Abstract
Snakebite being a quick progressing serious situation needs immediate and aggressive therapy. Snake venom antiserum is the only approved and effective treatment available, but for selected snake species only. The requirement of trained staff for administration and serum reactions make the therapy complicated. In tropical countries where snakebite incidence is high and healthcare facilities are limited, mortality and morbidities associated with snake envenomation are proportionately high. Traditional compilations of medical practitioners’ personal journals have wealth of plant-based snake venom antidotes. Relatively, very few plants or their extractives have been scientifically investigated for neutralization of snake venom or its components. None of these investigations presents enough evidence to initiate clinical testing of the agents. This review focuses on curating Indian traditional snake envenomation therapies, identifying plants involved and finding relevant evidence across modern literature to neutralize snake venom components. Traditional formulations, their method of preparation and dosing have been discussed along with the investigational approach in modern research and their possible outcomes. A safe and easily administrable small molecule of plant origin that would protect or limit the spread of venom and provide valuable time for the victim to reach the healthcare centre would be a great lifesaver.
1. Introduction
Snakebite envenomation is a fast advancing and serious situation, incomparable to any other acute disorder. The management of venomous snakebite is a complex process, and the victim’s physiological condition worsens quickly over time, demanding an aggressive treatment. Snake venom is a complex protein mixture, and every protein expresses its own biochemical activity. Few of the snake venom proteins are toxins while others are nontoxin proteins and enzymes. There is a great difference between the actions of the venoms of different snake species [1]. Neurotoxins are considered the most potent type of toxins as they quickly develop muscle paralysis and death occurs due to respiratory failure. Cytotoxins on the other hand induce haemorrhage and myonecrosis, causing severe local tissue damage at the bite site leading to permanent loss of organ function [1]. The nontoxin proteins from snake venoms induce various autopharmacological actions and may hamper the blood coagulation cascade. The other enzymes present in the snake venom act by inducing capillary damage leading to local tissue damage (hyaluronidases, phospholipases), various anti- and procoagulant actions (phospholipase A, proteinases) or induction of vasoconstriction and pain [2].
Snake envenomation has been classified as high-priority neglected tropical disease by the World Health Organization (WHO) [3,4]. A global initiative to reduce snakebite mortalities and morbidities to 50% by 2030 has also been launched [3,5]. In tropical countries, healthcare facilities in the rural areas might be scarce, and the victim might take significant time to reach the nearest facility. Reaching the nearest healthcare facility in the shortest possible time is of immense importance to avoid morbidities, permanent loss of function or death [6]. Once at the healthcare facility, the patient is critically observed for the vitals and treated accordingly with the snake venom antiserum (SVA) simply called antivenom. SVA is the only proven and recognized therapy for snake venom envenomation. First-aid measures such as immobilization of the bite site and restrictions on active movements of the victim are very important [7].
Before the discovery of SVA in the late nineteenth century [8] and its commercial availability later, snakebites were treated in various traditional ways that included physical therapies and mostly plant-based therapies. In the earlier days, practitioners of indigenous medicine in India used to maintain journals about their medical experiences. Mostly these journals were maintained secretly and passed to the next generation in the same household. Compilations of these journals were called Aushadhi Baad, meaning the medicinal collection. The practitioners would not disclose a particular therapy or an agent used therein to others. It was believed that certain therapies, if shared (with others), would lose their healing power [9], or the healer would lose their importance. The second reason appears to be more credible and would have resulted in the loss of several effective therapeutics to time.
In traditional Indian medicine (including Ayurveda), as most practised medicinal agents were derived from plant sources, books mentioning the use of plants in treating different ailments are available. These agents are known as Vanaushadhi (herbal medicine) in Marathi. Plant-based therapies provide clues for the discovery of newer molecules to a great extent. The terms Gharguti Aushadhe (home remedies), Gharcha Vaidya (home doctor) and Aajicha Batwa (grandma’s wallet) are popular for collections of therapies that are either first-aid or therapies that can be easily administered by a nonpractitioner.
Compilations of various folk and traditional therapies are available. These compilations include therapies obtained by interviewing tribal people or therapies from available traditional literature [10,11,12,13,14,15,16,17,18,19]. The correct interpretation of available information and further research in the same direction is essential for the discovery of newer therapeutic molecules. A few plant extracts or isolated compounds have been tested systematically for the potential to neutralize snake venom, but there is a long way ahead for these molecules to have clinical utility.
2. Materials and Methods
In the current work, therapies for snake envenomation were gathered from traditional compilations, Vanaushadhi Gunadarsha Vol. 1–7 [20] and Aushadhi Baad Vol. 1–3 [9]. As these books garner therapies from historical times, the language is more traditional than the one used in the present day. These therapies are translated into English, and the plant species used therein are identified using internet search engines. Some vernacular plant names that were more traditional were not identified through internet sources and were identified from another book, Gharguti Aushadhe [21]. This book lists various traditionally used therapeutic plants along with their properties, method of use, dose and, in some cases, binomial names, as well. Validation of the binomial names of the plant species was performed by referring to the International Plant Name Index, available at https://ipni.org (accessed during 1–15 March 2022) and/or Plants of the World Online, available at https://powo.science.kew.org (accessed during 1–15 March 2022). Related species and changes in binomial names of the same species of the plant were identified from this exercise.
Individual plant species were then subjected to a modern literature search for parallel mention of snake venom remedies from other traditional or folk literature. Relevant pharmacological, therapeutic or molecular investigations of the plant species or their isolated chemicals against whole or components of snake venoms were searched and documented simultaneously. For this exercise, major scientific databases including Academia, CAB Abstracts, Chemical Abstracts Service, CiteSeerX, Cochrane Library, Crossref, EMBASE, Europe PMC, Index Copernicus, Indian Citation Index, J-Gate, MEDLINE, PubMed Central, ResearchGate and ScienceDirect were accessed in combination with Google Scholar search engine. This extensive search was carried out using binomial names of the plants coupled with the terms, “snake venom”, “cobra venom”, viper venom”, “snake poison”, “cobra poison”, “snake bite”, “cobra bite”, “krait bite”, “venomous bite” and “poisonous bite”. Wherever one plant was identified by multiple binomial names, all available names were included in the search. Manual screening of the bibliographies of the selected references was also performed to identify source information and other potential studies.
For any reference to qualify for inclusion in the current work, the following criteria were applied.
- The article mentions the use of the whole plant, its part or extracted component as a therapy to treat snake envenomation and has ethnobotanical or folklore evidence.
- The article describes pharmacological research related to the neutralization of snake venom or its component by the specified plant, its part or extracted component.
- Multiple articles that cross-refer the same source of traditional use or pharmacological research without additional or unique particulars were not included.
This work mainly focuses on lesser investigated plants having potential for snake venom neutralization and discusses the rationality of the therapies mentioned. Plant-derived chemicals with potential pharmacological action have not been discussed to a greater extent.
3. The Traditional Therapies for Snake Envenomation
For the treatment of snake envenomation, a total of 51 therapies were identified of which 36 include single plant species, 13 include two to four plant species and 2 are large formulations including eight or more plant species. The therapies were listed, and the plant species were identified for further investigation in modern literature. Unlike modern medicine, therapies found in the traditional literature do not always have the exact dose or composition mentioned. As most of these were private journals held by the medical practitioners, they knew “how to” and “how much to” for these therapies. An attempt has been made to document all possible information regarding the therapies. The formulation technique, dose and direction for therapies along with identified plant species are listed in Table 1 and Table 2.

Table 1.
Snake-bite therapies comprising single plant species.

Table 2.
Snake-bite therapies comprising 2–4 plant species.
3.1. Large Formulations for Snake Envenomation
Few of the traditional Indian formulations comprised multiple ingredients and were sometimes intended to be used for a variety of ailments. It was either the dose of the formulation or its further treatment that decided what it would be used to cure. Similarly, a couple of formulations were noticed that were claimed for treatment of snake envenomation: Nagachya Golya (cobra pills) [9] (p. 171) and Vyadhiharak Vatika (curative pills) [9] (p. 61). Nagachya Golya is a formulation comprising 10 ingredients, of which 9 are plant-based. Formulation: Powdered Indian aconite 10 g, Mount Atlas daisy 10 g, long peppercorns 10 g, black peppercorns 5 g, nutmeg 10 g and mace 5 g should be combined with juice made from musk 5 g, ginger juice 20 g, holy basil leaf juice 30 g and betel leaf juice 10 g. The dough should be made, and small pills with a diameter of about 2–3 mm should be prepared [9] (p. 171). Identified plant species from the formulation are listed in Table 3. The information regarding the dose and administration of these pills was not found. The only nonplant ingredient in this formulation is musk, which is an aromatic substance obtained from the glandular secretions of male musk deer—Moschus cupreus (Moschidae). Musk is very precious, and finding genuine musk is hard in the present day as the musk deer is an endangered [22] animal species.

Table 3.
Plant species used in Nagachya Golya and Vyadhiharak Vatika.
The second formulation, Vyadhiharak Vatika, is a generalized formulation used for various ailments. It is a 26-ingredient formulation including 21 plant species and 4 mineral substances. Formulation: aconite, borax, purified orpiment, long pepper, chebulic myrobalan, Indian gooseberry, Ceylon leadwort, sulphur, purging croton, Mount Atlas daisy, freshwater mangrove, castor, liquorice, babreng, sacred fig, Indian barberry, Indian aconite, henbane, mace, nutmeg, opium poppy, rock salt, pushkarmool, asafoetida and garlic should be soaked and levigated in false daisy juice for three days. Rosary-pea-sized pills should be prepared [9] (p. 61). To cure snakebite, one pill should be levigated with water and instilled into the eyes [9] (p. 61). Identified plant species from the formulation are listed in Table 3. Borax, orpiment, sulphur and rock salt are the mineral ingredients used in this formulation. The quantities of the ingredients to be taken are not mentioned in the literature and hence, as per convention, it should be regarded that all ingredients are taken in equal weight ratio. These formulations were prepared and stored by traditional practitioners for a longer time and used quickly when necessary.
3.2. Therapeutic Anomalies
Surprisingly, some of the mentioned therapies use an emetic and claim that the victim would vomit out snake venom, which is practically unjustifiable [9] (pp. 15, 124, 260), [20] (V. 1, p. 54) (V. 5, p. 357). Only orally ingested poisons can be thrown out via emesis to avoid further entry into the systemic circulation. Snakebite introduces venom directly into tissues and systemic circulation, which never can be thrown out via vomit. Though such therapies can be weeded out, they have been mentioned for documentation. In another atypical therapy, the snakebite victim should be made to sit on a platform made up of cow dung, and a stream of cold water should be poured on their body while administering the medication [20] (V. 3, p. 164). There appears to be no direct link between this physical component and the remaining plant-based therapy. Though these parts of therapies appear unjustified, some scientific research may be conducted before rendering the idea futile.
3.3. Toxicity of Plants
Many plants are known to have compounds with potent pharmacological action or severe toxicity. The dose of the isolated component or plant-based formulation decides toxic or therapeutic action. Many plants covered in Table 1, Table 2 and Table 3 have toxic potential and should be used with caution to achieve the desired effect. Castor—Ricinus communis—has a toxic water-soluble glycoprotein, ricin, that is considered a potential chemical weapon [23]. Ricin is present in the castor beans, and the above-listed therapies mention either the use of castor leaves juice or castor oil. It might be concluded that the toxicity of ricin would not be a problem in these formulations. Latex of apple of Sodom—Calotropis procera—is also known to have toxic proteins. However, the toxicity of these proteins is profound with oral administration [24]. Most methods listed above mention topical use of latex that might not be harmful. Aristolochic acid from Aristolochia sp. is reported as nephrotoxic, mutagenic and carcinogenic. It produces quick-progressing nephritis resulting in renal failure [25]. Cases of bristly luffa—Luffa echinate—toxicity have been reported upon oral consumption; however, after proper treatment, the patient did not have long-term ill effects [26]. Other plants such as purging croton—Croton tiglium—and Colocynth—Citrullus colocynthis—have toxicity reports [27,28]. While researching these plants, their margin of toxicity should be well considered while titrating the dose.
4. Snake Envenomation Prophylaxis
As alluring as it may sound, traditional literature also cites prophylactic remedies for snake envenomation. Being immune to snake envenomation might be fascinating for the snake handlers and farm workers. There are increasing incidences of snakebites among experienced snake handlers [29], and no prophylactic remedy is available yet. Having specific antibodies against snake venom seems the only viable hypothesis today as prophylactic. To achieve prophylaxis for snake envenomation, the agent should be well circulated through the systemic circulation, not be cytotoxic and should quickly inhibit systemic as well as local effects of venomous snakebite. Literature mentions Azadirachta indica A.Juss. (Meliaceae) or neem to be prophylactic against snake envenomation. If neem leaves are chewed daily in the morning, snake venom will not affect in case of a bite [20]. Another reference mentions “brushing the teeth daily with the stick of Azadirachta indica A.Juss. makes the body resistant to snake venom” [30]. However, no clinical or in vivo evidence is available to support the claim.
In Indian mythology, the concept of Vishakanya is noteworthy [31,32]. A Vishakanya is a lady who has venom running in her blood which she could infuse into someone’s body and kill. As the say goes, a small amount of snake venom is put on a stone, and the girl would lick it. The quantity of venom is increased gradually, and she would become a Vishakanya. It is hard to believe that venom would be retained in her body and can be used to affect someone else. However, it seems possible that with the sublingual introduction of sublethal doses of snake venom, antibodies were developed, rendering her immune to any future snake envenomation. It would be fair to think that the Vishakanya might handle snakes fearlessly and make them bite the target person. Modern literature evidence on Vishakanya is not available.
5. Snake Repellents
While searching for the prospective snake venom antidotes, a few remedies to repel snakes were also found. One of those is the entry deterrent described as, “To keep snakes away, a root of Indian fumitory should be kept at home” [9]. Indian fumitory—Fumaria indica Lam. (Papaveraceae)—is used in Ayurvedic medicine to treat ailments such as pain, diarrhoea and fever and has pharmacological evidence [33], but its use as snake repellent has not been mentioned earlier. Similarly, Asafoetida—Ferula assa-foetida L. (Apiaceae)—is also mentioned to repel snakes [34]. Apple of Sodom—Calotropis procera Aiton (Apocynaceae)—is also referred to as snake repellent. It is said that venomous snakes including cobras cannot withstand the smell of Calotropis procera, and thus snake charmers use it for controlling snakes [35]. One more remedy used to shrug away snakes from the hiding says, “Small pieces of the horn of Sambar deer should be burned. The snake (cobra) flees because it can’t stand the smoke” [9]. This is one of the few remedies that use agents of an animal origin. Sambar deer—Rusa unicolor (Cervidae)—is a threatened [36] animal found in the Indian subcontinent. Sambar stags have highly precious horns. Traditionally Sambar horns were used in the treatment of various diseases [37], but now their use has declined to almost nothing.
6. Routes of Administration
Traditionally, the routes of administration of medications were limited, and the injectable routes were not practised then. Oral, topical, nasal, ophthalmic, urethral, vaginal and rectal routes were in routine use in the earlier days [38]. Oral administration and topical application on the affected part were the most common routes used. During a snakebite, the venom is released into the tissues and has direct access to the systemic circulation of the victim. The bite site, bite intensity, amount of venom injected and type of venom decide the further course of spread, morbidity or mortality. A superficial bite in subcutaneous or muscular areas delays the spread of venom to other vital organs through systemic circulation and provides grace time to the victim for treatment. However, an intense bite with close access to a vein quickly deteriorates the situation and starts affecting the victim’s vital organs. In such cases, even short delay would result in death or permanent disabilities [39]. Dry bites have also been observed where venomous snakes might not release venoms during a bite in rare instances. There are no systemic symptoms during the dry bites, but local tissue inflammation is still observed. Management of snakebite is tricky due to all these factors, and accordingly selected agents are used. Various routes of administration practised in the earlier days do not seem practical in such acute situations where the spread of venom is faster and the availability of the drug is slower. Routes of administration described in the enlisted therapies for snake envenomation are discussed below.
6.1. Oral
Like most of the therapies, the oral route is the most common route of administration found in the listed therapies. Out of 51 therapies, 37 involve oral administration of formulations. Most of these formulations are either aqueous or are juices of plant parts. Most of the therapies mention the formulation of paste by trituration or levigation of the plant part. In modern pharmaceutical formulations, a penetration enhancer is added to improve or accelerate the absorption of the main drug. It is expected that the earlier multi-ingredient formulations had ingredients to augment the effect of the main drug and mask the taste or reduce any side effects. As it has been established in modern literature that ingredients such as black pepper, long pepper or ginger are used to improve the bioavailability of the principle content of the formulation [40,41], similar ingredients are used in the traditional formulations, as well. Additionally, some of the additives are proven to have pharmacological effects [42,43,44].
One particular therapy mentions the administration of colocynth root (Citrullus colocynthis) in Paan/Vida. Its most basic preparation is made with betel leaf combined with slacked lime, areca nut (Areca catechu) and Kattha (cold crystalized aqueous extractives of Acacia catechu heartwood). Consumption of Paan increases salivation, and probably some contents are absorbed by the buccal route. The intention behind the administration of colocynth in Paan might be faster absorption into the systemic circulation and maybe taste masking. The simple Paan preparation is mostly enriched with other ingredients such as fennel seeds (Foeniculum vulgare), clove buds (Syzygium aromaticum), cardamom seeds (Elettaria cardamomum), cinnamon (dried inner bark of Cinnamomum verum), tobacco (Nicotiana tabacum), rosary pea leaves (Abrus precatorius), dried coconut (Cocos nucifera kernel), Dhana dal (roasted and split coriander seeds), Gulkand (the sweet preserve of rose petals), menthol and many more. Though consumption of Paan is socially well accepted in India and a few other Asian countries, long-term consumption is associated with an increased risk of oral ailments including cancer [45,46,47,48]. In another therapy, oral administration of colocynth extract/juice is expected to cause emesis and purgative action. Probably to suppress emesis, in earlier therapy, it is directed to be consumed in Paan.
6.2. Topical
Effects on the local tissue near snakebite are manifested immediately after the bite. Local tissue effects are profound in cases of semipoisonous snakebites, as well. To tackle the local effects of snakebite or to neutralize the amount of venom that is yet to enter the systemic circulation, topical application on skin or affected tissue is an important route of administration. It seems to be the second-most practised route among the enlisted therapies. Application of formulation on the skin or bite area is mentioned in 17 therapies. Most of the formulations mentioned are aqueous or make use of juice from fresh plant parts. Commonly, pastes prepared by levigation of fresh or dried plant parts (in water) are used. Occasionally, grated fresh or processed plant parts are also applied directly to the wound. Application of formulations on the skin in a thick or thin layer, rubbing liquid and using a poultice were common practices in earlier days. Thick layers of formulation and poultices were applied on, surrounding or near bite sites. Local absorption of the medication might reduce inflammation, neutralize localized venom or prevent its further spread. These therapies seem rational and are the fastest to reach and neutralize venom before it reaches the systemic circulation causing further complications. A few therapies also involve rubbing the formulations away from the bite site. Quick absorption of the medication from the skin and probably into the systemic circulation might be the goal of such therapies. Another goal might be to protect vital organs in the abdominal region.
In a therapy known from Ceylon (Sri Lanka), the use of leeches is mentioned. These leeches are to be applied around the bite site along with the application of key lime juice [9] (p. 114). Leeches were used in traditional Indian medicine, and in the modern setup, their use is increasing, mainly for poorly healing wounds [49]. Leeches are known to suck blood from the area, and it is rational to think that localized venom would also be sucked out with it. This might prevent further entry of venom into systemic circulation but carries a side effect of excessive bleeding [50]. The type of venom would be of key importance. The use of this therapy in neurotoxic envenomation might still prove beneficial over cytotoxic envenomation where the coagulation cascade is hampered. Leeches also inject anticoagulants locally to facilitate drawing blood. In such cases, excessive bleeding might be fatal. In the older days when chances of death were much higher, such therapy still might have saved a few lives. Case reports of leech therapy in chronic snakebite wounds are available [51], but no direct evidence on the use of leeches to tackle snake envenomation was found. In contrast, some snake venom proteins have direct relevance to components injected by leeches [52]. Thus, in some instances, leech therapy might quickly worsen snakebite wounds locally.
6.3. Ocular
Following the most commonly used oral and topical routes, ocular administration comes in third place, with fewer mentions in various therapies. Instillation of aqueous formulations (drops) is most commonly found in the ocular therapies, where plant parts are levigated and diluted with water to form a suspension or a solution. In fewer therapies administration of Anjana (cream, ointment or paste for instillation in eyes) [38] is recommended. Plant parts are powdered, triturated or levigated with water or juice from the same or other plants to form Anjana.
In the case of snake envenomation, the local effect of instillation in the eyes does not seem very effective as a treatment. The medication should be absorbed into the systemic circulation for therapeutic effect. As the instilled medication travels through the lacrimal duct, into the nasal mucosa and pharynx, it absorbs into the systemic circulation. A small amount of drug also may absorb systemically through the conjunctival and other ocular blood vessels [53]. Some of the ingredients such as Indian soapberry or garlic, mentioned in the eye instillation therapies, might cause severe irritation to the eyes. This might be by design to increase the permeability of blood capillary, thereby facilitating faster absorption. Indian soapberry is mentioned as an antidote for various poisons and venoms, and it causes severe eye irritation. To tackle the irritation to the eyes, it is mentioned that clarified butter should be instilled later. Moreover, since large quantities cannot be administered through this route, the drug has to be highly potent, and the formulation should be concentrated.
6.4. Nasal
Nasal drug delivery for the treatment of snakebite is quite uncommon, and only a couple of therapies mention nasal delivery. Both solids and liquids can be administered through the nasal route. One of the therapies involves the instillation of a diluted aqueous paste into the nose while another mentions insufflation of a finely powdered mixture of ingredients. Insufflation would be possible if the snakebite victim is well awake and can quickly sniff the powder mixture. Passive administration by this route would be difficult. Though systemic drug delivery is possible with the nasal route, the drug has to be potent, as large volumes cannot be administered via this route [54]. As hepatic first-pass metabolism is avoided by nasal drug delivery [55], it seems favourable for some molecules to reach systemic circulation and neutralize venom components.
7. Contemporary Exploration of Plants as Antidotes for Snake Venom
The various snake envenomation treatments listed in Table 1 and Table 2 involve 66 plant species from 39 families. The highest number of species (five each), were from Apocynaceae and Asteraceae; four each from Euphorbiaceae; three each from Cucurbitaceae, Fabaceae, Piperaceae, Solanaceae and Zingiberaceae; two each from Malvaceae, Moraceae, Papaveraceae, Ranunculaceae and Rutaceae; and the rest were one from each family.
While identifying plant species and their binomial names based on the reference from traditional literature, sometimes more than one plant species was identified for one traditional name. A classic example is Rui, which directs to two closely connected species, Calotropis procera and Calotropis gigantea. In such cases, the modern literature search was conducted for both species. In addition, binomial names of some plants are revised occasionally by botanists, and the literature is available for both older and revised names. In such cases, the modern literature search was performed for all available names of the plant. Table 4 exhibits ethnobotanical and pharmacological evidence for each plant species as a therapy for snake envenomation.

Table 4.
Ethnobotanical and pharmacological evidence of plant species used in the treatment of snake envenomation in the modern literature.
8. Plant Parts
Many articles from modern literature give a fair idea about plant parts used as snake venom antidotes. These references are from both traditional mentions and pharmacological research. Different plant parts might hold specific chemicals that bring about desired pharmacological effect making them important for the particular action. Figure 1 depicts the frequency of various plant parts appearing as snake venom antidotes from modern literature. The highest number of mentions were found for extracted or isolated components followed by roots and leaves. Various remedies from the traditional literature mention utility of roots and leaves as useful plant parts. The remedies where no specific plant part was referred are also significant in number. Seeds, fruits and stem bark hold intermediate places in utility, and all other plant parts were sparsely used in the therapies.
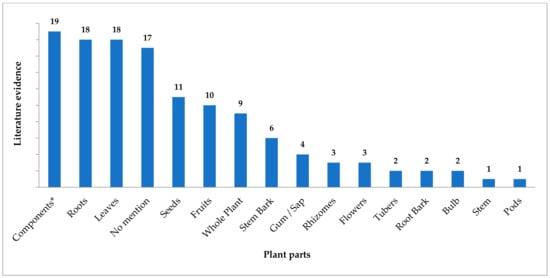
Figure 1.
Plant parts as an antidote for snake envenomation from modern literature. * Extracted components or chemicals.
Various traditional systems of medicine including Ayurveda have recited the importance of plant roots in medicine. Numerous bioactive compounds have been derived from the roots of various plant species [236]. Modified roots act as repositories of the components absorbed from the soil and synthesized within the plant, and many of them have medically important bioactives. Similarly, leaves are centres of biosynthesis of pharmacologically active components and utilize the constituents absorbed from the environment. All other plant parts have bioactives in relatively lower concentrations, and the same has been reflected in the current work.
9. Snake Species with Modern Evidence of Venom Neutralization
Table 4 depicts pharmacological evidence of snake venom neutralization by plants from modern literature. Various researchers have used in vivo, in vitro, and in silico methods or their combination to assess the neutralization of snake venoms by plants. The most references are available for Naja naja venom [64,74,94,96,108,173,203,212,226], followed by Daboia russelii venom [42,44,77,104,106,107,109,110,144,145,156,173]. Both these snake species are perfect representatives of their families Elapidae and Viperidae, respectively, and are the most occurring venomous snake species in the Indian subcontinent. Surprisingly, venoms of other two common snake species, Bungarus caeruleus [96,173] and Echis carinatus [75,78,104,173,226], have been studied less frequently. Only two more snake species from the Indian subcontinent, Naja kaouthia [104,144,167] and Naja naja karachiensis [60,61,62,128], have been found to have evidence for plant-based snake venom neutralization. All other venomous snake species are from tropical African or South American regions. Based on the mechanism of antagonism, the references were grouped into the following three categories.
9.1. Pharmacological Action
Snake venoms, being complex mixtures of toxins, enzymes and proteins, act on various tissues and systems simultaneously. Inhibition of venom lethality is the most important pharmacological outcome of any therapeutic agent for snake envenomation, and the highest number of references (25) was observed for this action. As snake venoms interfere with the coagulation cascade to a large extent, antihaemorrhagic (17) and anticoagulant (9) properties were subsequent. Immunomodulatory and hepatoprotective actions and competitive inhibition were a few more actions mentioned in the reports. Reduction in median lethal dose (LD50) of venom is the most studied method while studying pharmacological action followed by modulation of the coagulation cascade. Figure 2 depicts the number of appearances in references for plants that have pharmacological action against the venoms of respective snake species, and Table 5 enlists snake species wise references (label: Action).
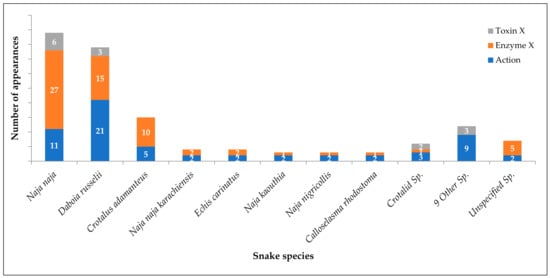
Figure 2.
Modern literature evidence on snake species-specific inhibition of venom and its components.

Table 5.
Snake species-wise literature references indicating pharmacological action, enzyme inhibition and toxin inhibition.
9.2. Enzyme Inhibition
The most important peptides interfering with the normal physiological function of the body are the enzymes present in snake venoms. The impact of plant derivatives or extracts on individual enzymes has been widely studied by researchers while searching for a plant-based snake venom antidote. Prospective studies have indicated positive results of inhibition of various enzymes such as Phospholipase (5) and Phospholipase A2 (17), Fibrinolytic enzymes (9), Protease (7), Superoxide dismutase (5), Lipid peroxidase (5), Acetylcholinesterase (3), 5′-nucleotidase (2), Hyaluronidase (2), L-amino acid oxidase (2) and ATPase (1). Phospholipase A2 is a highly investigated enzyme responsible for an inflammatory response that increases the permeation of other venom components into the systemic circulation. Refer to Figure 2 and Table 5 for snake venom enzyme inhibition by plants (label: Enzyme X).
9.3. Toxin Inhibition
Toxins in snake venom impair critical physiological functions in the victim’s body. Though the majority of references were found on enzyme inhibition, a few references on toxin inhibition were found. Naja naja venom being most investigated, and the highest number of reports was on neutralization of neurotoxins (6). As Daboia russelii was the second-highest investigated species, following neurotoxins, and reports on myotoxins (5), cardiotoxins (2) and cytotoxin (1) were recorded. Refer to Figure 2 and Table 5 for snake venom toxin inhibition by plants (label: Toxin X).
10. Plant Species with Modern Evidence of Snake Venom Neutralization
Of the 66 plant species identified from traditional literature, 17 have modern evidence indicating the neutralization of various snake venom components. Aristolochia indica precedes with most references [17,69,74,86,88,97,98,99,100,101,102,103,104,105,106,107,108,109,110] in both traditional and modern literature. In Marathi, it is known as Saapsund. The first half of the name Saap means snake, and it occurs in many traditional references as a cure for snakebite. It shares the first position with Emblica officinalis [64,118,143,144,145] which is a medicinally important plant in India. Various references demonstrate its effectiveness as an antidote for snake venom. Azadirachta indica [17,64,69,74,151,164,165,166,167,168,169,170,171,172,173] and Rauvolfia serpentina [17,18,69,87,88,89,90,91,92,93,94,95,96] hold second place in pharmacological evidence. Figure 3 depicts the number of appearances in references for each plant species as a snake venom antidote (labels as mentioned in the earlier section).
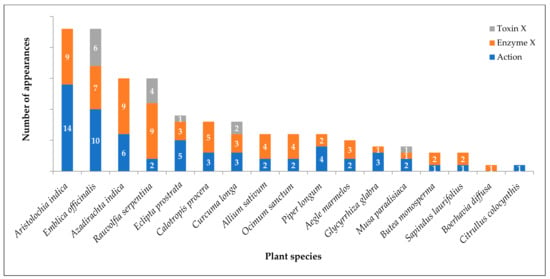
Figure 3.
Modern literature evidence on plant species that inhibit snake venom and its components.
11. Opportunities with a Few Additional Plant Species
Although there is a wealth of literature on plant-based therapies for snake envenomation, some plant species are notably absent. In the modern literature, 18 of the 66 plant species discussed in this work have no evidence as an antidote for snake envenomation. These plants have been investigated for other pharmacological activities but not for snake venom. This opens up research opportunities and indicates that most of these plant species are medicinally important. Table 6 lists these plant species and their families. These plants should be investigated for potential small molecules acting as snake venom antidote.

Table 6.
Plant species with no modern literature evidence as snake envenomation antidote.
12. The Way Forward
All plant-based therapies mentioned in the traditional or modern literature lack viable clinical evidence of being significantly effective against snake envenomation. Chemical components derived from whole plants or their parts have only been tried as a therapy against snake envenomation in either in vivo animal models, in vitro models or a few in silico models. The experimental design of these studies does not mimic the actual post-snakebite situation [237]. Some of the studies mentioned in vitro mixing of plant extracts with snake venom before injection in animals, while other studies mention the administration of test agent immediately after envenomation. For preliminary screening, such an experimental setup might work, but eventually, models mimicking actual clinical situations should be developed. The majority of the agents found effective in the initial studies might be rendered ineffective in the actual situations. Effective experimental models and in-depth studies are needed for an agent to even qualify for a clinical study. Apart from the herbal front, biotechnological approaches are also being explored. Monoclonal antibodies or other proteins are being tested as possible antidotes. An article mentions the use of a ribosomal peptide made up of 11 amino acids that can neutralize rattlesnake venom [238]. However, once again, this warrants premixing of proteins with venom before injecting into the animals.
Many plant species from Table 4 and the entire list in Table 6 have no pharmacological evidence of being useful as snake venom antidotes. Various traditional therapies describe combinations of multiple plant products in which one or more plant derivatives might have a principal active constituent(s) while others might be adjuvants such as bioavailability enhancers. Similar to modern medicine, Ayurveda has mentioned the concept of Yogavahi in its traditional literature, which is a bioavailability enhancement. Glycyrhhiza glabra L., Piper longum L. and Zingiber officinale Rosc. have been used classically as bioavailability enhancers [239]. Some plants might also be useful in reducing toxicity or other side effects imparted by the principal constituent plant. A detailed study based on plant constituents and their known roles from modern literature would help classify the plants and would create a platform for further experimental investigations.
In the rural areas of tropical countries, access to sophisticated medical treatment is scanty, or medical help is so far, that a snakebite victim succumbs on the way to medical facilities. Administration of snake venom antiserum is another challenge, and the dose titration is usually based on the symptoms presented by the victim [237] and tolerance to the SVA during therapy. Immediate hypersensitivity reactions associated with equine serum proteins make therapy more complicated [240]. World Health Organization (WHO) has published clear guidelines for standardization and refining of snake venom antisera [241], which have reduced the reactions. The availability of intensive care units and expert clinicians is primary in the treatment making the use of SVA. Though the availability of SVA has improved over the years, having it handy at the right time in the right place is crucial. There still is a big limitation to this SVA available in India as it is limited only to the “Big Four” snake species. It is effective only against venoms of Naja naja, Bungarus caeruleus, Daboia russelii and Echis carinatus. This polyvalent snake venom antiserum has very limited or no use in bites of other venomous snake species, and there is a dire need to develop antisera for the venoms of other clinically important snake species, as well [242]. The coastline of the Indian and Pacific Oceans faces another peculiar threat of sea snake envenomation. Warm currents of the Indian Ocean have many venomous sea snake species [243]. Accidental exposure of fishermen to sea snakes makes them vulnerable to envenomation. Sea snake venom is more toxic than that of terrestrial snakes, including cobras, but a sufficient quantity of venom is seldom injected during the bite [244]. Thus, overall mortality of sea snake envenomation is lower. Myoglobinuria is a common symptom that differentiates sea snakes from land snakes [244]. Though antiserum is available for sea snakes internationally and in most South-East Asian countries, its availability in India is uncommon.
The mental trauma associated with snakebite also plays a vital role in worsening clinical symptoms. The more anxious the patient is, the more are the chances of exacerbation of clinical symptoms. The stress associated with snakebite brings psychoneuroendocrinological effects into action [245]. Even today, snakebite is believed to be the act of evil or the wrath of the goddess in some rural cultures, and the victims are taken to local healers who are believed to have magical powers. Some social groups are working on superstition eradication and educating people to seek immediate medical help postsnakebite as the delay can result in severe morbidity or mortality. During field work, one such event was witnessed in the Konkan region of Maharashtra, where a farm worker snakebite victim was taken to a healer instead of seeking medical help. Deprived of survival hopes, the victim was suffering from tachycardia and palpitations. The victim’s coworkers killed the snake after a bite and carried it with them. In absence of the Vaidu (traditional healer), another person approached them and carefully looked at the situation. He chanted some mantras, prepared lemonade and asked the victim to sit across and drink the lemonade. He continued chanting and made the victim drink another glass of lemonade a few minutes later. The victim started feeling better, and the tachycardia and palpitations were relieved. After everyone was relieved of the death threat, the person revealed his identity as being a person from a superstition eradication group. He explained how the semipoisonous snake caused local tissue inflammation, was never a threat to the life and the tachycardia and palpitations were due to severe anxiety. The victim was then taken to a healthcare centre and treated further. The local people would never have taken the victim to the healthcare centre if it was not done this way.
Snake envenomation is a real neglected tropical disease. Its incidence is not as common as diseases such as diabetes or hypertension, as these lifestyle disorders do not kill a person overnight and have a lot of potential for the sale of medications to manage the disease over a long time. Snake envenomation, on the other hand, is an acute situation for which the time frame for its management is very short, and consumption of drugs is limited. Naturally, due to high return potential, research investment is much higher for lifestyle disorders in comparison to that of snake envenomation. A small molecule with a high margin of safety, which can be self-administered by a victim or a primary healthcare worker, would be a great life-saver in case of snake envenomation. Natural resources such as plants and traditional literature might have highly effective hidden gems. A lot of research would be necessary before any such molecule becomes clinically useful.
Author Contributions
Conceptualization and design of the work: A.M.D. and S.B.B.; methodology: A.M.D., K.V.S. and S.B.B.; data curation: A.M.D.; analysis and interpretation: A.M.D. and S.B.B.; writing—original draft preparation: A.M.D.; writing—review and editing: K.V.S. and S.B.B.; supervision, K.V.S. and S.B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are thankful to all those who helped in procuring the traditional literature and helped understand and translate it.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tu, A.T. Overview of Snake Venom Chemistry. In Natural Toxins II; Advances in Experimental Medicine and Biology; Singh, B.R., Tu, A.T., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 37–62. [Google Scholar] [CrossRef]
- Iwanaga, S.; Suzuki, T. Enzymes in Snake Venom. In Snake Venoms; Lee, C.-Y., Ed.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 61–158. [Google Scholar] [CrossRef]
- WHO. Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 5 September 2021).
- Snake-bite envenoming: A priority neglected tropical disease. Lancet 2017, 370, P2. [CrossRef]
- Williams, D.J.; Abul Faiz, M.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Wen Fan, H.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef] [PubMed]
- Punde, D.P. Management of snake-bite in rural Maharashtra: A 10-year experience. Natl. Med. J. India 2005, 18, 71. [Google Scholar] [PubMed]
- Mehta, S.R.; Sashindran, V.K. Clinical Features and Management of Snake Bite. Med. J. Armed Forces India 2002, 58, 247. [Google Scholar] [CrossRef]
- Meenatchisundaram, S.; Michael, A. Snake bite and therapeutic measures: Indian scenario. Indian J. Sci. Technol. 2009, 2, 69–73. [Google Scholar] [CrossRef]
- Pade, S.D.; Patil, P.B.; Gadre, D.V.; Padhye-Gurjar, A.B. Aushadhi Baad; Rajesh Prakashan: Pune, India, 2010; Volume 1–3. [Google Scholar]
- Martz, W. Plants with a reputation against snakebite. Toxicon 1992, 30, 1131–1142. [Google Scholar] [CrossRef]
- Mors, W.B.; do Nascimento, M.C.; Ruppelt Pereira, B.M.; Pereira, N.A. Plant natural products active against snake bite—The molecular approach. Phytochemistry 2000, 55, 627–642. [Google Scholar] [CrossRef]
- Houghton, P.J.; Osibogun, I.M. Flowering plants used against snakebite. J. Ethnopharmacol. 1993, 39, 1–29. [Google Scholar] [CrossRef]
- Coe, F.G.; Anderson, G.J. Snakebite ethnopharmacopoeia of eastern Nicaragua. J. Ethnopharmacol. 2005, 96, 303–323. [Google Scholar] [CrossRef]
- Soares, A.M.; Ticli, F.K.; Marcussi, S.; Lourenco, M.V.; Januario, A.H.; Sampaio, S.V.; Giglio, J.R.; Lomonte, B.; Pereira, P.S. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 2005, 12, 2625–2641. [Google Scholar] [CrossRef]
- Mebs, D. Notes on the traditional use of plants to treat snake bite in northern Papua New Guinea. Toxicon 2000, 38, 299–302. [Google Scholar] [CrossRef]
- Kanojia, A.; Chaudhari, K.S.; Gothecha, V.K. Medicinal plants active against snake envenomation. Int. J. Res. Ayurveda Pharm. 2012, 3, 363–366. [Google Scholar]
- Samy, R.P.; Thwin, M.M.; Gopalakrishnakone, P.; Ignacimuthu, S. Ethnobotanical survey of folk plants for the treatment of snakebites in Southern part of Tamilnadu, India. J. Ethnopharmacol. 2008, 115, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Khyade, M.S.; Takate, Y.A.; Divekar, M.V. Plants Used as an Antidote Against Snakebite in Akole Taluka of Ahmednagar District (MS), India. J. Nat. Remedies 2011, 11, 182–192. [Google Scholar]
- Kadel, C.; Jain, A.K. Folklore claims on snakebite among some tribal communities of Central India. Indian J. Tradit. Knowl. 2008, 7, 296–299. [Google Scholar]
- Pade, S.D. Vanaushadhi Gunadarsha; Shri Gajanan Book Depot: Pune, India, 1893; Volume 1–7. [Google Scholar]
- Sathe, K.N. Gharguti Aushadhe, 16th ed.; Shailaja Anil Sathe: Mumbai, India, 2003. [Google Scholar]
- Timmins, R.J.; Duckworth, J.W. Moschus cupreus. In The IUCN Red List Threatened Species 2015; 2015; p. e.T136750A61979453. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Dickers, K.J.; Rice, P.; Griffiths, G.D.; Vale, J.A. Ricin Poisoning. Toxicol. Rev. 2003, 22, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.F.; Mota, É.F.; Silva, A.C.M.; Tomé, A.R.; Silva, M.Z.; de Brito, D.; Porfírio, C.T.; Oliveira, A.C.; Lima-Filho, J.V.; Ramos, M.V. Latex proteins from Calotropis procera: Toxicity and immunological tolerance revisited. Chem. Biol. Interact. 2017, 274, 138–149. [Google Scholar] [CrossRef]
- Anger, E.E.; Yu, F.; Li, J. Aristolochic acid-induced nephrotoxicity: Molecular mechanisms and potential protective approaches. Int. J. Mol. Sci. 2020, 21, 1157. [Google Scholar] [CrossRef]
- Giri, S.; Lokesh, C.R.; Sahu, S.; Gupta, N. Luffa echinata: Healer plant or potential killer? J. Postgrad. Med. 2014, 60, 72. [Google Scholar] [CrossRef]
- Stirpe, F.; Pession-Brizzi, A.; Lorenzoni, E.; Strocchi, P.; Montanaro, L.; Sperti, S. Studies on the proteins from the seeds of Croton tiglium and of Jatropha curcas. Toxic properties and inhibition of protein synthesis in vitro. Biochem. J. 1976, 156, 1–6. [Google Scholar] [CrossRef]
- Barth, A.; Müller, D.; Dürrling, K. In vitro investigation of a standardized dried extract of Citrullus colocynthis on liver toxicity in adult rats. Exp. Toxicol. Pathol. 2002, 54, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar] [PubMed]
- Naidu, M.T.; Babu, N.C.; Venkaiah, M. Ethnic remedies against snake bite from Kotia hills of Vizianagaram district, Andhra Pradesh, India. Indian J. Nat. Prod. Resour. 2013, 4, 194–196. [Google Scholar]
- Roy, K. India’s Historic Battles from Alexander the Great to Kargil; Permanent Black: Delhi, India, 2004; p. 201. [Google Scholar]
- Penzer, N.M.; Bhaṭṭa, S. Poison Damsels: Folklore of the World; Arno Press: New York, NY, USA, 1980; p. 319. [Google Scholar]
- Rao, C.V.; Verma, A.R.; Gupta, P.K.; Madhavan, V. Anti-inflammatory and anti-nociceptive activities of Fumaria indica whole plant extract in experimental animals. Acta Pharm. 2007, 57, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Alm, T. Dyvelsdrek Ferula assa-foetida i folketradisjonen i Norge-med noen klassiske sidesprang. Blyttia 2004, 62, 14–48. [Google Scholar]
- Wadhwani, B.D.; Mali, D.; Vyas, P.; Nair, R.; Khandelwal, P. A review on phytochemical constituents and pharmacological potential of Calotropis procera. RSC Adv. 2021, 11, 35854–35878. [Google Scholar] [CrossRef]
- Timmins, R.; Kawanishi, K.; Giman, B.; Lynam, A.; Chan, B.; Steinmetz, R.; Sagar Baral, H.; Samba Kumar, N. Rusa unicolor. In IUCN Red List Threatened Species 2015; (Errata Version Published in 2015); 2015; p. e.T41790A85628124. [Google Scholar] [CrossRef]
- Widyowati, R.; Suciati, S.; Haryadi, D.M.; Chang, H.-I.; Suryawan, I.N.; Utama, A.W. The effect of Rusa unicolor antler deer extracts from East Kalimantan in bone turnover cell models. Turk. J. Pharm. Sci. 2020, 17, 440–445. [Google Scholar] [CrossRef]
- Savrikar, S.S.; Ravishankar, B. Bhaishajya Kalpanaa—The Ayurvedic pharmaceutics—An overview. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 174–184. [Google Scholar] [CrossRef][Green Version]
- Reid, H.A.; Theakston, R.D.G. The management of snake bite. Bull. World Health Organ. 1983, 61, 885–895. [Google Scholar]
- Patil, V.M.; Das, S.; Balasubramanian, K. Quantum chemical and docking insights into bioavailability enhancement of curcumin by piperine in pepper. J. Phys. Chem. A 2016, 120, 3643–3653. [Google Scholar] [CrossRef]
- Khajuria, A.; Zutshi, U.; Bedi, K. Permeability characteristics of piperine on oral absorption-an active alkaloid from peppers and a bioavailability enhancer. Indian J. Exp. Biol. 1998, 36, 46–50. [Google Scholar] [PubMed]
- Shenoy, P.A.; Nipate, S.S.; Sonpetkar, J.M.; Salvi, N.C.; Waghmare, A.B.; Chaudhari, P.D. Anti-snake venom activities of ethanolic extract of fruits of Piper longum L. (Piperaceae) against Russell’s viper venom: Characterization of piperine as active principle. J. Ethnopharmacol. 2013, 147, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.A.; Nipate, S.S.; Sonpetkar, J.M.; Salvi, N.C.; Waghmare, A.B.; Chaudhari, P.D. Production of high titre antibody response against Russell’s viper venom in mice immunized with ethanolic extract of fruits of Piper longum L. (Piperaceae) and piperine. Phytomedicine 2014, 21, 159–163. [Google Scholar] [CrossRef]
- Ghag-Sawant, M.; More, T.V.; Samant, L.S.; Chowdhary, A.S. Study of neutralization of enzymatic activity of Daboia russelii venom by various plant extracts and their combinations using in vitro methods. Int. J. Pharm. Sci. Res. 2016, 7, 2531–2536. [Google Scholar] [CrossRef]
- Dave, B.J.; Trivedi, A.H.; Adhvatyu, S.G. Role of areca nut consumption in the cause of oral cancers. A cytogenetic assessment. Cancer 1992, 70, 1017–1023. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Trivedy, C.; Peters, T.J. Areca nut use: An independent risk factor for oral cancer: The health problem is under-recognised. Br. Med. J. 2002, 324, 799–800. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 85, 334. [Google Scholar]
- Merchant, A.; Husain, S.S.M.; Hosain, M.; Fikree, F.F.; Pitiphat, W.; Siddiqui, A.R.; Hayder, S.J.; Haider, S.M.; Ikram, M.; Chuang, S.K.; et al. Paan without tobacco: An independent risk factor for oral cancer. Int. J. Cancer 2000, 86, 128–131. [Google Scholar] [CrossRef]
- Kumar, S.; Dobos, G.J.; Rampp, T. Clinical significance of leech therapy in Indian medicine. J. Evid. Based Complement. Altern. Med. 2013, 18, 152–158. [Google Scholar] [CrossRef]
- Adams, S.L. The medicinal leech: A page from the annelids of internal medicine. Ann. Intern. Med. 1988, 109, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, D.; Aurich, M.; Pasalar, M.; Rampp, T. Medicinal leech therapy in venous congestion and various ulcer forms: Perspectives of Western, Persian and Indian medicine. J. Tradit. Complement. Med. 2020, 10, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Snake venom components affecting blood coagulation and the vascular system: Structural similarities and marked diversity. Curr. Pharm. Des. 2007, 13, 2872–2886. [Google Scholar] [CrossRef] [PubMed]
- Ghate, D.; Edelhauser, H.F. Ocular drug delivery. Expert Opin. Drug. Deliv. 2006, 3, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kublik, H.; Vidgren, M.T. Nasal delivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev. 1998, 29, 157–177. [Google Scholar] [CrossRef]
- Ghori, M.U.; Mahdi, M.H.; Smith, A.M.; Conway, B.R. Nasal drug delivery systems: An overview. Am. J. Pharmacol. Sci. 2015, 3, 110–119. [Google Scholar] [CrossRef]
- Dorrigiv, M.; Zareiyan, A.; Hosseinzadeh, H. Garlic (Allium sativum) as an antidote or a protective agent against natural or chemical toxicities: A comprehensive update review. Phytother. Res. 2020, 34, 1770–1797. [Google Scholar] [CrossRef]
- Nencini, C.; Franchi, G.G.; Cavallo, F.; Micheli, L. Protective effect of Allium neapolitanum Cyr. versus Allium sativum L. on acute ethanol-induced oxidative stress in rat liver. J. Med. Food 2010, 13, 329–335. [Google Scholar] [CrossRef]
- Venugopal, P.V.; Venugopal, T.V. Antidermatophytic activity of garlic (Allium sativum) in vitro. Int. J. Dermatol. 1995, 34, 278–279. [Google Scholar] [CrossRef]
- Asante-Kwatia, E.; Mensah, A.Y.; Fobi, E. An ethnobotanical study on medicinal plants used as antidote for snakebite and as snake repellent in the Ejisu-Juabeng District of Ghana. Res. J. Pharmacogn. 2021, 8, 53–62. [Google Scholar] [CrossRef]
- Asad, M.H.H.B.; Durr-E-Sabih; Yaqab, T.; Murtaza, G.; Hussain, M.S.; Hussain, M.S.; Nasir, M.T.; Azhar, S.; Khan, S.A.; Hussain, I. Phospholipases A2: Enzymatic assay for snake venom (Naja naja karachiensis) with their neutralization by medicinal plants of Pakistan. Acta Pol. Pharm. Drug Res. 2014, 71, 625–630. [Google Scholar]
- Asad, M.H.H.B.; Murtaza, G.; Ubaid, M.; Sajjad, A.; Mehmood, R.; Mahmood, Q.; Ansari, M.M.; Karim, S.; Mehmood, Z.; Hussain, I. Naja naja karachiensis envenomation: Biochemical parameters for cardiac, liver, and renal damage along with their neutralization by medicinal plants. Biomed Res. Int. 2014, 2014, 970540. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.H.H.B.; Razi, M.T.; Najamus-Saqib, Q.; Nasim, S.J.; Murtaza, G.; Hussain, I. Anti-venom potential of Pakistani medicinal plants: Inhibition of anticoagulation activity of Naja naja karachiensis toxin. Curr. Sci. 2013, 105, 1419–1424. [Google Scholar]
- Rahmy, T.R.; Hemmaid, K.Z. Prophylactic action of garlic on the histological and histochemical patterns of hepatic and gastric tissues in rats injected with a snake venom. J. Nat. Toxins 2001, 10, 137–165. [Google Scholar] [PubMed]
- Kuriakose, B.B.; Aleykutty, N.A.; Nitha, B. Evaluation of venom neutralising capacity of Indian medicinal plants by in vitro methods. Asian J. Pharm. Health Sci. 2012, 2, 552–554. [Google Scholar]
- Arif, M.; Rahman, M.A.; Imran, M.; Khalid, M.; Khushtar, M. An insight of Spondias mangifera willd: An underutilized medicinal plant with immense nutraceutical and therapeutic potentials. Int. J. Res. Pharm. Sci. 2015, 6, 100–109. [Google Scholar]
- Bahrami, G.; Soltani, R.; Sajjadi, S.-E.; Kanani, M.-R.; Naderi, R.; Ghiasvand, N.; Shokoohinia, Y. Essential oil composition of Ferula assa-foetida L. fruits from Western Iran. J. Rep. Pharm. Sci. 2013, 2, 90–97. [Google Scholar]
- Lenin; Rao, M.R.K.; Prabhu, K.; Bindu; Elizabeth, R.A.A.; Dinakar, S. The study of antioxidant activities of an Ayurvedic medicine Ayaskriti. Pharm. Lett. 2016, 8, 203–211. [Google Scholar]
- Sharma, R.; Thakur, G.S.; Sanodiya, B.S.; Savita, A.; Pandey, M.; Sharma, A.; Bisen, P.S. Therapeutic potential of Calotropis procera: A giant milkweed. IOSR J. Pharm. Biol. Sci. 2012, 4, 42–57. [Google Scholar] [CrossRef]
- Gomes, A.; Das, R.; Sarkhel, S.; Mishra, R.; Mukherjee, S.; Bhattacharya, S.; Gomes, A. Herbs and herbal constituents active against snake bite. Indian J. Exp. Biol. 2010, 48, 865–878. [Google Scholar]
- Murti, Y.; Yogi, B.; Pathak, D. Pharmacognostic standardization of leaves of Calotropis procera (Ait.) R. Br.(Asclepiadaceae). Int. J. Ayurveda Res. 2010, 1, 14–17. [Google Scholar] [CrossRef]
- Poonam; Punia, G. A review on varieties of Arka—Calotropis procera (Aiton) Dryand. and Calotropis gigantea (L.) Dryand. Glob. J. Res. Med. Plant Indig. Med. 2013, 2, 392–400. [Google Scholar]
- Alagesaboopathi, C. Ethnomedicinal plants and their utilization by villagers in Kumaragiri hills of Salem district of Tamilnadu, India. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 222–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, S.; Kumari, A.; Sharma, M. Comparative GC-MS analysis of bioactive compounds in methanolic extract of Calotropis gigantea (L.) WT Aiton leaf and latex. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1823–1827. [Google Scholar]
- Shwetha, V.; Veena, S.M.; Govindappa, M.; Zameer, F.; Francois, N.N.; More, S.S. In vitro neutralization of Naja naja venom enzymes by folk medicinal plant extracts. J. Biol. Act. Prod. Nat. 2019, 9, 278–288. [Google Scholar] [CrossRef]
- Aslam, N.; Fatima, S.; Khalid, S.; Hussain, S.; Qayum, M.; Afzal, K.; Asad, M.H.H.B. Anti-5′-Nucleotidases (5′-ND) and Acetylcholinesterase (AChE) Activities of Medicinal Plants to Combat Echis carinatus Venom-Induced Toxicities. Biomed Res. Int. 2021, 2021, 6631042. [Google Scholar] [CrossRef] [PubMed]
- Kaladhar, D.S.V.G.K.; Duddukuri, G.R.; Ramesh, K.; Varahalarao Vadlapudi, V.; Yarla, N.S. In vitro protease Inhibition, Modulation of PLA2 Activity and Protein Interaction Studies of Calotropis gigantea. J. Clin. Cell. Immunol. 2013, 4, 1000165. [Google Scholar] [CrossRef]
- Parthasarathy, V.; Menon, A.R.; Devaranavadagi, B. Identification of Daboia russelii snake venom Phospholipase A2 [PLA2] inhibitors present in methanolic root extract of Calotropis gigantea. Int. J. Pharm. Res. 2020, 178. [Google Scholar] [CrossRef]
- Sani, I.; Bello, F.; Fakai, I.M.; Abdulhamid, A. Evaluation of antisnake venom activities of some medicinal plants using albino rats. Sch. Int. J. Tradit. Complement. Med. 2020, 3, 111–117. [Google Scholar] [CrossRef]
- Bhadane, B.S.; Patil, M.P.; Maheshwari, V.L.; Patil, R.H. Ethnopharmacology, phytochemistry, and biotechnological advances of family Apocynaceae: A review. Phytother. Res. 2018, 32, 1181–1210. [Google Scholar] [CrossRef]
- Rahuman, H.B.H.; Dhandapani, R.; Palanivel, V.; Thangavelu, S.; Paramasivam, R.; Muthupandian, S. Bioengineered phytomolecules-capped silver nanoparticles using Carissa carandas leaf extract to embed on to urinary catheter to combat UTI pathogens. PLoS ONE 2021, 16, e0256748. [Google Scholar] [CrossRef]
- Shamim, S.; Ahmad, S.I. Pharmacodynamic study on acute hypotensive activities of Carissa carandas extract in normal rats. Pak. J. Pharm. Sci. 2012, 25, 577–582. [Google Scholar] [PubMed]
- Tayoub, G.; Sulaiman, H.; Alorfi, M. Analysis of Oleandrin in Oleander extract (Nerium oleander) by HPLC. J. Nat. Prod. 2014, 7, 73–78. [Google Scholar]
- Mookerjee, D. A Case of Poisoning by Sheth Kurrubbee, the White Oleander. Ind. Med. Gaz. 1866, 1, 258A. [Google Scholar] [PubMed]
- Sinha, S.N.; Biswas, K. A concise review on Nerium oleander L.—An important medicinal plant. Trop. Plant Res. 2016, 3, 408–412. [Google Scholar]
- Siddiqui, S.; Hafeez, F.; Begum, S.; Siddiqui, B.S. Isolation and structure of two cardiac glycosides from the leaves of Nerium oleander. Phytochemistry 1986, 26, 237–241. [Google Scholar] [CrossRef]
- Makhija, I.K.; Khamar, D. Anti-snake venom properties of medicinal plants. Pharm. Lett. 2010, 2, 399–411. [Google Scholar]
- BBRG, V.L.; Nagavardhanam, N.; Pradesh, A.; Rani, G. Diversity of medicinal flora in and around Kolleru lake. Int. J. Adv. Res. Ideas Innov. Technol. 2018, 4, 5–10. [Google Scholar]
- Rahmatullah, M.; Mollik, M.A.H.; Ali, M.; Abbas, M.F.B.; Jahan, R.; Chowdhury, M.H.; Seraj, S.; Miajee Zumeu, A.A.K.; Bashar, A.B.M.A.; Chowdhury, A.R. An ethnomedicinal survey of Vitbilia village in Sujanagar sub-district of Pabna district, Bangladesh. Am. Eurasian J. Sustain. Agric. 2010, 4, 302–308. [Google Scholar]
- Sandey, H.; Sharma, A. Study on ethnomedicinal plants of Achanakmar-Amarkantak Tiger reserve of Chhattisgarh. J. Sci. Lett. 2016, 1, 216–222. [Google Scholar]
- Monachino, J. Rauvolfia serpentina—Its history, botany and medical use. Econ. Bot. 1954, 8, 349–365. [Google Scholar] [CrossRef]
- Trivedi, M.P.; Kumari, R. Ethno-botanical and Germinational Aspects of Rauvolfia serpentina (L.) Benth. Ex Kurz. Our Nature 2011, 9, 176–178. [Google Scholar] [CrossRef]
- Panda, D.; Kumar, S.; Padhan, B.; Nayak, K. Phytochemical evaluation of ethnomedicinal plants used against snake bite by the tribal people of koraput, Odisha, India. Ann. Ayurvedic Med. 2020, 9, 12–21. [Google Scholar]
- Dey, A.; De, J.N. Ethnobotanical aspects of Rauvolfia serpentina (L). Benth. ex Kurz. in India, Nepal and Bangladesh. J. Med. Plants Res. 2011, 5, 144–150. [Google Scholar]
- Sivaraman, T.; Sreedevi, N.S.; Meenachisundharam, S.; Vadivelan, R. Neutralizing potential of Rauvolfia serpentina root extract against Naja naja venom. Braz. J. Pharm. Sci. 2020, 56, e18050. [Google Scholar] [CrossRef]
- Sreekumar, S.; Nisha, N.; Biju, C.; Krishnan, P. Identification of potential lead compounds against cobra venom in Rauvolfia serpentina (L.) Benth. Ex kurz through molecular docking. Int. J. Pharm. Res. Dev. 2014, 6, 32–43. [Google Scholar]
- Joshi, Y.N.; Sodal, I.; Kale, T.; Patange, S.; Gote, V. Identification of Potential Lead Compounds Against Snake Neurotoxin in Rauvolfia serpentina Through Molecular Docking. Int. J. Sci. Res. Sci. Technol. 2020, 7, 180–186. [Google Scholar] [CrossRef]
- Dey, A.; Hazra, A.K.; Mukherjee, A.; Nandy, S.; Pandey, D.K. Chemotaxonomy of the ethnic antidote Aristolochia indica for aristolochic acid content: Implications of anti-phospholipase activity and genotoxicity study. J. Ethnopharmacol. 2021, 266, 113416. [Google Scholar] [CrossRef]
- Gómez-Betancur, I.; Gogineni, V.; Salazar-Ospina, A.; León, F. Perspective on the therapeutics of anti-snake venom. Molecules 2019, 24, 3276. [Google Scholar] [CrossRef]
- Padhy, G.K. A Review of Aristolochia indica: Ethnomedicinal Uses, Phytochemistry, Pharmacological and Toxicological Effects. Curr. Tradit. Med. 2021, 7, 372–386. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Peshin, S.S. Do herbal medicines have potential for managing snake bite envenomation? Toxicol. Int. 2012, 19, 89. [Google Scholar] [CrossRef]
- Rajashekharan, S.; Pushpangadan, P.; Kumar, P.K.R.; Jawahar, C.R.; Nair, C.P.R.; Amma, L.S. Ethno medico botanical studies of cheriya arayan and valiya arayan (Aristolochia indica, Linn; Aristolochia tagala, Cham). Anc. Sci. Life 1989, IX, 99–106. [Google Scholar]
- Minu, V.; Harsh, V.; Ravikant, T.; Paridhi, J.; Noopur, S. Medicinal plants of Chhattisgarh with anti-snake venom property. Int. J. Curr. Pharm. Rev. Res. 2012, 3, 1–10. [Google Scholar]
- Dey, A.; De, J.N. Anti-snake venom botanicals used by the ethnic groups of Purulia District, West Bengal, India. J. Herbs Spices Med. Plants 2012, 18, 152–165. [Google Scholar] [CrossRef]
- Alam, M. Inhibition of toxic effects of viper and cobra venom by Indian medicinal plants. Pharmacol. Pharm. 2014, 5, 48216. [Google Scholar] [CrossRef]
- Modak, B.K.; Gorai, P.; Pandey, D.K.; Dey, A.; Malik, T. An evidence based efficacy and safety assessment of the ethnobiologicals against poisonous and non-poisonous bites used by the tribals of three westernmost districts of West Bengal, India: Anti-phospholipase A2 and genotoxic effects. PLoS ONE 2020, 15, e0242944. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bhattacharyya, D. Characterization of the aqueous extract of the root of Aristolochia indica: Evaluation of its traditional use as an antidote for snake bites. J. Ethnopharmacol. 2013, 145, 220–226. [Google Scholar] [CrossRef]
- Meenatchisundaram, S.; Parameswari, G.; Michael, A. Studies on antivenom activity of Andrographis paniculata and Aristolochia indica plant extracts against Daboia russelli venom by in vivo and in vitro methods. Indian J. Sci. Technol. 2009, 2, 76–79. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. Inhibition of Naja naja venom hyaluronidase: Role in the management of poisonous bite. Life Sci. 2006, 78, 1433–1440. [Google Scholar] [CrossRef]
- Vishwanath, B.S.; Rao, A.G.A.; Gowda, T.V. Interaction of phospholipase A2 from Vipera russelli venom with aristolochic acid: A circular dichroism study. Toxicon 1987, 25, 939–946. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bera, I.; Chakraborty, S.; Ghoshal, N.; Bhattacharyya, D. Aristolochic acid and its derivatives as inhibitors of snake venom L-amino acid oxidase. Toxicon 2017, 138, 1–17. [Google Scholar] [CrossRef]
- Ashok, P.; Koti, B.C.; Thippeswamy, A.H.M.; Tikare, V.P.; Dabadi, P.; Viswanathaswamy, A.H.M. Evaluation of antiinflammatory activity of Centratherum anthelminticum (L.) Kuntze seed. Indian J. Pharm. Sci. 2010, 72, 697. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Rajakumar, G.; Lee, J.-H.; Kim, S.-H.; Thiruvengadam, M. Ethnopharmacological uses, phytochemistry, biological activities, and biotechnological applications of Eclipta prostrata. Appl. Microbiol. Biotechnol. 2017, 101, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Pithayanukul, P.; Laovachirasuwan, S.; Bavovada, R.; Pakmanee, N.; Suttisri, R. Anti-venom potential of butanolic extract of Eclipta prostrata against Malayan pit viper venom. J. Ethnopharmacol. 2004, 90, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Mors, W.B.; Do Nascimento, M.C.; Parente, J.; Da Silva, M.H.; Melo, P.A.; Suarez-Kurtz, G. Neutralization of lethal and myotoxic activities of South American rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae). Toxicon 1989, 27, 1003–1009. [Google Scholar] [CrossRef]
- Melo, P.A.; Do Nascimento, M.C.; Mors, W.B.; Suarez-Kurtz, G. Inhibition of the myotoxic and hemorrhagic activities of crotalid venoms by Eclipta prostrata (Asteraceae) extracts and constituents. Toxicon 1994, 32, 595–603. [Google Scholar] [CrossRef]
- Pithayanukul, P.; Lapett, B.; Bavovada, R.; Pakmanee, N.; Suttisri, R. Inhibition of Proteolytic and Hemorrhagic Activities by Ethyl Acetate Extract of Eclipta prostrata. Against Malayan Pit Viper Venom. Pharm. Biol. 2007, 45, 282–288. [Google Scholar] [CrossRef]
- Chopra, R.N.; Dikshit, B.B.; Chowhan, J.S. Berberine and Berberine-containing plants in pharmacology and therapeutics. Ind. Med. Gaz. 1932, 67, 194–197. [Google Scholar]
- Kamal, G.C.; Gururaj, C.H. A review on Bilwadi Agada and its indications. World J. Pharm. Pharm. Sci. 2021, 10, 338–347. [Google Scholar] [CrossRef]
- Giri, A.; Mundhe, S.; Shimpi, M.; Gujrathi, D.S. Herbal antidotes for the management of snake bite. World J. Pharm. Pharm. Sci. 2019, 9, 735–743. [Google Scholar] [CrossRef]
- Omara, T.; Kagoya, S.; Openy, A.; Omute, T.; Ssebulime, S.; Kiplagat, K.M.; Bongomin, O. Antivenin plants used for treatment of snakebites in Uganda: Ethnobotanical reports and pharmacological evidences. Trop. Med. Health 2020, 48, 1–16. [Google Scholar] [CrossRef]
- Saran, P.L.; Choudhary, R.; Solanki, I.S.; Devi, G. Traditional medicaments through papaya in North eastern plains zone of India. Indian J. Tradit. Knowl. 2015, 14, 537–543. [Google Scholar]
- Hukkeri, V.I.; Joshi, M.P.; Deshpande, M.N.; Nagare, S.K.; Korgaonkar, A.M. Phyto-pharmacological review of Terminalia chebula Retz. Nat. Prod. Indian J. 2010, 6, 24–28. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; Blatter, E., Caius, J.F., Mhaskar, K.S., Eds.; Lalit Mohan Basu: Allahabad, India, 1935; Volume II. [Google Scholar]
- Kapoor, M.; Kaur, N.; Sharma, C.; Kaur, G.; Kaur, R.; Batra, K.; Rani, J. Citrullus colocynthis an Important Plant in Indian Traditional System of Medicine. Pharmacogn. Rev. 2020, 14, 22–27. [Google Scholar] [CrossRef]
- Meena, M.C.; Meena, R.K.; Patni, V. Ethnobotanical studies of Citrullus colocynthis (Linn.) Schrad.—An important threatened medicinal herb. J. Med. Plants 2014, 2, 15–22. [Google Scholar]
- Kumar, V.; Rathee, P.; Kohli, K.; Chaudhary, H.; Rathee, S. Phytochemical and biological potential of indrayan: An overview. Pharmacogn. Rev. 2009, 3, 193. [Google Scholar]
- Jabeen, S.; Al Mahruqi, Z.M.H.; Nadeem, F.; Khalid, T. Bitter Apple (Citrullus colocynthis)—A Review of a Wild Plant Growing from Asia to Africa with High Medicinal Potentials. Int. J. Chem. Biochem. Sci. 2017, 11, 65–70. [Google Scholar]
- Asad, M.H.H.B.; Razi, M.T.; Murtaza, G.; Azhar, S.; Khan, S.A.; Saqib, Q.N.U.; Hussain, I. Antihaemorrhagic potential of Citrullus colocynthis schrad (cucurbitaceae) against Naja naja karachiensis (Black Pakistan cobra) venom. J. Med. Plants Res. 2012, 6, 3455–3458. [Google Scholar] [CrossRef]
- Wagh, V.V.; Jain, A.K. Traditional herbal remedies among Bheel and Bhilala tribes of Jhabua district Madhya Pradesh. Int. J. Biol. Technol. 2010, 1, 20–24. [Google Scholar]
- Das, D.R.; Sachan, A.K.; Shuaib, M.; Imtiyaz, M. Phyto-pharmacology of Momordca dioica: A review. Asian J. Pharm. Res. Dev. 2016, 4, 1–6. [Google Scholar]
- Patel, M.G.; Ishnava, K.B. Momordica dioica Roxb. (spine gourd): Multiple shoot induction from nodal cultures and its antidiabetic activity. J. Med. Plants Stud. 2015, 3, 82–88. [Google Scholar]
- Pawar, S. Traditional phytotherapy for animal bite among the tribal’s in Jalgaon district Maharashtra. Life Sci. Leafl. 2012, 8, 40–43. [Google Scholar]
- Weerasinghe, M.G.W.K.; Dahanayake, N. Momordica dioica Roxb. (Spine Gourd)- An underutilized vegetable and medicinal plant in Sri Lanka. Int. J. Minor Fruits Med. Aromat. Plants 2021, 7, 100–104. [Google Scholar] [CrossRef]
- Rupachandra, S.; Selvam, M.P.; Muthukumaran, N.; Senthilkumar, S.; Vaidhyalingam, S.; Dharshene, K. In Vitro Assessment of Cytotoxic Activity of Bioactive Peptides from Momordica dioica and Solanum trilobatum against Human Colon Cancer Cells. Biomed. Pharmacol. J. 2021, 14, 1007–1018. [Google Scholar] [CrossRef]
- Lim, T.K. Dioscorea bulbifera. In Edible Medicinal and Non-Medicinal Plants; Springer: Amsterdam, The Netherlands, 2016; Volume 10, pp. 235–252. [Google Scholar] [CrossRef]
- Esha, P.; Padiya, R.; Acharya, R.; Chauhan, M. Pharmacognostical Evaluation of Croton roxburghii Balak. (Euphorbiaceae) Bark. Ayurpharm Int. J. Ayurveda Allied Sci. 2013, 2, 58–62. [Google Scholar]
- Panda, S.K.; Dutta, S.K.; Bastia, A.K. Antibacterial activity of Croton roxburghii Balak. against the enteric pathogens. J. Adv. Pharm. Technol. Res. 2010, 1, 419–422. [Google Scholar] [CrossRef]
- Chatatikun, M.; Yamauchi, T.; Yamasaki, K.; Aiba, S.; Chiabchalard, A. Anti melanogenic effect of Croton roxburghii and Croton sublyratus leaves in α-MSH stimulated B16F10 cells. J. Tradit. Complement. Med. 2019, 9, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mandal, L.; Bose, S. Pharmacognostic Standardization and Quantitative Estimation of some Isolated Phytoconstituents from Croton oblongifolius Roxb. J. PharmSciTech 2011, 1, 10–15. [Google Scholar]
- Mahmoud Aboulthana, W.; Youssef, A.; El-Feky, A.M.; El-Sayed Ibrahim, N.; Seif, M.M.; Kamal Hassan, A. Evaluation of antioxidant efficiency of Croton tiglium L. seeds extracts after incorporating silver nanoparticles. Egypt. J. Chem. 2019, 62, 181–200. [Google Scholar] [CrossRef]
- Simm, P.L. The supposed remedies for snake-bites. J. Soc. Arts 1856, 5, 101. [Google Scholar]
- Félix-Silva, J.; Silva-Junior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.d.F. Medicinal plants for the treatment of local tissue damage induced by snake venoms: An overview from traditional use to pharmacological evidence. Evid. Based Complement. Altern. Med. 2017, 2017, 5748256. [Google Scholar] [CrossRef]
- Khan, K.H. Roles of Emblica officinalis in medicine—A review. Bot. Res. Int. 2009, 2, 218–228. [Google Scholar]
- Alam, M.I.; Gomes, A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J. Ethnopharmacol. 2003, 86, 75–80. [Google Scholar] [CrossRef]
- Sarkhel, S.; Chakravarty, A.K.; Das, R.; Gomes, A.; Gomes, A. Snake venom neutralising factor from the root extract of Emblica officinalis Linn. Orient. Pharm. Exp. Med. 2011, 11, 25–33. [Google Scholar] [CrossRef]
- Garaniya, N.; Bapodra, A. Ethno botanical and Phytophrmacological potential of Abrus precatorius L.: A review. Asian Pac. J. Trop. Biomed. 2014, 4, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Wambebe, C.; Amosun, S.L. Some neuromuscular effects of the crude extracts of the leaves of Abrus precatorius. J. Ethnopharmacol. 1984, 11, 49–58. [Google Scholar] [CrossRef]
- Vaidya, S.M.; Singh, A.R.; Patel, V.G.; Khan, N.A.; Yewale, R.P.; Kale, D.M.K. A review on herbs against snake venom. J. Pharmacogn. Phytochem. 2018, 7, 5–9. [Google Scholar] [CrossRef]
- Bhakta, S.; Das, S.K. The medicinal values of Abrus precatorius: A review study. J. Adv. Biotechnol. Exp. Ther. 2020, 3, 84–91. [Google Scholar] [CrossRef]
- Bhatia, M.; Siddiqui, N.; Gupta, S. Abrus precatorius (L.): An evaluation of traditional herb. J. Pharm. Res. 2013, 3, 3295–3315. [Google Scholar]
- Sajon, S.R.; Sana, S.; Rana, S. Anti-venoms for snake bite: A synthetic and traditional drugs review. J. Pharmacogn. Phytochem. 2017, 6, 190–197. [Google Scholar]
- Burli, D.A.; Khade, A.B. A comprehensive review on Butea monosperma (Lam.) Kuntze. Pharmacogn. Rev. 2007, 1, 333–337. [Google Scholar]
- Patil, M.V.; Pawar, S.; Patil, D.A. Ethnobotany of Butea monosperma (Lam.) Kuntze in North Maharashtra, India. Nat. Prod. Radiance 2006, 5, 323–325. [Google Scholar]
- Yadav, R.S.; Sharma, S.; Pasi, A.K.; Patel, S. Butea monosperma (PALASH): Plant Review with Their Phytoconstituents and Pharmacological applications. IOSR J. Pharm. Biol. Sci. 2020, 15, 18–23. [Google Scholar] [CrossRef]
- Firdaus, R.; Mazumder, A. Review on Butea monosperma. Int. J. Res. Pharm. Chem. 2012, 2, 1035–1039. [Google Scholar]
- Tarannum, S.; Mohamed, R.; Vishwanath, B.S. Inhibition of testicular and Vipera russelli snake venom hyaluronidase activity by Butea monosperma (Lam) Kuntze stem bark. Nat. Prod. Res. 2012, 26, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.M.; Patel, B.R. Phyto Pharmacological Perspective of Yashtimadhu (Glycyrrhiza glabra Linn.) A Review. Int. J. Pharm. Biol. Sci. Arch. 2013, 4, 833–841. [Google Scholar]
- Assafim, M.; Ferreira, M.S.; Frattani, F.S.; Guimarães, J.A.; Monteiro, R.Q.; Zingali, R.B. Counteracting effect of glycyrrhizin on the hemostatic abnormalities induced by Bothrops jararaca snake venom. Br. J. Pharmacol. 2006, 148, 807–813. [Google Scholar] [CrossRef]
- Avinash, K.O.; Pradeep, S.; Shivamallu, C.; Gopenath, T.S.; Kumar, M.N.K.; Kanthesh, B.M. In silico screened flavanoids of Glycyrrhiza glabra Inhibit cPLA2 and sPLA2 in LPS stimulated macrophages. Bull. Environ. Pharmacol. Life Sci. 2021, 10, 14–24. [Google Scholar]
- Jitin, R.; Singh, S.P.; Naz, A. An ethnomedicinal survey of Orchha wildlife sanctuary region of Tikamgarh District, Madhya Pradesh, India. J. Bot. Res. 2013, 4, 31–34. [Google Scholar]
- Joshi, R.K.; Setzer, W.N.; Da Silva, J.K. Phytoconstituents, traditional medicinal uses and bioactivities of Tulsi (Ocimum sanctum Linn.): A review. Am. J. Essent. Oil Nat. Prod. 2017, 5, 18–21. [Google Scholar]
- Iqbal Chowdhury, I.; Rahman, M.A.; Hashem, M.A.; Bhuiyan, M.M.H.; Hajjar, D.; Alelwani, W.; Makki, A.A.; Haque, M.A.; Tangpong, J.; Bakhtiar, M.T.B. Supplements of an aqueous combination of Justicia adhatoda and Ocimum tenuiflorum boost antioxidative effects and impede hyperlipidemia. Anim. Models Exp. Med. 2020, 3, 140–151. [Google Scholar] [CrossRef]
- Zaman, K.A.; Khalid, A.A. Free radical scavenging activity of some Bangladeshi medicinal plants. Pharmacologyonline 2015, 3, 29–32. [Google Scholar]
- Venkatachalapathi, A.; Sangeeth, T.; Ali, M.A.; Tamilselvi, S.S.; Paulsamy, S.; Al-Hemaidc, F.M. Ethnomedicinal assessment of Irula tribes of Walayar valley of Southern Western Ghats, India. Saudi J. Biol. Sci. 2018, 25, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Prasad, M.; Sah, N.K. Anticancer biology of Azadirachta indica L. (neem): A mini review. Cancer Biol. Ther. 2011, 12, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.V.S.; Neelima, P. Neem (Azadirachta indica): A Review on Medicinal Kalpavriksha. Int. J. Econ. Plants 2022, 9, 59–63. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Doley, R.; Saikia, D. Isolation of a snake venom phospholipase A2 (PLA2) inhibitor (AIPLAI) from leaves of Azadirachta indica (Neem): Mechanism of PLA2 inhibition by AIPLAI in vitro condition. Toxicon 2008, 51, 1548–1553. [Google Scholar] [CrossRef]
- Sani, I.; Umar, R.A.; Hassan, S.W.; Faruq, U.Z.; Bello, F.; Aminu, H.; Sulaiman, A. Hepatoprotective effect of Azadirachta indica leaf fractionated extracts against snake venom toxicity on albino rats. Saudi J. Biomed. Res. 2020, 5, 112–117. [Google Scholar] [CrossRef]
- Sani, I.; Umar, R.A.; Hassan, S.W.; Faruq, U.Z.; Bello, F.; Abdulhamid, A. Inhibition of snake venom enzymes and antivenom adjuvant effects of Azadirachta indica A. Juss. (Meliaceae) leaf extracts. European J. Med. Plants 2020, 31, 114–128. [Google Scholar] [CrossRef]
- Arafa, N.M.S.; Mubarak, S.A. Antivenom Activity Exploration of Azadirachta indica. Cienc. Tec. Vitivinic. 2017, 32, 110–125. [Google Scholar]
- Shrirangasami, S.R.; Murugaragavan, R.; Rakesh, S.S.; Ramesh, P.T. Chemistry behind in neem (Azadirachta indica) as medicinal value to living forms—A review. J. Pharmacogn. Phytochem. 2020, 9, 467–469. [Google Scholar] [CrossRef]
- Dagar, P.; Mishra, A. Molecular docking analysis of modified gedunin from neem with snake venom enzymes. Bioinformation 2021, 17, 776–783. [Google Scholar] [CrossRef]
- Vasudev, S.; More, V.S.; Ananthraju, K.S.; More, S.S. Potential of herbal cocktail of medicinal plant extracts against ‘big four’snake venoms from India. J. Ayurveda Integr. Med. 2021, 12, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Bisht, G.S. Some novel folk treatments among the tribes of Uttar Pradesh. Anc. Sci. Life 1999, 18, 250–253. [Google Scholar] [PubMed]
- Tahir, A.; Khan, N.A.; Mansha, M.Z.; Ikram, K.; Aslam, H.M.U.; Aatif, H.M.; Hanif, C.M.S.; Ashfaq, M. Yield analysis of oyster mashroom (Pleurotus ostreatus) on Ficus religiosa leaves in combination with agricultural waste materials. Pure Appl. Biol. 2021, 10, 12–18. [Google Scholar] [CrossRef]
- Tarun, S.; Ramamurthy, A.; Parul, A.; Malvika, S. Medicinal Plants Used In Various Indian Traditional Customs. Int. J. Ayurvedic Herb. Med. 2016, 6, 2326–2332. [Google Scholar]
- Lim, T.K. Musa acuminata (AAA Group) ‘Dwarf Cavendish’. In Edible Medicinal and Non-Medicinal Plants; Springer: Amsterdam, The Netherlands, 2012; Volume 3, pp. 502–527. [Google Scholar] [CrossRef]
- Borges, M.H.; Alves, D.L.F.; Raslan, D.S.; Piló-Veloso, D.; Rodrigues, V.M.; Homsi-Brandeburgo, M.I.; De Lima, M.E. Neutralizing properties of Musa paradisiaca L. (Musaceae) juice on phospholipase A2, myotoxic, hemorrhagic and lethal activities of crotalidae venoms. J. Ethnopharmacol. 2005, 98, 21–29. [Google Scholar] [CrossRef]
- Zeeshan, U.; Barkat, M.Q.; Mahmood, H.K. Phytochemical and antioxidant screening of Cassia angustifolia, Curcuma zedoaria, Embelia ribes, Piper nigrum, Rosa damascena, Terminalia belerica, Terminalia chebula, Zingiber officinale and their effect on stomach and liver. Matrix Sci. Pharm. 2018, 2, 15–20. [Google Scholar] [CrossRef]
- Pandey, A.K.; Shukla, P.K. Role of Medicinal Plants in Health Care and Rural Economy in the Tribals of Satpura Plateau Region of Central India. Indian For. 2008, 134, 1438–1446. [Google Scholar]
- Shankar, R.; Lavekar, G.S.; Deb, S.; Sharma, B.K.; Rawat, M.S. Distribution, conservation and folk uses of Vaibidang (Embelia ribes Burm. f.). Int. J. Biodivers. Conserv. 2012, 4, 525–529. [Google Scholar] [CrossRef]
- Arya, V.; Kumari, S.; Sharma, S. Plants Used in Reptile Bites with Emphasis on Snake Bite. Rasamruta 2015, 7, 1435778196. [Google Scholar]
- Gupta, J.; Ali, M. Chemical constituents of Boerhavia diffusa Linn. roots. Indian J. Chem. 1998, 37B, 912–917. [Google Scholar]
- Mishra, S.; Aeri, V.; Gaur, P.K.; Jachak, S.M. Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. Biomed. Res. Int. 2014, 2014, 808302. [Google Scholar] [CrossRef] [PubMed]
- Guha, G.; Rajkumar, V.; Mathew, L.; Kumar, A. The antioxidant and DNA protection potential of Indian tribal medicinal plants. Turk. J. Biol. 2011, 35, 233–242. [Google Scholar] [CrossRef]
- Sandey, H.; Sharma, L. Phytosociological study of ethno medicinal leafy vegetable flora of district Kondagaon Chhattisgarh. J. Emerg. Technol. Innov. Res. 2019, 6, 422–435. [Google Scholar]
- Giresha, A.S.; Pramod, S.N.; Sathisha, A.; Dharmappa, K. Neutralization of Inflammation by Inhibiting In vitro and In vivo Secretory Phospholipase A2 by Ethanol Extract of Boerhaavia diffusa L. Pharmacogn. Res. 2017, 9, 174–181. [Google Scholar] [CrossRef]
- Hegde, D.M. Sesame. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, p. 479. [Google Scholar] [CrossRef]
- Nagori, K.; Singh, M.K.; Alexander, A.; Kumar, T.; Dewangan, D.; Badwaik, H.; Tripathi, D. Piper betle L.: A review on its ethnobotany, phytochemistry, pharmacological profile and profiling by new hyphenated technique DART-MS (Direct Analysis in Real Time Mass Spectrometry). J. Pharm. Res. 2011, 4, 2991–2997. [Google Scholar]
- Upasani, S.V.; Beldar, V.G.; Tatiya, A.U.; Upasani, M.S.; Surana, S.J.; Patil, D.S. Ethnomedicinal plants used for snakebite in India: A brief overview. Integr. Med. Res. 2017, 6, 114–130. [Google Scholar] [CrossRef]
- Dasgupta, A.; Datta, P. Medicinal species of Piper, pharmacognostic delimitation. Q. J. Crude Drug Res. 1980, 18, 17–25. [Google Scholar] [CrossRef]
- Lakshmi, V.; Kumar, R.; Agarwal, S.K.; Dhar, J.D. Antifertility activity of Piper longum Linn. in female rats. Nat. Prod. Res. 2006, 20, 235–239. [Google Scholar] [CrossRef]
- Biswas, K.R.; Khan, T.; Monalisa, M.N.; Swarna, A.; Ishika, T.; Rahman, M.; Rahmatullah, M. Medicinal plants used by folk medicinal practitioners of four adjoining villages of Narail and Jessore districts, Bangladesh. Am. Eurasian J. Sustain. Agric. 2011, 5, 23–33. [Google Scholar]
- Raman, R.; Raman, A.; Manohar, P.R. The arsenic and mercury-containing Tanjore pills used in treating snake bites in the 18th century Madras Presidency. Curr. Sci. 2014, 106, 1759–1763. [Google Scholar]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Chaudhari, G.S. A review on Plumbago zeylanica Linn.—A divine medicinal plant. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 119–127. [Google Scholar]
- Singh, U.P.; Yadav, P.; Goutam, M.P. Antimicrobial Efficacy of Traditionally Used Plant Chitrak Plumbago zeylanica. Asian Man 2018, 12, 161–172. [Google Scholar] [CrossRef]
- Shivanna, M.B.; Mangala, K.R.; Parinitha, M. Ethno-medicinal knowledge of Lambani community in Chikmagalur district of Karnataka, India. J. Med. Aromat. Plant Sci. 2008, 30, 105–108. [Google Scholar]
- Bhavya, J.; Vineetha, M.S.; More, V.S.; Zameer, F.; Muddapur, U.; More, S.S.; Govindappa, M. Ethnomedicinal plants and isolated compounds against Snake venom activity: A review. Indian J. Nat. Prod. Resour. 2022, 12, 491–505. [Google Scholar]
- Mandal, S.; Rath, J. Phytochemical and antioxidant activities of ethno-medicinal plants used by Fisher folks of Chilika lagoon for Indigenous Phytotherapy. J. Pharmacogn. Phytochem. 2015, 3, 55–65. [Google Scholar]
- Eshwara, J.H.K.; Kulathunga, R.D.H.; Gunarathna, E.D.T.P. Standardization of Nagaraja Guliya; A Sri Lankan Traditional Formula Used in Poisons of Animal Origin (Jangama Visha). Asian J. Adv. Res. Rep. 2020, 13, 49–59. [Google Scholar] [CrossRef]
- Jiji Mol, V.C.; Shanmugapriya, P. Scientific validation on Siddha purification process of Nabhi. Int. J. Pharm. Sci. Res. 2017, 8, 1790–1795. [Google Scholar] [CrossRef]
- Dhaliya, R.; Patil, S.; Netravathi, A. Evaluation of Effect of Vatsanabha (Aconitum Ferox Wall.) As a Prativisha against Cobra Venom (Naja naja) Toxicity: An Experimental Study. Indian J. Anc. Med. Yoga 2018, 11, 57–65. [Google Scholar] [CrossRef]
- Tsering, J.; Tag, H.; Gogoi, B.J.; Veer, V. Traditional anti-poison plants used by the Monpa tribe of Arunachal Pradesh. In Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization; Veer, V., Gopalakrishnan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–203. [Google Scholar] [CrossRef]
- Bhar, K.; Mondal, S.; Suresh, P. An eye-catching review of Aegle marmelos L. (Golden Apple). Pharmacogn. J. 2019, 11, 207–224. [Google Scholar] [CrossRef]
- Gupta, R. Ethnobotanical studies of Bael (Aegle marmelos): A sacred plant of Hindus. Int. J. Herb. Med. 2016, 4, 114–115. [Google Scholar]
- Bhatti, R.; Singh, J.; Saxena, A.K.; Suri, N.; Ishar, M.P.S. Pharmacognostic standardisation and antiproliferative activity of Aegle marmelos (L.) Correa leaves in various human cancer cell lines. Indian J. Pharm. Sci. 2013, 75, 628–634. [Google Scholar] [PubMed]
- Singh, E.A.; Kamble, S.Y.; Bipinraj, N.K.; Jagtap, S.D. Medicinal plants used by the Thakar tribes of Raigad district, Maharashtra for the treatment of snake-bite and scorpion-bite. Int. J. Phytother. Res. 2012, 2, 26–35. [Google Scholar]
- Mali, S.S.; Dhumal, R.L.; Havaldar, V.D.; Shinde, S.S.; Jadhav, N.Y.; Gaikwad, B.S. A systematic review on Aegle marmelos (Bael). Res. J. Pharmacogn. Phytochem. 2020, 12, 31–36. [Google Scholar] [CrossRef]
- Malviya, R.; Kumar, A.; Singh, A.; Kulkarni, G.T. Pharmacological screening, Ayurvedic values and commercial utility of Aegle marmelos. Int. J. Drug Dev. Res. 2012, 4, 28–37. [Google Scholar]
- Yogesh, G.; Jha, A.K. Ethnobotanical Studies of Sacred Aegle marmelos Plant. Int. J. Adv. Res. Ideas Innov. Technol. 2017, 3, 498–501. [Google Scholar]
- Nisha, N.C.; Sreekumar, S.; Evans, D.A.; Biju, C.K. In vitro and in silico validation of anti-cobra venom activity and identification of lead molecules in Aegle marmelos (L.) Correa. Curr. Sci. 2018, 114, 1214–1221. [Google Scholar] [CrossRef]
- Taleballa, L.H. Stimulation of Lime’s (Citrus aurantifolia) Seedlings Growth by Macro and Micro-nutrients, Hormones and Botanical Treatments. Master’s Thesis, Sudan University of Science and Technology, Khartoum, Sudan, 2019. [Google Scholar]
- Sharma, A. A Study of Ethnobotany with Reference to Traditional Knowledge of India. Int. J. Multidiscip. Res. Sci. Eng. Technol. 2018, 1, 51–57. [Google Scholar]
- Mali, P.R. Ethnobotanical studies of Peth and Trimbakeshwar district Nashik, Maharashtra, India. Trends Life Sci. 2012, 1, 35–37. [Google Scholar]
- Ediriweera, E.R.H.S.S.; Premakeerthi, W.M.S.A.; Perera, A.M.H.Y. A literary review on Sapindus trifoliatus (Gaspenela) and its medicinal values. Int. J. Ayurveda Pharm. Res. 2021, 9, 51–55. [Google Scholar] [CrossRef]
- Gandhi, A.; Adiga, A.; Hegde, P.L.; Pradeep. Comprehensive review on Arishtaka (Sapindus trifoliatus L. and S. mukorossi Gaertn). J. Ayurveda Integr. Med. Sci. 2022, 7, 158–164. [Google Scholar]
- Ntume, R.; Anywar, G.U. Ethnopharmacological survey of medicinal plants used in the treatment of snakebites in Central Uganda. Curr. Life Sci. 2015, 1, 6–14. [Google Scholar]
- Kishore, K. Monograph of tobacco (Nicotiana tabacum). Indian J. Drugs 2014, 2, 5–23. [Google Scholar]
- Bharathi, Y.; Jaffarbasha, S.; Manjunath, J. Variability, correlation and path analysis for seed yield and its component traits in Bidi Tobacco (Nicotiana tabacum L.). J. Res. PJTSAU 2018, 46, 99–102. [Google Scholar]
- Bhattacharjee, P.; Bhattacharyya, D. Medicinal plants as snake venom antidotes. J. Exp. Appl. Anim. Res. 2013, 1, 156–181. [Google Scholar] [CrossRef]
- Ameya, G.; Manilal, A.; Merdekios, B. In vitro antibacterial activity and phytochemical analysis of Nicotiana tabacum L. extracted in different organic solvents. Open Microbiol. J. 2017, 11, 352–359. [Google Scholar] [CrossRef]
- Binorkar, S.V.; Jani, D.K. Traditional Medicinal Usage of Tobacco—A Review. Spatula DD 2012, 2, 127–134. [Google Scholar] [CrossRef]
- Velayudhan, K.C.; Dikshit, N.; Nizar, M.A. Ethnobotany of turmeric (Curcuma longa L.). Indian J. Tradit. Knowl. 2012, 11, 607–614. [Google Scholar]
- Singh, S.; Sankar, B.; Rajesh, S.; Sahoo, K.; Subudhi, E.; Nayak, S. Chemical composition of turmeric oil (Curcuma longa L. cv. Roma) and its antimicrobial activity against eye infecting pathogens. J. Essent. Oil Res. 2011, 23, 11–18. [Google Scholar] [CrossRef]
- Chethankumar, M.; Srinivas, L. New biological activity against phospholipase A2 by Turmerin, a protein from Curcuma longa L. J. Biol. Chem. 2008, 389, 299–303. [Google Scholar] [CrossRef]
- Ferreira, L.A.F.; Henriques, O.B.; Andreoni, A.A.S.; Vital, G.R.F.; Campos, M.M.C.; Habermehl, G.G.; de Moraes, V.L.G. Antivenom and biological effects of ar-turmerone isolated from Curcuma longa (Zingiberaceae). Toxicon 1992, 30, 1211–1218. [Google Scholar] [CrossRef]
- Dileep, K.V.; Tintu, I.; Sadasivan, C. Molecular docking studies of curcumin analogs with phospholipase A2. Interdiscip. Sci. Comput. Life Sci. 2011, 3, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Majumdar, R.S.; Alam, P. Systematics evaluations of morphological traits, chemical composition, and antimicrobial properties of selected varieties of Elettaria cardamomum (L.) Maton. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Dattatray, S.; Kamini, B.; Mohanlal, J. The Queen of Spices and Ayurveda: A brief review. Int. J. Res. Ayurveda Pharm. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Upasani, M.S.; Upasani, S.V.; Beldar, V.G.; Beldar, C.G.; Gujarathi, P.P. Infrequent use of medicinal plants from India in snakebite treatment. Integr. Med. Res. 2018, 7, 9–26. [Google Scholar] [CrossRef]
- Raj, L.F.A.; Jayalakshmy, E. Biosynthesis and characterization of zinc oxide nanoparticles using root extract of Zingiber officinale. Orient. J. Chem. 2015, 31, 51–56. [Google Scholar] [CrossRef]
- Grys, A.; Łowicki, Z.; Parus, A. Medicinal properties of ginger (Zingiber officinale Roscoe). Postępy Fitoter. 2010, 2010, 42–45. [Google Scholar]
- Selvanayagam, Z.E.; Gnanavendhan, S.G.; Balakrishna, K.; Rao, R.B. Antisnake venom botanicals from ethnomedicine. J. Herbs Spices Med. Plants 1995, 2, 45–100. [Google Scholar] [CrossRef]
- Das, A.; Dan, V.M.; Varughese, G.; Varma, A. Roots of Medicinal Importance. In Root Engineering: Basic and Applied Concepts; Morte, A., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 443–467. [Google Scholar] [CrossRef]
- Trim, S.A.; Trim, C.M.; Williams, H.F.; Vaiyapuri, S. The failures of ethnobotany and phytomedicine in delivering novel treatments for snakebite envenomation. Toxins 2020, 12, 774. [Google Scholar] [CrossRef]
- Komives, C.F.; Sanchez, E.E.; Rathore, A.S.; White, B.; Balderrama, M.; Suntravat, M.; Cifelli, A.; Joshi, V. Opossum peptide that can neutralize rattlesnake venom is expressed in Escherichia coli. Biotechnol. Prog. 2017, 33, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Tripathi, J.S.; Rai, N.P. An appraisal of the bioavailability enhancers in Ayurveda in the light of recent pharmacological advances. Ayu 2016, 37, 3. [Google Scholar] [CrossRef] [PubMed]
- Cupo, P.; Azevedo-Marques, M.M.; de Menezes, J.B.; Hering, S.E. Immediate hypersensitivity reactions after intravenous use of antivenin sera: Prognostic value of intradermal sensitivity tests. Rev. Inst. Med. Trop. Sao Paulo 1991, 33, 115–122. [Google Scholar] [CrossRef] [PubMed]
- WHO. Progress in the Characterization of Venoms and Standardization of Antivenoms; World Health Organization: Geneva, Switzerland, 1981. [Google Scholar]
- Laxme, R.R.S.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the ‘big four’: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLOS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef]
- Phillips, C.M. Sea snake envenomation. Dermatol. Ther. 2002, 15, 58–61. [Google Scholar] [CrossRef]
- Bhise, S.B.; Bhide, M.B. Presynaptic action of Hydrophis cyanocinctus venom. Bull. Haffkine Inst. 1978, 6, 92–95. [Google Scholar]
- Ehlert, U.; Gaab, J.; Heinrichs, M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus-pituitary-adrenal axis. Biol. Psychol. 2001, 57, 141–152. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).