Programmatic Implementation of Contact Investigation in Eight African Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approach
2.2. Study Site and Population
2.3. Data Collection
2.3.1. Kick-Off Meeting
2.3.2. Tools Adaptation, National Training
2.3.3. Procedures for CI
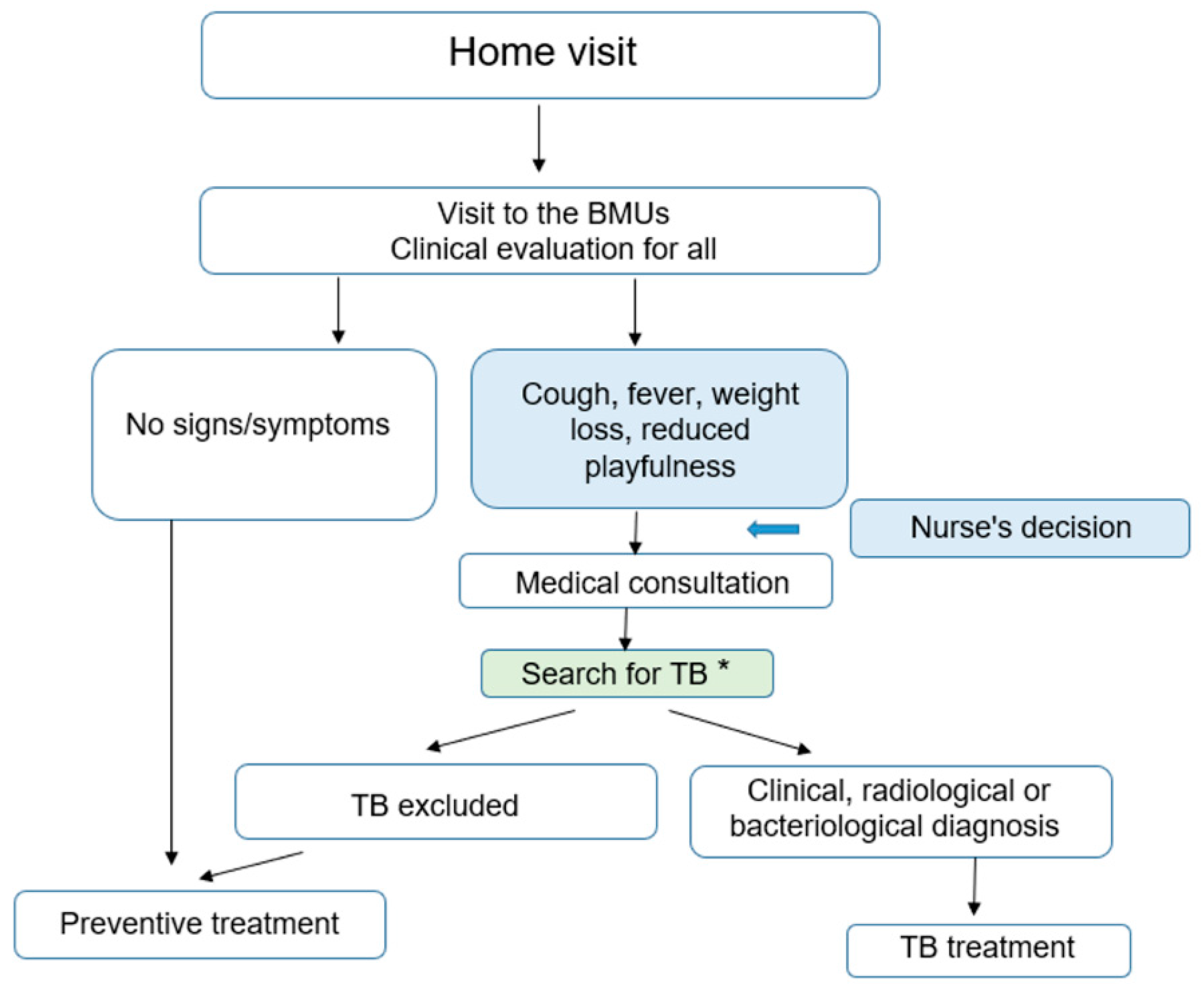
- Children <5 years were clinically re-evaluated and the nurse read the results of the CXR when available. The nurse referred children with any radiographic abnormality or with any signs or symptoms suggestive of TB to a doctor. The latter conducted a full clinical examination, re-interpreted the CXR and requested a collection of sputum specimens for smear microscopy and Xpert MTB/RIF testing. The decision to administer anti-tuberculosis treatment was then taken by the doctor on the basis of this assessment. Children without evidence of active disease after this evaluation were immediately started on TB preventive treatment (see Figure 2).
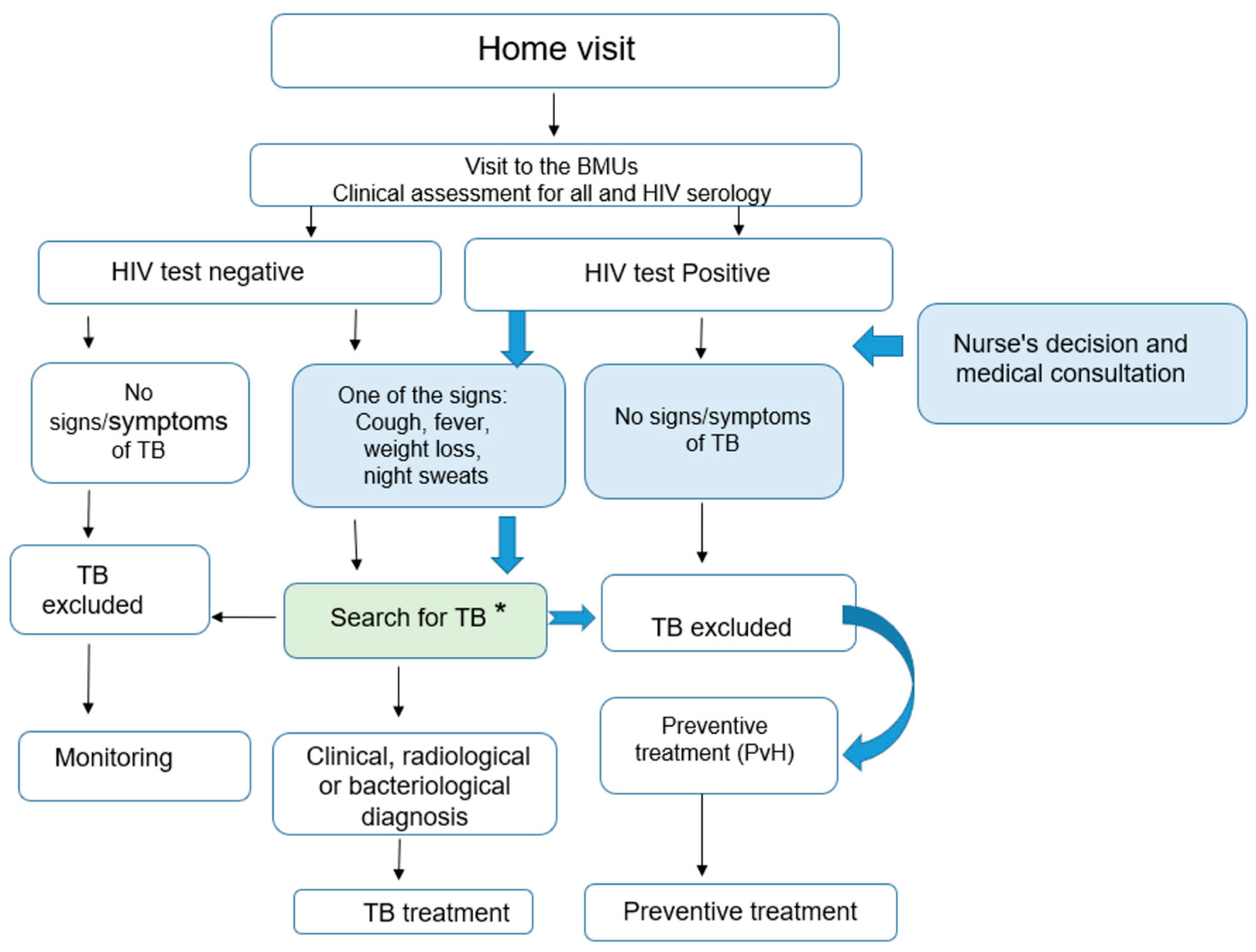
- Adults and adolescents were re-evaluated and the nurse read the results of the CXR. HIV tests were performed (see Figure 3):
- ○
- If the HIV test was negative and there were no signs and symptoms of TB, TB was excluded, and no preventive therapy was provided to these contacts. They were informed about the symptoms and signs of TB and requested to come back to the BMU if any of these symptoms and signs occurred. For the other adults and adolescents, if there were any signs or symptoms suggestive of TB with any radiographic abnormality, the contacts were referred to a doctor who conducted a complete clinical examination, re-interpreted the CXR and requested sputum specimen collection for smear microscopy and Xpert MTB/RIF testing. The decision to administer anti-tuberculosis treatment was then taken by the doctor on the basis of this assessment.
- ○
- In contrast, if the HIV Test was positive and there were no signs and symptoms suggestive of TB and radiography was normal, TB was excluded and the PLHIV contact received TB preventive treatment. If there were any signs or symptoms suggestive of TB with any radiographic abnormality the contacts were referred to a doctor who conducted a full clinical examination, re-interpreted the CXR and requested a collection of sputum specimens for smear microscopy and Xpert MTB/RIF testing. The decision to administer anti-tuberculosis treatment was then taken by the doctor on the basis of this assessment.
2.3.4. Tuberculosis Treatment and Preventive Treatment
2.4. Data Analysis
2.5. Ethics
3. Results
3.1. Proportion of Home Visits Performed among Index Cases
3.2. Proportion of Contacts Living at Home and on Preventive Treatment or TB Treatment
3.2.1. Children under 5 Years
3.2.2. PLHIV
3.3. Ratio of the Number of Contacts Living at Home to the Number of Index Cases
3.4. Scale Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Fox, G.J.; Barry, S.E.; Britton, W.J.; Marks, G.B. Contact investigation for tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2013, 41, 140–156. [Google Scholar] [CrossRef]
- Morrison, J.; Pai, M.; Hopewell, P.C. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Infect. Dis. 2008, 8, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Velleca, M.; Malekinejad, M.; Miller, C.; Abascal Miguel, L.; Reeves, H.; Hopewell, P.; Fair, E. The yield of tuberculosis contact investigation in low-and middle-income settings: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Velen, K.; Shingde, R.V.; Ho, J.; Fox, G.J. The effectiveness of contact investigation among contacts of tuberculosis patients: A systematic review and meta-analysis. Eur. Respir. J. 2021, 58, 2100266. [Google Scholar] [CrossRef] [PubMed]
- Schwoebel, V.; Koura, K.G.; Adjobimey, M.; Gnanou, S.; Wandji, A.G.; Gody, J.C.; Delacourt, C.; Detjen, A.; Graham, S.M.; Masserey, E.; et al. Tuberculosis contact investigation and short-course preventive therapy among young children in Africa. Int. J. Tuberc. Lung. Dis. 2020, 24, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.B.; Wynn-Williams, N. Infectivity of pulmonary tuberculosis in relation to sputum status. Am. Rev. Tuberc. 1954, 69, 724–732. [Google Scholar] [PubMed]
- Daley, C.L.; Small, P.M.; Schecter, G.F.; Schoolnik, G.K.; McAdam, R.A.; Jacobs, W.R., Jr.; Hopewell, P.C. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: An analysis using restriction-fragment—Length polymorphisms. N. Engl. J. Med. 1992, 326, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Selwyn, P.A.; Hartel, D.; Lewis, V.A.; Schoenbaum, E.E.; Vermund, S.H.; Klein, R.S.; Walker, A.T.; Friedland, G.H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 1989, 320, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Guwatudde, D.; Nakakeeto, M.; Jones-Lopez, E.; Maganda, A.; Chiunda, A.; Mugerwa, R.; Ellner, J.; Bukenya, G.; Whalen, C.C. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am. J. Epidemiol. 2003, 158, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Ayaz, A.; Murtaza, A.; Ahmad, S.; Hasan, R.; Pfau, R. Childhood tuberculosis in household contacts of newly diagnosed TB patients. PLoS ONE 2012, 7, e40880. [Google Scholar] [CrossRef] [PubMed]
- Egere, U.; Togun, T.; Sillah, A.; Mendy, F.; Otu, J.; Hoelscher, M.; Heinrich, N.; Hill, P.; Kampmann, B. Identifying children with tuberculosis among household contacts in The Gambia. Int. J. Tuberc. Lung Dis. 2017, 21, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.; Wejse, C.; Oliveira, I.; Andersen, A.; Vieira, F.; Carlos, L.; Vieira, C.; Aaby, P.; Gustafson, P. Adherence to isoniazid preventive therapy in children exposed to tuberculosis: A prospective study from Guinea-Bissau. Int. J. Tuberc. Lung Dis. 2011, 15, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, D.; Zalwango, S.; Okware, B.; Nsereko, M.; Kisingo, H.; Malone, L.; Lancioni, C.; Okwera, A.; Joloba, M.; Mayanja-Kizza, H. Contact investigation for active tuberculosis among child contacts in Uganda. Clin. Infect. Dis. 2013, 57, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Hesseling, A.; Beyers, N.; Enarson, D.; Rusen, I.; Lombard, C.; Van Wyk, S. Routine programmatic delivery of isoniazid preventive therapy to children in Cape Town, South Africa. Public Health Action 2013, 3, 199–203. [Google Scholar] [CrossRef]
- Iwelunmor, J.; Newsome, V.; Airhihenbuwa, C.O. Framing the impact of culture on health: A systematic review of the PEN-3 cultural model and its application in public health research and interventions. Ethn. Health 2014, 19, 20–46. [Google Scholar] [CrossRef] [PubMed]
| Country | N Index Cases | N Index Cases Visited at Home Visit | Ratio |
|---|---|---|---|
| Benin | 813 | 583 | 72% |
| Burkina Faso | 1525 | 1261 | 83% |
| Cameroon | 2114 | 660 | 31% |
| Guinea | 4613 | 1351 | 29% |
| Niger | 2382 | 1673 | 70% |
| Central African Republic | 5086 | 1145 | 23% |
| Senegal | 4100 | 1231 | 30% |
| Togo | 1312 | 1145 | 87% |
| Total | 21,945 | 9049 | 41% |
| Country | <5 Years Living at Home | <5 Years on TPT | <5 Years on TB Treatment | Proportion of children <5 Years on TPT or TB Treatment |
|---|---|---|---|---|
| Benin | 564 | 560 | 4 | 100% |
| Burkina Faso | 1713 | 1143 | 13 | 67% |
| Cameroon | 956 | 936 | 20 | 100% |
| Guinea | 3884 | 3677 | 207 | 100% |
| Niger | 1885 | 1542 | 92 | 87% |
| CAR 1 | 3043 | 3022 | 21 | 100% |
| Senegal | 1709 | 1571 | 17 | 93% |
| Togo | 536 | 532 | 4 | 100% |
| Total | 14,290 | 12983 | 378 | 93% |
| Country | PLHIV Living at Home | PLHIV on TPT | PLHIV on TB Treatment | Proportion of PLHIV on TPT or TB Treatment |
|---|---|---|---|---|
| Benin | 20 | 13 | 6 | 95% |
| Burkina Faso | 22 | 13 | 9 | 100% |
| Cameroon | 70 | 64 | 6 | 100% |
| Guinea | 6 | 3 | 0 | 50% |
| Niger | 4 | 4 | 0 | 100% |
| CAR 1 | 104 | 99 | 5 | 100% |
| Senegal | 11 | 10 | 1 | 100% |
| Togo | 50 | 46 | 2 | 96% |
| Total | 287 | 252 | 29 | 98% |
| Country | N Index Cases | <5 Years Living at Home | Ratio |
|---|---|---|---|
| Benin | 813 | 564 | 0.69 |
| Burkina Faso | 1525 | 1713 | 1.12 |
| Cameroon | 2114 | 956 | 0.45 |
| Guinea | 4613 | 3884 | 0.84 |
| Niger | 2382 | 1885 | 0.79 |
| CAR 1 | 5086 | 3043 | 0.60 |
| Senegal | 4100 | 1709 | 0.42 |
| Togo | 1313 | 533 | 0.41 |
| Total | 21,946 | 14,287 | 0.65 |
| Country | N Index Cases | PLVIH Living at Home | Ratio |
|---|---|---|---|
| Benin | 813 | 20 | 0.025 |
| Burkina Faso | 1525 | 22 | 0.014 |
| Cameroon | 2114 | 70 | 0.033 |
| Guinea | 4613 | 6 | 0.001 |
| Niger | 2382 | 4 | 0.002 |
| CAR 1 | 5086 | 104 | 0.020 |
| Senegal | 4100 | 11 | 0.003 |
| Togo | 1313 | 52 | 0.040 |
| Total | 21,946 | 289 | 0.013 |
| Country | Current Situation |
|---|---|
| Benin | Scale-up is done under GF funding for children under 5 years only. |
| Burkina Faso | National scale-up ongoing under the Global Fund funding. The GF covers 10/13 regions in 2022 and 13/13 regions in 2023. The Union provided funds for the multiplication of the tools. |
| Cameroon | Progressive national scale-up under Global Fund funding (NFM3). In 2022, 6 regions out of 10 are already covered. The four other regions will be covered in 2023. |
| Guinea | Partial scale-up under the Global Fund grant |
| Niger | Partial scale-up started in 2022 (training of stakeholders and conducting investigations around the cases on the new Global fund grant 2022–2024 and COVID-19 RM contingency plan). |
| Togo | Partial scale-up through the Union’s funds |
| CAR 1 | Scale-up has not yet started |
| Senegal | Scale-up planned under the Global Fund grant but has not yet started |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koura, K.G.; Mbitikon, O.B.; Fiogbé, A.A.; Ouédraogo, A.R.; Kuate Kuate, A.; Magassouba, A.S.; Soumana, A.; Hermana, G.; Gning, B.; Dogo, M.F.; et al. Programmatic Implementation of Contact Investigation in Eight African Countries. Trop. Med. Infect. Dis. 2023, 8, 29. https://doi.org/10.3390/tropicalmed8010029
Koura KG, Mbitikon OB, Fiogbé AA, Ouédraogo AR, Kuate Kuate A, Magassouba AS, Soumana A, Hermana G, Gning B, Dogo MF, et al. Programmatic Implementation of Contact Investigation in Eight African Countries. Tropical Medicine and Infectious Disease. 2023; 8(1):29. https://doi.org/10.3390/tropicalmed8010029
Chicago/Turabian StyleKoura, Kobto G., Olivia B. Mbitikon, Attannon A. Fiogbé, Abdoul R. Ouédraogo, Albert Kuate Kuate, Aboubacar S. Magassouba, Alphazazi Soumana, Georges Hermana, Barnabé Gning, Mohammed F. Dogo, and et al. 2023. "Programmatic Implementation of Contact Investigation in Eight African Countries" Tropical Medicine and Infectious Disease 8, no. 1: 29. https://doi.org/10.3390/tropicalmed8010029
APA StyleKoura, K. G., Mbitikon, O. B., Fiogbé, A. A., Ouédraogo, A. R., Kuate Kuate, A., Magassouba, A. S., Soumana, A., Hermana, G., Gning, B., Dogo, M. F., Andefa, M., & Badoum, G. (2023). Programmatic Implementation of Contact Investigation in Eight African Countries. Tropical Medicine and Infectious Disease, 8(1), 29. https://doi.org/10.3390/tropicalmed8010029







