | 0 | 1 | 100 | 1 | ||
| None | 0 | 0 | 198 | 100 | 198 | ||
| Shadow | Morning | 0 | 0 | 95 | 100 | 95 | |
| Afternoon | 0 | 0 | 2 | 100 | 2 | ||
| Both periods | 3 | 1.6 | 183 | 98.4 | 186 | nr | |
| Absent | 0 | 0 | 45 | 100 | 45 | ||
| Soil type | Sand | 3 | 1 | 299 | 99 | 302 | F = 1 |
| Clay soil | 0 | 0 | 26 | 100 | 26 | p = 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus, A.V.; Sevá, A.d.P.; Guedes, P.E.B.; dos Santos, K.C.; Harvey, T.V.; de Oliveira, G.M.S.; Bitar, T.V.; Ferreira, F.; Albuquerque, G.R.; Carlos, R.S.A. Spatial Distribution of Off-Host Stages of Tunga penetrans in the Soil within the Home Range of Nine Infected Dogs in An Endemic Tourist Area in Brazil. Trop. Med. Infect. Dis. 2023, 8, 98. https://doi.org/10.3390/tropicalmed8020098
de Jesus AV, Sevá AdP, Guedes PEB, dos Santos KC, Harvey TV, de Oliveira GMS, Bitar TV, Ferreira F, Albuquerque GR, Carlos RSA. Spatial Distribution of Off-Host Stages of Tunga penetrans in the Soil within the Home Range of Nine Infected Dogs in An Endemic Tourist Area in Brazil. Tropical Medicine and Infectious Disease. 2023; 8(2):98. https://doi.org/10.3390/tropicalmed8020098
Chicago/Turabian Stylede Jesus, Anderson Vieira, Anaiá da Paixão Sevá, Paula Elisa Brandão Guedes, Katharine Costa dos Santos, Tatiani Vitor Harvey, Gabriela Mota Sena de Oliveira, Thammy Vieira Bitar, Fernando Ferreira, George Rêgo Albuquerque, and Renata Santiago Alberto Carlos. 2023. "Spatial Distribution of Off-Host Stages of Tunga penetrans in the Soil within the Home Range of Nine Infected Dogs in An Endemic Tourist Area in Brazil" Tropical Medicine and Infectious Disease 8, no. 2: 98. https://doi.org/10.3390/tropicalmed8020098
APA Stylede Jesus, A. V., Sevá, A. d. P., Guedes, P. E. B., dos Santos, K. C., Harvey, T. V., de Oliveira, G. M. S., Bitar, T. V., Ferreira, F., Albuquerque, G. R., & Carlos, R. S. A. (2023). Spatial Distribution of Off-Host Stages of Tunga penetrans in the Soil within the Home Range of Nine Infected Dogs in An Endemic Tourist Area in Brazil. Tropical Medicine and Infectious Disease, 8(2), 98. https://doi.org/10.3390/tropicalmed8020098







