The Resistance Patterns in E. coli Isolates among Apparently Healthy Adults and Local Drivers of Antimicrobial Resistance: A Mixed-Methods Study in a Suburban Area of Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
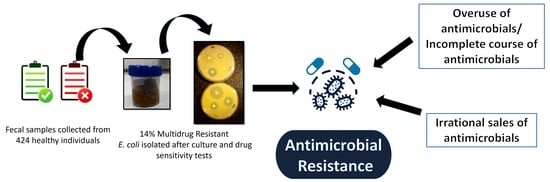
2.3. Study Population and Sampling
2.4. Study Variables, Sources and Data Collection
2.5. Data Analysis
3. Results
3.1. Quantitative Findings
3.2. Qualitative Findings
3.2.1. Issues Related to Community and Individual Behavior
- Lack of awareness on antimicrobials and AMR among community members
Yes, the doctor prescribes antimicrobials. But I think we can discontinue the medicines once the symptoms subside. I don’t feel like continuing the medicine once I start feeling better. P(3)
The main problem is that the patient is not aware that the full dose of the medicine needs to be completed, when symptoms subside after having 2-3 tablets, they throw or stop taking the remaining medicine. They are not aware of the consequences. I feel public awareness is the most important thing needed. (WC_6)
- 2.
- Self-medication practices
What people usually do is, if they have symptoms similar to previously cured illness, they visit the drugstore directly and ask for the same old medicine, which increases the frequency of using the medicine even though it is not required. (FGD2-P13)
We ask if the doctor has written any prescription or have they been taking the antimicrobials regularly. Most of them practice self-medication and some of them are health care workers too. (P001)
- 3.
- Financial constraints
A culture test determines whether the antimicrobial will work for the particular disease or not, but it is very expensive. A general culture will cost you more than 1200 and the report will be ready only after 2-3 days. In such a case, it becomes difficult for an individual to afford the cost for laboratory investigations. (H001)
Some of these antimicrobials are a little expensive, they can’t afford a complete course of medicine, and they can’t take it all at once. One course can cost him up to Rs1500-2,000. He may not have the money to complete this course, so he takes one half course of antimicrobials. (P002)
- 4.
- Overuse of antimicrobials/Incomplete course of antimicrobials
People visit medical/pharmacy even if they have a simple fever and the pharmacist gives antimicrobials to them without proper check-ups and urine/fecal examinations. They are overusing antimicrobials. They think if they visit the hospital instead, it will cost a lot for all examinations. That’s why antimicrobials are being overused. I guess there is no single person in the village who has not taken antimicrobials. (WC_1)
3.2.2. Factors Related to Laws and Regulations
- Lack of guidelines to monitor the selling of antimicrobials
The medicine shops recommend them to take some strong medicine that is why people here use lots of antimicrobials......There is a system prevalent that even for a simple type of disease, people go to medicinal shops in Banepa and then take strong antimicrobials..........there is no monitoring or surveillance because they take medicine from medicine shops without tests and prescriptions, there is such a system in Nepal that is why people openly use antimicrobials. (H002)
- 2.
- Unregistered pharmacies/shops
In terms of resistance, unregistered pharmacies should be taken under control….I have seen grocery stores in Dhulikhel selling paracetamols, syrups and amoxicillin. I was surprised to see that. Such practices should not be allowed at any cost. (H003)
Yes. It is not ‘might be selling’, such unlicensed persons are selling antimicrobials and other drugs. We have found it once or twice. (WC-6)
- 3.
- Irrational sales and use of antimicrobials
If we go to the pharmacy for the common cold, they will recommend a lot of antimicrobials. It is said that patients with cold do not have to take any medicine, as they will recover in seven or ten days without medicine. Nowadays, the pharmacy will directly prescribe you strong medicine like azithromycin. When using such drugs, we should think twice. They lie that we sell drugs on prescription if we go for inspection. This is challenging because the truth is hidden. And another challenge is that consumers these days are very clever because people themselves come to buy Amoxicillin and ranitidine directly without a prescription. Grocery stores also sell antimicrobials. Because of these things we are facing a lot of challenges. (PM002)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- So, A.D.; Ruiz-Esparza, Q.; Gupta, N.; Cars, O. 3Rs for innovating novel antibiotics: Sharing resources, risks, and rewards. BMJ 2012, 344, e1782. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.; Coast, J. The true cost of antimicrobial resistance. BMJ 2013, 346, f1493. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, A.; Memon, B.I.; Mukhopadhyay, A.; Li, J.; Tambyah, P.A. The costs of nosocomial resistant gram negative intensive care unit infections among patients with the systemic inflammatory response syndrome-a propensity matched case control study. Antimicrob. Resist. Infect. Control 2015, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Morales, E.; Cots, F.; Sala, M.; Comas, M.; Belvis, F.; Riu, M.; Salvadó, M.; Grau, S.; Horcajada, J.P.; Montero, M.M. Hospital costs of nosocomial multi-drug resistant Pseudomonas aeruginosa acquisition. BMC Health Serv. Res. 2012, 12, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laxminarayan, R.; Malani, A.; Howard, D.; Smith, D.L. Extending the Cure: Policy Responses to the Growing Threat of Antibiotic Resistance; Taylor & Francis Group: Abingdon, UK, 2010. [Google Scholar] [CrossRef]
- Hu, Y.; Rubin, J.; Mussio, K.; Riley, L.W. Risk factors for faecal carriage of multidrug-resistant Escherichia coli in a college community: A penalised regression model. J. Glob. Antimicrob. Resist. 2021, 26, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Mellata, M. Human and avian extraintestinal pathogenic Escherichia coli: Infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013, 10, 916–932. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.K.; Pinyon, J.L.; Anantham, S.; Hall, R.M. Commensal Escherichia coli of healthy humans: A reservoir for antibiotic-resistance determinants. J. Med. Microbiol. 2010, 59, 1331–1339. [Google Scholar] [CrossRef]
- Nji, E.; Kazibwe, J.; Hambridge, T.; Joko, C.A.; Larbi, A.A.; Damptey, L.A.O.; Nkansa-Gyamfi, N.A.; Stålsby Lundborg, C.; Lien, L.T.Q. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci. Rep. 2021, 11, 3372. [Google Scholar] [CrossRef]
- London, N.; Nijsten, R.; Stobberingh, E. Carriage of antibiotic-resistantEscherichia coli by healthy volunteers during a 15-week period. Infection 1994, 22, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.; Stobberingh, E.; Philips, J.; Houben, A. Antibiotic resistance ofEscherichia coli in fecal samples of healthy people in two different areas in an industrialized country. Infection 1992, 20, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Van de Mortel, H.; Jansen, E.; Dinant, G.; London, N.; Prü, E.P.; Stobberingh, E. The prevalence of antibiotic-resistant faecalEscherichia coli in healthy volunteers in Venezuela. Infection 1998, 26, 292–297. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention Control. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf (accessed on 2 February 2022).
- Kaesbohrer, A.; Schroeter, A.; Tenhagen, B.A.; Alt, K.; Guerra, B.; Appel, B. Emerging antimicrobial resistance in commensal Escherichia coli with public health relevance. Zoonoses Public Health 2012, 59, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Szmolka, A.; Nagy, B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front. Microbiol. 2013, 4, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlet, J. The gut is the epicentre of antibiotic resistance. Antimicrob. Resist. Infect. Control 2012, 1, 39. [Google Scholar] [CrossRef] [Green Version]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef]
- Science Daily. Available online: www.sciencedaily.com/releases/2019/07/190723182245.htm (accessed on 2 February 2022).
- Wasyl, D.; Hoszowski, A.; Szulowski, K.; Zając, M. Antimicrobial resistance in commensal Escherichia coli isolated from animals at slaughter. Front. Microbiol. 2013, 4, 221. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhao, Z.-C.; Wang, M.-H.; Huang, X.-H.; Pan, Y.-H.; Cao, Y.-P. Antimicrobial resistance and integrons of commensal Escherichia coli strains from healthy humans in China. J. Chemother. 2014, 26, 190–192. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Basnyat, B.; Pokharel, P.; Dixit, S.; Giri, S. Antibiotic Use, Its Resistance in Nepal and Recommendations for Action: A Situation Analysis. J. Nepal Health Res. Counc. 2015, 13, 102–111. [Google Scholar] [PubMed]
- Acharya, K.P.; Wilson, R.T. Frontiers. Available online: https://www.frontiersin.org/articles/10.3389/fmed.2019.00105 (accessed on 16 March 2022).
- Malla, S.; Dumre, S.P.; Shakya, G.; Kansakar, P.; Rai, B.; Hossain, A.; Nair, G.B.; Albert, M.J.; Sack, D.; Baker, S. The challenges and successes of implementing a sustainable antimicrobial resistance surveillance programme in Nepal. BMC Public Health 2014, 14, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Public Health Laboratory. Antimicrobial Resistance (Amr) Surveillance Programme; Monthly Bulletin. 2017. Available online: https://www.nphl.gov.np/images/post-pictures/1495344122-amr-bulletin-april.PDF (accessed on 26 May 2022).
- It Is Federal Democratic Republic Nepal, Not Just Nepal, Parliamentary Committee Says. Available online: https://kathmandupost.com/national/2020/11/09/it-is-federal-democratic-republic-nepal-not-just-nepal-parliamentary-committee-says (accessed on 24 May 2022).
- Shrestha, A. Key Highlights from the Census Report 2021; Nepal Economic Forum. Available online: https://nepaleconomicforum.org/key-highlights-from-the-census-report-2021/#:~:text=In%202021%2C%20the%20urban%20population,to%20a%2036.8%25%20in%202011 (accessed on 24 May 2022).
- Thapa, R.; Bam, K.; Tiwari, P.; Sinha, T.K.; Dahal, S. Implementing federalism in the health system of Nepal: Opportunities and challenges. Int. J. Health Policy Manag. 2019, 8, 195. [Google Scholar] [CrossRef]
- Global Antibiotic Resistance Partnership-Nepal Working Group: Nepal PublicHealth Foundation: Nepal. Situation Analysis and Recommendations: Antibiotic Use and Resistance in Nepal; Nepal Public Health Foundation: Kathmandu, Nepal, 2016. [Google Scholar]
- Lwanga, S.K.; Lemeshow, S.; World Health Organization. Sample Size Determination in Health Studies: A Practical Manual; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Antimicrobial Susceptibility Systems; HiMediaLaboratories. Available online: https://www.himedialabs.com/HML/images/literature/pdf/100000027/68.pdf (accessed on 12 June 2022).
- Kabir, S.M.S. Basic Guidelines for Research: An Introductory Approach for All Disciplines, 1st ed.; Book Zone Publication: Chittagong, Bangaladesh, 2016. [Google Scholar]
- Adenipekun, E.O.; Jackson, C.R.; Ramadan, H.; Iwalokun, B.A.; Oyedeji, K.S.; Frye, J.G.; Barrett, J.B.; Hiott, L.M.; Woodley, T.A.; Oluwadun, A. Prevalence and multidrug resistance of Escherichia coli from community-acquired infections in Lagos, Nigeria. J. Infect. Dev. Ctries. 2016, 10, 920–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations, New York. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld/publication (accessed on 12 June 2022).
- Mapanguy, C.C.M.; Adedoja, A.; Kecka, L.G.V.; Vouvoungui, J.C.; Nguimbi, E.; Velavan, T.P.; Ntoumi, F. High prevalence of antibiotic-resistant Escherichia coli in Congolese students. Int. J. Infect. Dis. 2021, 103, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Amin, M.B.; Roy, S.; Asaduzzaman, M.; Islam, M.; Navab-Daneshmand, T.; Mattioli, M.C.; Kile, M.L.; Levy, K.; Julian, T.R. Fecal colonization with multidrug-resistant E. coli among healthy infants in rural Bangladesh. Front. Microbiol. 2019, 10, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, I.-F.; Lee, W.-Y.; Wang, J.-L.; Hung, C.-H.; Hu, H.-H.; Hung, W.-Y.; Hung, Y.-J.; Chen, W.-C.; Shen, Y.-T.; Cheng, M.-F. Fecal carriage of multidrug-resistant Escherichia coli by community children in southern Taiwan. BMC Gastroenterol. 2018, 18, 86. [Google Scholar] [CrossRef]
- Benz, I.; Schmidt, M.A. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 1989, 57, 1506–1511. [Google Scholar] [CrossRef] [Green Version]
- Tawfick, M.M.; Elshamy, A.A.; Mohamed, K.T.; El Menofy, N.G. Gut Commensal Escherichia coli, a High-Risk Reservoir of Transferable Plasmid-Mediated Antimicrobial Resistance Traits. Infect. Drug Resist. 2022, 15, 1077. [Google Scholar] [CrossRef]
- McInnes, R.S.; McCallum, G.E.; Lamberte, L.E.; van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef]
- Shakya, P.; Barrett, P.; Diwan, V.; Marothi, Y.; Shah, H.; Chhari, N.; Tamhankar, A.J.; Pathak, A.; Lundborg, C.S. Antibiotic resistance among Escherichia coli isolates from stool samples of children aged 3 to 14 years from Ujjain, India. BMC Infect. Dis. 2013, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.R.; Lindahl, L.F.; Diwan, V.; Marrone, G.; Lundborg, C.S. High levels of drug resistance in commensal E. coli in a cohort of children from rural central India. Sci. Rep. 2019, 9, 6682. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Chaudhury, R.R. Antibiotic resistance in India: Drivers and opportunities for action. PLoS Med. 2016, 13, e1001974. Available online: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001974 (accessed on 12 June 2022). [CrossRef] [PubMed] [Green Version]
- World Health Organization. Community-Based Surveillance of Antimicrobial Use and Resistance in Resource-Constrained Settings: Report on Five Pilot Projects; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Park, S.H. Third-generation cephalosporin resistance in gram-negative bacteria in the community: A growing public health concern. Korean J. Intern. Med. 2014, 29, 27. [Google Scholar] [CrossRef]
- Wang, J.-T.; Chang, S.-C.; Chang, F.-Y.; Fung, C.-P.; Chuang, Y.-C.; Chen, Y.-S.; Shiau, Y.-R.; Tan, M.-C.; Wang, H.-Y.; Lai, J.-F. Antimicrobial non-susceptibility of Escherichia coli from outpatients and patients visiting emergency rooms in Taiwan. PLoS ONE 2015, 10, e0144103. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.-L.; Baldwin Toye, S.K.; Zvonar, R. Risk factors for and outcomes of bacteremia caused by extended-spectrum ß-Lactamase–producing Escherichia coli and Klebsiella species at a Canadian tertiary care hospital. Can. J. Hosp. Pharm. 2015, 68, 136. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Han, S.W.; Kim, K.W.; Song, D.Y.; Kwon, K.T. Third-generation cephalosporin resistance of community-onset Escherichia coli and Klebsiella pneumoniae bacteremia in a secondary hospital. Korean J. Intern. Med. 2014, 29, 49. [Google Scholar] [CrossRef]
- Jeanvoine, A.; Bouxom, H.; Leroy, J.; Gbaguidi-Haore, H.; Bertrand, X.; Slekovec, C. Resistance to third-generation cephalosporins in Escherichia coli in the French community: The times they are a-changin? Int. J. Antimicrob. Agents 2020, 55, 105909. [Google Scholar] [CrossRef]
- Zhen, X.; Stålsby Lundborg, C.; Sun, X.; Hu, X.; Dong, H. Clinical and economic impact of third-generation cephalosporin-resistant infection or colonization caused by Escherichia coli and Klebsiella pneumoniae: A multicenter study in China. Int. J. Environ. Res. Public Health 2020, 17, 9285. [Google Scholar] [CrossRef]
- Nys, S.; Okeke, I.N.; Kariuki, S.; Dinant, G.; Driessen, C.; Stobberingh, E. Antibiotic resistance of faecal Escherichia coli from healthy volunteers from eight developing countries. J. Antimicrob. Chemother. 2004, 54, 952–955. [Google Scholar] [CrossRef]
- Mkuhlu, N.A.; Chuks, I.B.; Chikwelu, O.L. Characterization and Antibiotic Susceptibility Profiles of Pathogenic Isolated from Diarrhea Samples within the Buffalo City Metropolitan Municipality, Eastern Cape, South Africa. Open Microbiol. J. 2020, 321–330. [Google Scholar] [CrossRef]
- Chaves, B.J.; Tadi, P. Gentamicin; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Parajuli, N.P.; Acharya, S.P.; Mishra, S.K.; Parajuli, K.; Rijal, B.P.; Pokhrel, B.M. High burden of antimicrobial resistance among gram negative bacteria causing healthcare associated infections in a critical care unit of Nepal. Antimicrob. Resist. Infect. Control 2017, 6, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Das, S.; Singh, S.; Gajamer, V.R.; Pradhan, N.; Lepcha, Y.D.; Tiwari, H.K. Prevalence of antibiotic resistance in commensal Escherichia coli among the children in rural hill communities of Northeast India. PLoS ONE 2018, 13, e0199179. [Google Scholar] [CrossRef] [PubMed]
- Shankar, R.P.; Partha, P.; Shenoy, N.K.; Easow, J.M.; Brahmadathan, K.N. Prescribing patterns of antibiotics and sensitivity patterns of common microorganisms in the Internal Medicine ward of a teaching hospital in Western Nepal: A prospective study. Ann. Clin. Microbiol. Antimicrob. 2003, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Cho, Y.-H.; Shim, B.S.; Lee, S.D. Risk factors for antimicrobial resistance among the Escherichia coli strains isolated from Korean patients with acute uncomplicated cystitis: A prospective and nationwide study. J. Korean Med. Sci. 2010, 25, 1205–1209. [Google Scholar] [CrossRef] [Green Version]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Catena, F.; Coccolini, F.; Craig Hardcastle, T.; Roques, C.; Salameh, P. Drivers of antibiotic resistance transmission in low-and middle-income countries from a “one health” perspective—A review. Antibiotics 2020, 9, 372. [Google Scholar] [CrossRef]
- Eurobarometer, S. Antimicrobial resistance. In Belgium: TNS Opinion & Social; Directorate General for Communication, European Union: Brussels, Belgium, 2010. [Google Scholar]
- Dahal, R.H.; Chaudhary, D.K. Microbial infections and antimicrobial resistance in Nepal: Current trends and recommendations. Open Microbiol. J. 2018, 12, 230. [Google Scholar] [CrossRef] [Green Version]
- Colson, A.R.; Megiddo, I.; Alvarez-Uria, G.; Gandra, S.; Bedford, T.; Morton, A.; Cooke, R.M.; Laxminarayan, R. Quantifying uncertainty about future antimicrobial resistance: Comparing structured expert judgment and statistical forecasting methods. PLoS ONE 2019, 14, e0219190. [Google Scholar] [CrossRef]
- Authority, E.F.S. Report for 2018 on the Results from the Monitoring of Veterinary Medicinal Product Residues and Other Substances in Live Animals and Animal Products; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 2397–8325. [Google Scholar]
- WHO. Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018; p. 9241514884. [Google Scholar]
- Drugs Act, 2035 (1978) Government of Nepal. Available online: www.lawcommission.gov.np/en/documents/2015/08/nepal-health-professional-council-act-2053-1997.pdf. (accessed on 12 June 2022).
- Rijal, K.R.; Banjara, M.R.; Dhungel, B.; Kafle, S.; Gautam, K.; Ghimire, B.; Ghimire, P.; Dhungel, S.; Adhikari, N.; Shrestha, U.T. Use of antimicrobials and antimicrobial resistance in Nepal: A nationwide survey. Sci. Rep. 2021, 11, 11554. [Google Scholar] [CrossRef]
- Mo, Y.; Seah, I.; Lye, P.S.P.; Kee, X.L.J.; Wong, K.Y.M.; Ko, K.K.K.; Ong, R.T.-H.; Tambyah, P.A.; Cook, A.R. Relating knowledge, attitude and practice of antibiotic use to extended-spectrum beta-lactamase-producing Enterobacteriaceae carriage: Results of a cross-sectional community survey. BMJ Open 2019, 9, e023859. [Google Scholar] [CrossRef] [Green Version]
- Acharya, K.P. National Action Plan for Antimicrobial Resistance in Nepal: Possibility of translating idea into reality. Open Microbiol. J. 2020, 14, 38–39. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for South-East Asia. Health Financing Profile 2017: Nepal; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Puvača, N.; de Llanos Frutos, R. Antimicrobial resistance in escherichia coli strains isolated from humans and Pet animals. Antibiotics 2021, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, S.H. The evolving response to antibiotic resistance (1945–2018). Palgrave Commun. 2018, 4, 124. [Google Scholar] [CrossRef]
- Karki, K.; Aryal, K.; Gyawali, P.; Pandit, A.; Thapa, R.; Maskey, J.; Mehta, R.; Poudel, P.; Giri, M.; Acharya, T. Quality of Drugs and Drug Use Patterns at Different Level of Health Care Settings in Nepal; Nepal Health Research Council: Kathmandu, Nepal, 2017. [Google Scholar]
- Acharya, J.; Zolfo, M.; Enbiale, W.; Kyaw, K.W.Y.; Bhattachan, M.; Rijal, N.; Shrestha, A.; Shrestha, B.; Madhup, S.K.; Raghubanshi, B.R. Quality assessment of an antimicrobial resistance surveillance system in a province of Nepal. Trop. Med. Infect. Dis. 2021, 6, 60. [Google Scholar] [CrossRef]


| Characteristics | Total | E. coli | Unadjusted | p Value | |||
|---|---|---|---|---|---|---|---|
| N | (%) ^ | n | (%) $ | PR | (95% CI) | ||
| Total | 424 | (100) | 364 | (85.9) | |||
| Age (in years) | |||||||
| 18–29 | 56 | (13.2) | 48 | (85.7) | 1.0 | (0.9–1.2) | 0.611 |
| 30–44 | 151 | (35.6) | 131 | (86.8) | 1.1 | (0.9–1.2) | 0.400 |
| 45–59 | 131 | (30.9) | 114 | (87.0) | 1.1 | (0.9–1.2) | 0.380 |
| ≥60 | 86 | (20.3) | 71 | (82.6) | 1 | ||
| Gender | |||||||
| Male | 133 | (31.4) | 112 | (84.2) | 1 | ||
| Female | 291 | (68.6) | 252 | (86.6) | 1.0 | (0.9–1.1) | 0.526 |
| Marital status | |||||||
| Never married | 19 | (4.5) | 14 | (73.7) | 1 | ||
| Currently married/cohabitating | 366 | (86.3) | 317 | (86.6) | 1.2 | (0.9–1.5) | 0.244 |
| separated/divorced/widow | 36 | (8.5) | 30 | (83.3) | 1.1 | (0.8–1.5) | 0.431 |
| Refused to answer | 3 | (0.7) | 3 | (100.0) | 1.4 | (1.0–1.8) | 0.026 |
| Ethnicity/Caste | |||||||
| Brahmin | 175 | (41.3) | 143 | (81.7) | 1 | ||
| Chhetri | 53 | (12.5) | 44 | (83.0) | 1.0 | (0.9–1.2) | 0.825 |
| Janajati | 176 | (41.5) | 159 | (90.3) | 1.1 | (1.0–1.2) | 0.021 |
| Dalit | 20 | (4.7) | 18 | (90.0) | 1.1 | (0.9–1.3) | 0.243 |
| Religion | |||||||
| Hindu | 337 | (79.5) | 283 | (84.0) | 1 | ||
| Buddhist | 73 | (17.2) | 68 | (93.2) | 1.1 | (1.0–1.2) | 0.009 |
| Others # | 14 | (3.3) | 13 | (92.9) | 1.1 | (0.9–1.3) | 0.197 |
| Education status | |||||||
| No formal education | 238 | (56.1) | 205 | (86.1) | 10 | (0.9–1.2) | 0.755 |
| Primary school 1 | 75 | (17.7) | 65 | (86.7) | 1.0 | (0.9–1.2) | 0.724 |
| Secondary school 2 | 58 | (13.7) | 49 | (84.5) | 1 | ||
| Higher secondary and above 3 | 53 | (12.5) | 45 | (85.0) | 1.0 | (0.9–1.2) | 0.951 |
| Occupation status | |||||||
| Agriculture/livestock | 302 | (75.5) | 263 | (87.1) | 1.2 | (0.9–1.7) | 0.141 |
| Business | 45 | (10.6) | 39 | (86.7) | 1.2 | (0.9–1.7) | 0.176 |
| Employed * | 20 | (4.7) | 14 | (70.0) | 1 | ||
| Unemployed | 57 | (13.4) | 48 | (84.2) | 1.2 | (0.9–1.6) | 0.240 |
| Ward | |||||||
| Ward-2 | 309 | (72.9) | 266 | (86.9) | 1.0 | (0.9–1.1) | 0.823 |
| Ward-6 | 115 | (36.6) | 98 | (85.2) | 1 | ||
| Access to improved sanitation | |||||||
| Yes | 422 | (99.5) | 362 | (85.8) | 1 | ||
| No | 2 | (0.5) | 2 | (100.0) | 1.2 | (1.1–1.2) | <0.001 |
| Presence of Livestock close to House | |||||||
| Yes | 329 | (77.6) | 287 | (87.2) | 1.1 | (1.0–1.2) | 0.173 |
| No | 95 | (22.4) | 77 | (81.1) | 1 | ||
| Consume water from open sources | |||||||
| Yes | 3 | (0.7) | 3 | (100.0) | 1.2 | (1.1–1.2) | <0.001 |
| No | 421 | (99.3) | 361 | (85.7) | |||
| Antimicrobial | Sensitive | Indeterminate | Resistant | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Cefotaxime | 294 | (79.9) | 17 | (4.6) | 57 | (15.5) |
| Ciprofloxacin | 277 | (75.3) | 52 | (14.1) | 39 | (10.6) |
| Tetracycline | 289 | (78.5) | 3 | (0.8) | 76 | (20.7) |
| Ampicillin | 116 | (31.5) | 106 | (28.8) | 149 | (40.5) |
| Chloramphenicol | 319 | (86.7) | 36 | (9.8) | 13 | (3.5) |
| Gentamicin | 366 | (99.5) | 0 | (0.0) | 2 | (0.5) |
| Cotrimoxazole | 313 | (85.1) | 1 | (0.3) | 54 | (14.7) |
| Characteristics | Total | MDR | Unadjusted | p Value | ||
|---|---|---|---|---|---|---|
| N | (%) $ | PR | (95% CI) | |||
| Total | 364 | 51 | (14.0) | |||
| Age (in years) | ||||||
| 18–29 | 48 | 7 | (14.6) | 1.5 | (0.6–4.0) | 0.435 |
| 30–44 | 131 | 20 | (15.3) | 1.5 | (0.7–3.5) | 0.291 |
| 45–59 | 114 | 17 | (14.9) | 1.5 | (0.7–3.5) | 0.328 |
| ≥60 | 71 | 7 | (9.9) | 1 | ||
| Gender | ||||||
| Male | 112 | 17 | (15.2) | 1.1 | (0.7–1.9) | 0.668 |
| Female | 252 | 34 | (13.5) | 1 | ||
| Marital status | ||||||
| Never married | 14 | 1 | (7.1) | 1 | ||
| Currently married/Cohabitating | 317 | 46 | (14.5) | 2.0 | (0.3–13.7) | 0.467 |
| Separated/divorced/Widow | 30 | 4 | (13.3) | 1.9 | (0.2–15.2) | 0.560 |
| Refused to answer | 3 | 0 | (0.0) | - | ||
| Ethnicity | ||||||
| Brahmin | 143 | 16 | (11.2) | 1 | ||
| Chhetri | 44 | 7 | (15.9) | 1.4 | (0.6–3.2) | 0.402 |
| Janajati | 159 | 24 | (15.1) | 1.4 | (0.7–2.4) | 0.321 |
| Dalit | 18 | 4 | (22.2) | 2.0 | (0.7–5.2) | 0.170 |
| Religion | ||||||
| Hindu | 283 | 42 | (14.8) | 1.3 | (0.6–2.6) | 0.521 |
| Buddhist | 68 | 8 | (11.8) | 1 | ||
| Others # | 13 | 1 | (7.7) | 0.7 | (0.1–5.0) | 0.676 |
| Education status | ||||||
| No formal education | 205 | 29 | (14.1) | 1.4 | (0.6–3.4) | 0.476 |
| Primary school 1 | 65 | 11 | (16.9) | 1.7 | (0.6–4.5) | 0.317 |
| Secondary school 2 | 49 | 5 | (10.2) | 1 | ||
| Higher secondary and above 3 | 45 | 6 | (13.3) | 1.3 | (0.4–4.0) | 0.639 |
| Occupation status | ||||||
| Agriculture/livestock | 263 | 34 | (12.9) | 1 | ||
| Business | 39 | 6 | (15.4) | 1.2 | (0.5–2.7) | 0.670 |
| Employed * | 14 | 3 | (21.4) | 1.7 | (0.6–4.7) | 0.347 |
| Unemployed | 48 | 8 | (16.7) | 1.3 | (0.6–2.6) | 0.481 |
| Ward | ||||||
| Ward-2 | 266 | 33 | (12.4) | 1 | ||
| Ward-6 | 98 | 18 | (18.4) | 1.5 | (0.9–2.5) | 0.144 |
| Access to sanitary latrine | ||||||
| Yes | 362 | 50 | (13.8) | 1 | ||
| No | 2 | 1 | (50.0) | 3.6 | (0.9–14.9) | 0.074 |
| Presence of livestock close to house | ||||||
| Yes | 287 | 36 | (12.5) | 1 | ||
| No | 77 | 15 | (19.5) | 1.6 | (0.9–2.7) | 1.115 |
| Consume water from open sources | ||||||
| Yes | 3 | 1 | (33.3) | 2.4 | (0.5–12.2) | 0.289 |
| No | 361 | 50 | (13.8) | 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, A.; Shrestha, R.; Koju, P.; Tamrakar, S.; Rai, A.; Shrestha, P.; Madhup, S.K.; Katuwal, N.; Shrestha, A.; Shrestha, A.; et al. The Resistance Patterns in E. coli Isolates among Apparently Healthy Adults and Local Drivers of Antimicrobial Resistance: A Mixed-Methods Study in a Suburban Area of Nepal. Trop. Med. Infect. Dis. 2022, 7, 133. https://doi.org/10.3390/tropicalmed7070133
Shrestha A, Shrestha R, Koju P, Tamrakar S, Rai A, Shrestha P, Madhup SK, Katuwal N, Shrestha A, Shrestha A, et al. The Resistance Patterns in E. coli Isolates among Apparently Healthy Adults and Local Drivers of Antimicrobial Resistance: A Mixed-Methods Study in a Suburban Area of Nepal. Tropical Medicine and Infectious Disease. 2022; 7(7):133. https://doi.org/10.3390/tropicalmed7070133
Chicago/Turabian StyleShrestha, Abha, Rajeev Shrestha, Pramesh Koju, Sudichhya Tamrakar, Anisha Rai, Priyanka Shrestha, Surendra Kumar Madhup, Nishan Katuwal, Archana Shrestha, Akina Shrestha, and et al. 2022. "The Resistance Patterns in E. coli Isolates among Apparently Healthy Adults and Local Drivers of Antimicrobial Resistance: A Mixed-Methods Study in a Suburban Area of Nepal" Tropical Medicine and Infectious Disease 7, no. 7: 133. https://doi.org/10.3390/tropicalmed7070133
APA StyleShrestha, A., Shrestha, R., Koju, P., Tamrakar, S., Rai, A., Shrestha, P., Madhup, S. K., Katuwal, N., Shrestha, A., Shrestha, A., Shrestha, S., K.C, S., Kharel, S., Tamang, P., Thekkur, P., & Shakya Shrestha, S. (2022). The Resistance Patterns in E. coli Isolates among Apparently Healthy Adults and Local Drivers of Antimicrobial Resistance: A Mixed-Methods Study in a Suburban Area of Nepal. Tropical Medicine and Infectious Disease, 7(7), 133. https://doi.org/10.3390/tropicalmed7070133