Dengue Epidemiology in Qatar from 2013–2021: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Dengue Case Management
2.2. Data Analysis
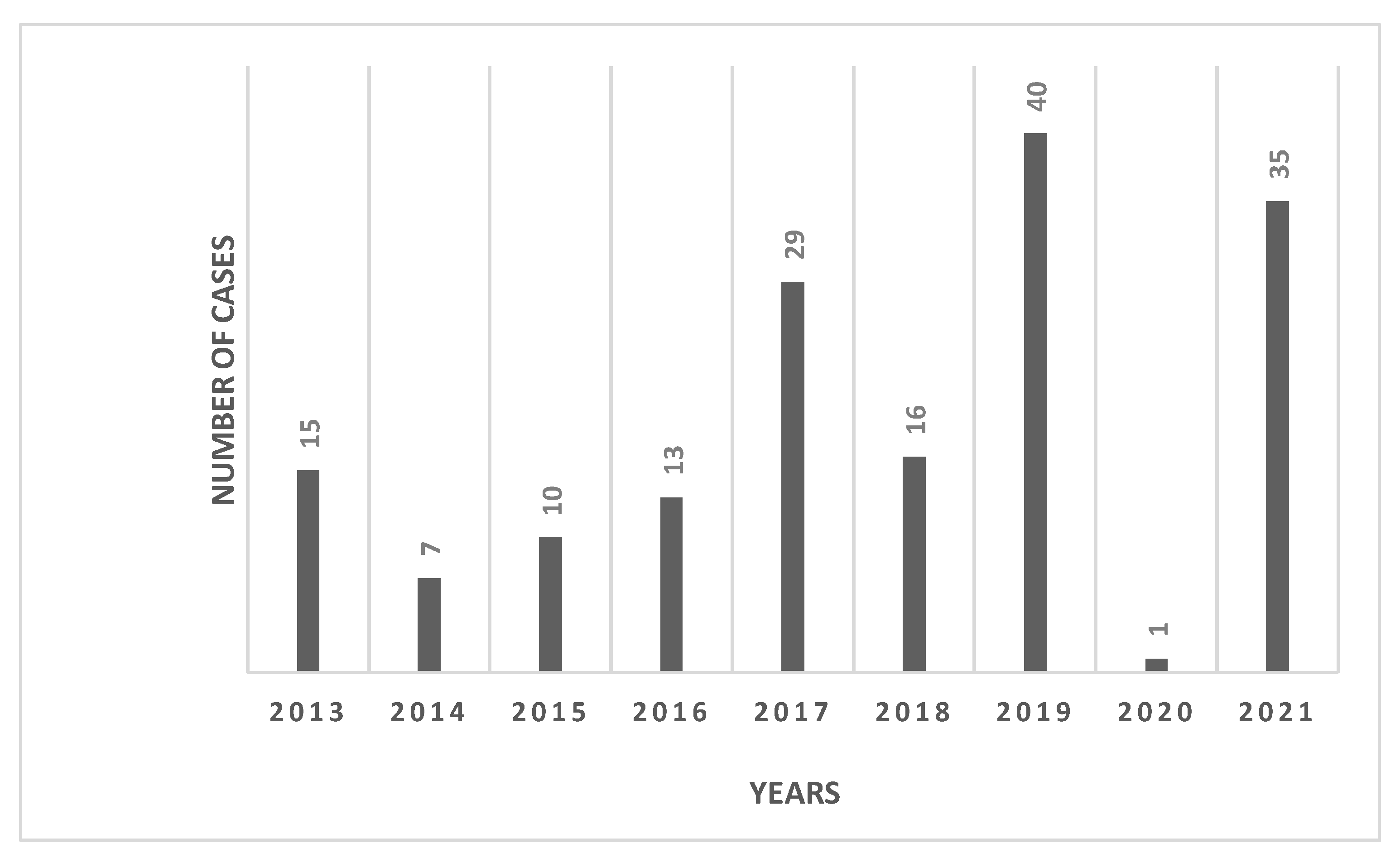
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 18 April 2022).
- World Health Organization. Ending NTD’s: Together towards 2030. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/ending-ntds-together-towards-2030 (accessed on 12 June 2022).
- Bennett, S.N. Taxonomy and Evolutionary Relationship of Flaviviruses; Ooi, E.E., Gubler, D.J., Vasudevan, S., Farrar, J., Eds.; CAB International: Wallingford, UK, 2014; Volume 322–333. [Google Scholar]
- Zanotto, P.M.; Gould, E.A.; Gao, G.F.; Harvey, P.H.; Holmes, E.C. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc. Natl. Acad. Sci. USA 1996, 93, 548–553. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control, 2nd ed.; WHO: Geneva, Switzerland, 2009; Available online: https://www.who.int/csr/resources/spublications/dengue/Denguepublication/en/ (accessed on 28 January 2020).
- Gubler, D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends. Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- Humphrey, J.M.; Al-Absi, E.S.; Hamdan, M.M.; Okasha, S.S.; Al-Trmanini, D.M.; El-Dous, H.G.; Dargham, S.R.; Schieffelin, J.; Abu-Raddad, L.J.; Nasrallah, G.K. Dengue and chikungunya seroprevalence among Qatari nationals and immigrants residing in Qatar. PLoS ONE 2019, 14, e0211574. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Yoon, I.-K. A review of Dengvaxia®: Development to deployment. Hum. Vaccin. Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Morrison, D.; Legg, T.J.; Billings, C.W.; Forrat, R.; Yoksan, S.; Lang, J. A Novel Tetravalent Dengue Vaccine Is Well Tolerated and Immunogenic against All 4 Serotypes in Flavivirus-Naive Adults. J. Infect. Dis. 2010, 201, 370–377. [Google Scholar] [CrossRef]
- Guy, B.; Guirakhoo, F.; Barban, V.; Higgs, S.; Monath, T.P.; Lang, J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 2010, 28, 632–649. [Google Scholar] [CrossRef]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.; Messina, J.P.; Reiner, R.C., Jr.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit. Vectors 2014, 7, 338. [Google Scholar] [CrossRef]
- Khormi, H.M.; Kumar, L. Climate change and the potential global distribution of Aedes aegypti: Spatial modelling using GIS and CLIMEX. Geospat. Health 2014, 8, 405–415. [Google Scholar] [CrossRef]
- Proestos, Y.; Christophides, G.K.; Ergüler, K.; Tanarhte, M.; Waldock, J.; Lelieveld, J. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Phil. Trans. R. Soc. B 2015, 370, 20130554. [Google Scholar] [CrossRef]
- Farag, E.A.B.A.; Bansal, D.; Mardini, K.; Sultan, A.A.; Al-Thani, M.H.J.; Al-Marri, S.A.; Al-Hajri, M.; Al-Romaihi, H.; Schaffner, F. Identification and characterisation of mosquitoes from different locations in Qatar in 2017–2019. Parasite 2021, 28, 84. [Google Scholar] [CrossRef]
- Fang, Y.; Tambo, E.; Xue, J.B.; Zhang, Y.; Zhou, X.N.; Khater, E.I.M. Detection of DENV-2 and Insect-Specific Flaviviruses in Mosquitoes Collected from Jeddah, Saudi Arabia. Front. Cell Infect. Microbiol. 2021, 11, 626368. [Google Scholar] [CrossRef]
- Tambo, E.; El Dessouky, A.G.; Khater, E.I.M. Innovative Preventive and Resilience Approaches Against Aedes-linked Vector-borne Arboviral Diseases Threat and Epidemics Burden in Gulf Council Countries. Oman Med. J. 2019, 34, 391–396. [Google Scholar] [CrossRef]
- Mosquito and Tick Bite Avoidance. Available online: https://www.hamad.qa/EN/Hospitals-and-services/Communicable-Disease-Center/Documents/Mosquito-and-Tick-Bite-Avoidance-EN.pdf (accessed on 14 September 2022).
- Ministry of Planning and Statistics Authority. Available online: https://www.psa.gov.qa/en/statistics1/StatisticsSite/Pages/KeyIndicators.aspx (accessed on 15 April 2022).
- Torresi, J.; Leder, K. Defining infections in international travellers through the GeoSentinel surveillance network. Nat. Rev. Microbiol. 2009, 7, 895–901. [Google Scholar] [CrossRef]
- Knope, K.; Giele, C. Increasing notifications of dengue in Australia related to overseas travel, 1991 to 2012. Commun. Dis. Intell. 2013, 37, 55–59. [Google Scholar]
- Chang, F.S.; Tseng, Y.T.; Hsu, P.S.; Chen, C.D.; Lian, I.B.; Chao, D.Y. Re-assess Vector Indices Threshold as an Early Warning Tool for Predicting Dengue Epidemic in a Dengue Non-endemic Country. PLoS Negl. Trop. Dis. 2015, 9, e0004043. [Google Scholar] [CrossRef]
- Ducheyne, E.; Tran Minh, N.N.; Haddad, N.; Bryssinckx, W.; Buliva, E.; Simard, F.; Malik, M.R.; Charlier, J.; De Waele, V.; Mahmoud, O.; et al. Current and future distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in WHO Eastern Mediterranean Region. Int. J. Health Geogr. 2018, 17, 4. [Google Scholar] [CrossRef]
- Himatt, S. Risk assessment of dengue fever in Qatar. J. Emerg. Med. 2016, 118. Available online: https://www.qscience.com/content/journals/10.5339/jemtac.2016.icepq.118#abstract_content (accessed on 14 September 2022).
- Armed Forces Pest Management Board (AFPMB). Regional Disease Vector Ecology Profile. 1999. Available online: https://permanent.access.gpo.gov/lps28798/mid_east.pdf (accessed on 3 February 2022).
- Schaffner, F.; Bansal, D.; Mardini, K.; Al-Marri, S.A.; Al-Thani, M.H.J.; Al-Romaihi, H.; Sultan, A.A.; Al-Hajri, M.; Farag, E.A.B.A. Vectors and vector-borne diseases in Qatar: Current status, key challenges and future prospects. J. Eur. Mosquito Control Assoc. 2021, 39, 3–13. [Google Scholar] [CrossRef]
- Lippi, C.A.; Stewart-Ibarra, A.M.; Loor, M.E.F.B.; Zambrano, J.E.D.; Lopez, N.A.E.; Blackburn, J.K.; Ryan, S.J. Geographic shifts in Aedes aegypti habitat suitability in Ecuador using larval surveillance data and ecological niche modeling: Implications of climate change for public health vector control. PLoS Negl. Trop. Dis. 2019, 13, e0007322. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Al Awaidy, S.T.; Khamis, F. Dengue Fever: An Emerging Disease in Oman Requiring Urgent Public Health Interventions. Oman Med. J. 2019, 34, 91–93. [Google Scholar] [CrossRef]
- Alhaeli, A.; Bahkali, S.; Ali, A.; Househ, M.S.; El-Metwally, A.A. The epidemiology of Dengue fever in Saudi Arabia: A systematic review. J. Infect. Public Health 2016, 9, 117–124. [Google Scholar] [CrossRef]
- Pagani, G.; Zanchetta, N.; Galimberti, L.; Oreni, L.; Passerini, S.; Giacomelli, A.; Cordier, L.; Gismondo, M.R.; Rizzardini, G.; Galli, M.; et al. Imported dengue fever: A 16-years retrospective analysis in Milan (Italy) and a brief review of the European literature. Infez. Med. 2020, 28, 243–252. [Google Scholar]
- Redondo-Bravo, L.; Ruiz-Huerta, C.; Gomez-Barroso, D.; Sierra-Moros, M.J.; Benito, A.; Herrador, Z. Imported dengue in Spain: A nationwide analysis with predictive time series analyses. J. Travel Med. 2019, 26, taz072. [Google Scholar] [CrossRef]
- Araújo, H.; Carvalho, D.; Ioshino, R.; Costa-da-Silva, A.; Capurro, M. Aedes aegypti Control Strategies in Brazil: Incorporation of New Technologies to Overcome the Persistence of Dengue Epidemics. Insects 2015, 6, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, A.; Letson, G. Dengue in the Middle East: A neglected, emerging disease of importance. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bin Ghouth, A.S.; Amarasinghe, A.; Letson, G.W. Dengue outbreak in Hadramout, Yemen, 2010: An epidemiological perspective. Am. J. Trop. Med. Hyg. 2012, 86, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Madani, T.A.; Abuelzein, E.T.M.E.; Al-Bar, H.M.S.; Azhar, E.I.; Kao, M.; Alshoeb, H.O.; Bamoosa, A.R. Outbreak of viral hemorrhagic fever caused by dengue virus type 3 in Al-Mukalla, Yemen. BMC Infect. Dis. 2013, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Yemen: Dengue Fever Outbreak (DREF n° MDRYE008)-Final Report, 5 March 2021. Available online: https://reliefweb.int/report/yemen/yemen-dengue-fever-outbreak-dref-n-mdrye008-final-report-05-march-2021 (accessed on 6 May 2022).
- Al-Abri, S.S.; Kurup, P.J.; Al Manji, A.; Al Kindi, H.; Al Wahaibi, A.; Al Jardani, A.; Mahmoud, O.A.; Al Balushi, L.; Al Rawahi, B.; Al Fahdi, F.; et al. Control of the 2018-2019 dengue fever outbreak in Oman: A country previously without local transmission. Int. J. Infect. Dis. 2020, 90, 97–103. [Google Scholar] [CrossRef]
- Melebari, S.; Bakri, R.; Hafiz, A.; Qabbani, F.; Khogeer, A.; Alharthi, I.; Alhazmi, S.; Almalki, Y.; Bulkhi, R.; Gammash, R.; et al. The epidemiology and incidence of dengue in Makkah, Saudi Arabia, during 2017–2019. Saudi Med. J. 2021, 42, 1173–1179. [Google Scholar] [CrossRef]
- Schaffner, F.; Bansal, D.; Al-Thani, M.H.J.; Al-Romaihi, H.; Farag, E.A.B.A. Preventing vector-borne diseases at major sport events: Addressing the challenges for FIFA 22 in Qatar. PLoS Negl. Trop. Dis. 2021, 15, e0009135. [Google Scholar] [CrossRef]

| Variables | TOTAL n = 166 (%) | Dengue IgM Positive n = 130 (%) | Dengue IgG Positive n = 96 (%) | Dengue IgM & IgG Positive n = 60 (%) |
|---|---|---|---|---|
| Sex | ||||
| Female | 40 (24) | 32 (25) | 25 (26) | 17 (28) |
| Male | 126 (76) | 98 (75) | 71 (74) | 43 (72) |
| Age | ||||
| <10 | 6 (4) | 6 (5) | 1 (1) | 1 (2) |
| 10–20 | 16 (10) | 14 (11) | 7 (7) | 5 (8) |
| 21–30 | 44 (27) | 33 (25) | 24 (25) | 13 (22) |
| 31–40 | 58 (35) | 44 (34) | 37 (39) | 23 (38) |
| 41–50 | 29 (17) | 23 (18) | 17(18) | 11 (18) |
| >50 | 13 (7) | 10 (8) | 10(10) | 7 (12) |
| Nationality | ||||
| South & Southeast Asian # | 146 (8) | 112 (86) | 87 (91) | 53 (88) |
| African Region $ | 7 (4) | 7 (5) | 4 (4) | 4 (7) |
| Others @ | 13 (8) | 11 (8) | 5 (5) | 3 (5) |
| Symptoms | ||||
| Fever | 165 (99) | 129 (99) | 96 (100) | 60 (100) |
| Headache | 46 (28) | 332 (25) | 30 (31) | 16 (27) |
| Joint Pain | 39 (23) | 29 (22) | 27 (28) | 17 (28) |
| Muscle Pain | 45 (27) | 34 (26) | 30 (31) | 19 (32) |
| Rash | 16 (10) | 13 (10) | 11 (11) | 8 (13) |
| Fatigue | 16 (10) | 12 (9) | 12 (13) | 8 (13) |
| Vomiting | 11 (6.61) | 6 (6) | 7 (7) | 4 (7) |
| Others | 14 (8) | 7 (5) | 12 (12) | 5 (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, E.A.; Jaffrey, S.; Daraan, F.; Al-Shamali, M.H.M.A.; Khan, F.Y.; Coyle, P.V.; Schaffner, F.; Al-Romaihi, H.E.; Al-Thani, M.; Bansal, D. Dengue Epidemiology in Qatar from 2013–2021: A Retrospective Study. Trop. Med. Infect. Dis. 2022, 7, 329. https://doi.org/10.3390/tropicalmed7110329
Farag EA, Jaffrey S, Daraan F, Al-Shamali MHMA, Khan FY, Coyle PV, Schaffner F, Al-Romaihi HE, Al-Thani M, Bansal D. Dengue Epidemiology in Qatar from 2013–2021: A Retrospective Study. Tropical Medicine and Infectious Disease. 2022; 7(11):329. https://doi.org/10.3390/tropicalmed7110329
Chicago/Turabian StyleFarag, Elmoubashar Abd, Shariq Jaffrey, Faisal Daraan, Maha Hammam M. A. Al-Shamali, Fahmi Y. Khan, Peter V. Coyle, Francis Schaffner, Hamad Eid Al-Romaihi, Mohammed Al-Thani, and Devendra Bansal. 2022. "Dengue Epidemiology in Qatar from 2013–2021: A Retrospective Study" Tropical Medicine and Infectious Disease 7, no. 11: 329. https://doi.org/10.3390/tropicalmed7110329
APA StyleFarag, E. A., Jaffrey, S., Daraan, F., Al-Shamali, M. H. M. A., Khan, F. Y., Coyle, P. V., Schaffner, F., Al-Romaihi, H. E., Al-Thani, M., & Bansal, D. (2022). Dengue Epidemiology in Qatar from 2013–2021: A Retrospective Study. Tropical Medicine and Infectious Disease, 7(11), 329. https://doi.org/10.3390/tropicalmed7110329







