Assessing the Need for Multiplex and Multifunctional Tick-Borne Disease Test in Routine Clinical Laboratory Samples from Lyme Disease and Febrile Patients with a History of a Tick Bite
Abstract
1. Introduction
2. Materials and Methods
2.1. Index Test and Interpretation
2.2. Ethics Statement
2.3. Reference Tests and Interpretation
2.4. Patient Categorization
2.5. Index Test and Interpretation
2.6. Statistical Analysis
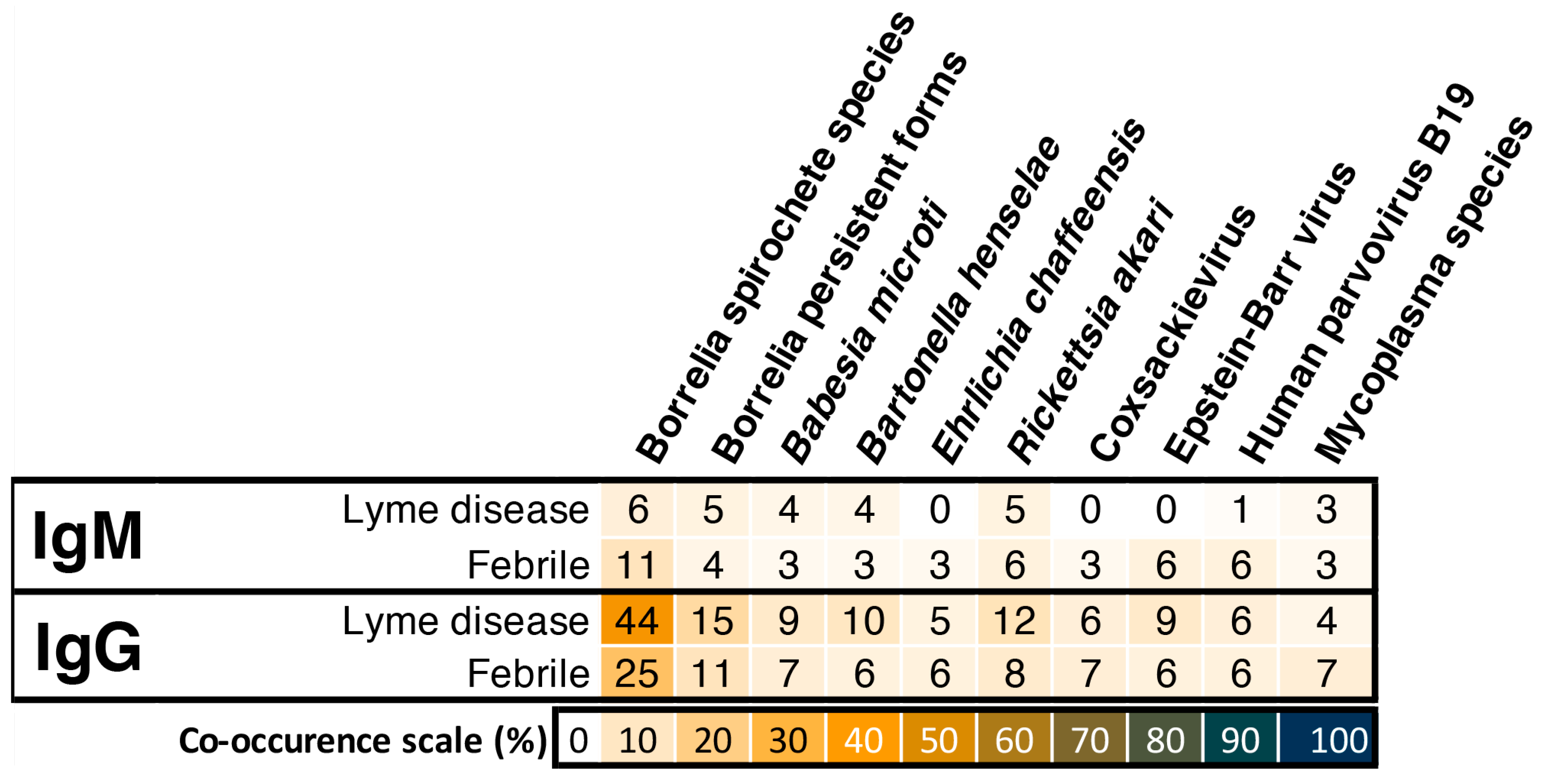
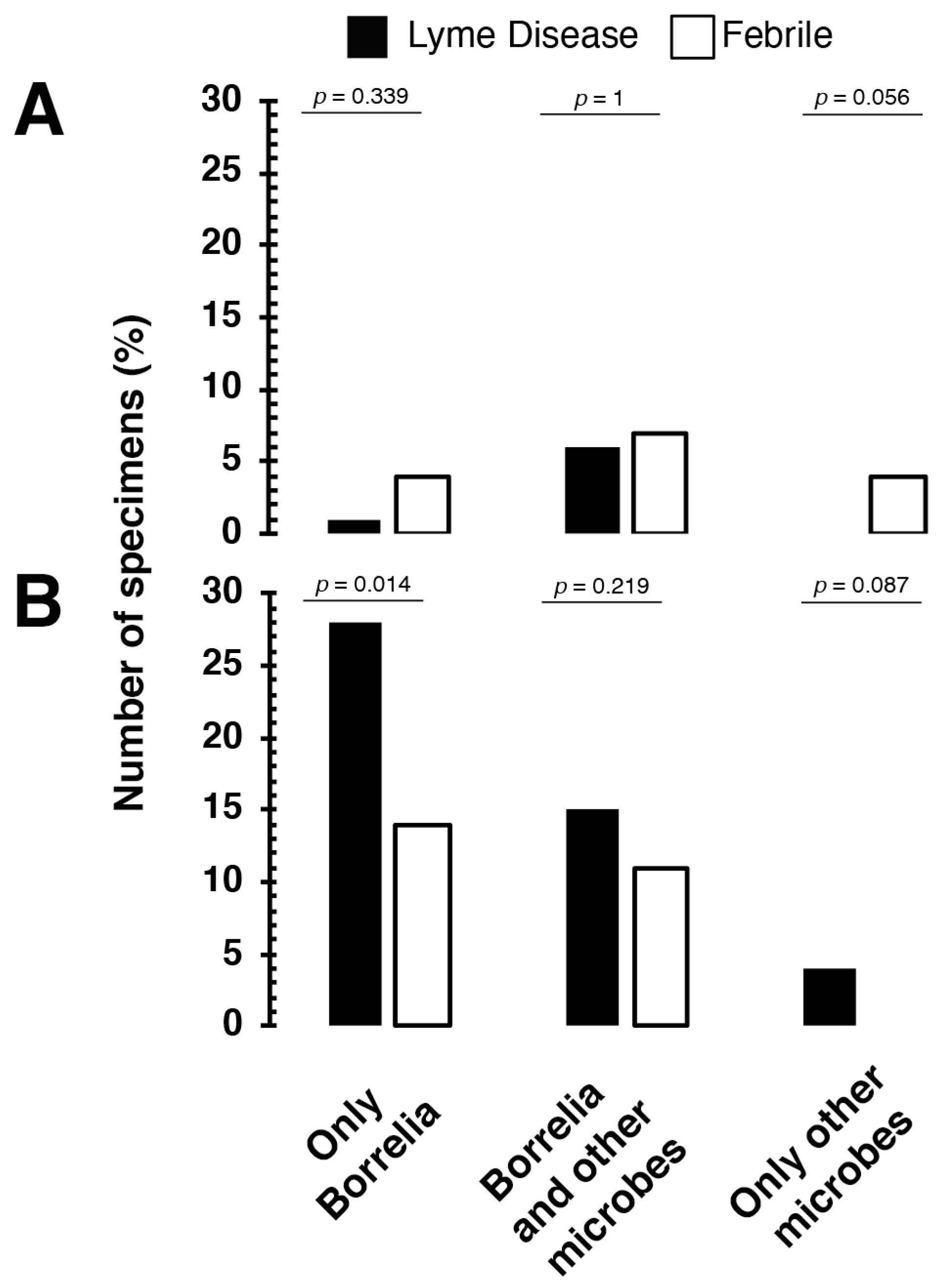
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguero-Rosenfeld, M.E.; Wang, G.; Schwartz, I.; Wormser, G.P. Diagnosis of lyme borreliosis. Clin. Microbiol Rev. 2005, 18, 484–509. [Google Scholar] [CrossRef]
- Wilske, B. Epidemiology and diagnosis of Lyme borreliosis. Ann. Med. 2005, 37, 568–579. [Google Scholar] [CrossRef]
- Aberer, E.; Kersten, A.; Klade, H.; Poitschek, C.; Jurecka, W. Heterogeneity of Borrelia burgdorferi in the skin. Am. J. Dermatopathol. 1996, 18, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.J. Lyme Disease: Neurology, Neurobiology, and Behavior. Clin. Infect. Dis. 2014, 58, 1267–1272. [Google Scholar] [CrossRef]
- Lempereur, L.; Shiels, B.; Heyman, P.; Moreau, E.; Saegerman, C.; Losson, B.; Malandrin, L. A retrospective serological survey on human babesiosis in Belgium. Clin. Microbiol. Infect. 2015, 21, 96.e1–96.e7. [Google Scholar] [CrossRef] [PubMed]
- Grab, D.J.; Nyarko, E.; Barat, N.C.; Nikolskaia, O.V.; Dumler, J.S. Anaplasma phagocytophilum-Borrelia burgdorferi Coinfection Enhances Chemokine, Cytokine, and Matrix Metalloprotease Expression by Human Brain Microvascular Endothelial Cells. Clin. Vaccine Immunol. 2007, 14, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human Ehrlichiosis and Anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Steffen, R. Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western/Central Europe and conclusions on vaccination recommendations. J. Travel Med. 2016, 23. [Google Scholar] [CrossRef][Green Version]
- Davidsson, M. The Financial Implications of a Well-Hidden and Ignored Chronic Lyme Disease Pandemic. Healthcare 2018, 6, 16. [Google Scholar] [CrossRef]
- Karim, S.; Budachetri, K.; Mukherjee, N.; Williams, J.; Kausar, A.; Hassan, M.; Adamson, S.; Dowd, S.E.; Apanskevich, D.; Arijo, A.; et al. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Neglect. Trop. D 2017, 11, e0005681. [Google Scholar] [CrossRef]
- Laaksonen, M.; Klemola, T.; Feuth, E.; Sormunen, J.J.; Puisto, A.; Mäkelä, S.; Penttinen, R.; Ruohomäki, K.; Hänninen, J.; Sääksjärvi, I.E.; et al. Tick-borne pathogens in Finland: Comparison of Ixodes ricinus and I. persulcatus in sympatric and parapatric areas. Parasite Vector 2018, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Sormunen, J.J.; Penttinen, R.; Klemola, T.; Hänninen, J.; Vuorinen, I.; Laaksonen, M.; Sääksjärvi, I.E.; Ruohomäki, K.; Vesterinen, E.J. Tick-borne bacterial pathogens in southwestern Finland. Parasite Vector 2016, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Sormunen, J.J.; Andersson, T.; Aspi, J.; Bäck, J.; Cederberg, T.; Haavisto, N.; Halonen, H.; Hänninen, J.; Inkinen, J.; Kulha, N.; et al. Monitoring of ticks and tick-borne pathogens through a nationwide research station network in Finland. Ticks Tick Borne Dis. 2020, 11, 101449. [Google Scholar] [CrossRef]
- DeLong, A.; Hsu, M.; Kotsoris, H. Estimation of cumulative number of post-treatment Lyme disease cases in the US, 2016 and 2020. BMC Public Health 2019, 19, 352. [Google Scholar] [CrossRef] [PubMed]
- Kugeler, K.J.; Eisen, R.J. Challenges in Predicting Lyme Disease Risk. JAMA Netw. Open 2020, 3, e200328. [Google Scholar] [CrossRef]
- Stone, B.L.; Tourand, Y.; Brissette, C.A. Brave New Worlds: The Expanding Universe of Lyme Disease. Vector-Borne Zoonot 2017, 17, 619–629. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). How Many People Get Lyme Disease? Available online: https://www.cdc.gov/lyme/stats/humancases.html (accessed on 16 February 2019).
- European Centre for Disease Prevention and Control. ECDC Comment: European Commission Updates Communicable Disease Surveillance List—Lyme Neuroborreliosis Now under EU/EEA Surveillance. Available online: https://www.ecdc.europa.eu/en/news-events/ecdc-comment-european-commission-updates-communicable-disease-surveillance-list-lyme (accessed on 28 May 2019).
- Parliament, E. European Parliament Resolution of 15 November 2018 on Borrelia Infection. Strasbourg 2018. Available online: https://www.europarl.europa.eu/doceo/document/TA-8-2018-0465_SV.html?redirect (accessed on 28 May 2019).
- Eldin, C.; Raffetin, A.; Bouiller, K.; Hansmann, Y.; Roblot, F.; Raoult, D.; Parola, P. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Médecine Mal. Infect 2019, 49, 121–132. [Google Scholar] [CrossRef]
- Waddell, L.A.; Greig, J.; Mascarenhas, M.; Harding, S.; Lindsay, R.; Ogden, N. The Accuracy of Diagnostic Tests for Lyme Disease in Humans, A Systematic Review and Meta-Analysis of North American Research. PLoS ONE 2016, 11, e0168613. [Google Scholar] [CrossRef]
- Leeflang, M.M.; Ang, C.W.; Berkhout, J.; Bijlmer, H.A.; Bortel, W.V.; Brandenburg, A.H.; Burgel, N.D.V.; Dam, A.P.V.; Dessau, R.B.; Fingerle, V.; et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta-analysis. BMC Infect Dis. 2016, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Branda, J.A.; Strle, K.; Nigrovic, L.E.; Lantos, P.M.; Lepore, T.J.; Damle, N.S.; Ferraro, M.J.; Steere, A.C. Evaluation of Modified 2-Tiered Serodiagnostic Testing Algorithms for Early Lyme Disease. Clin. Infect. Dis. 2017, 64, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR. Morb. Mortal. Wkly. Rep. 1995, 44, 590–591. [Google Scholar]
- Garg, K.; Meriläinen, L.; Franz, O.; Pirttinen, H.; Quevedo-Diaz, M.; Croucher, S.; Gilbert, L. Evaluating polymicrobial immune responses in patients suffering from tick-borne diseases. Sci. Rep. 2018, 8, 15932. [Google Scholar] [CrossRef]
- Connally, N.P.; Hinckley, A.F.; Feldman, K.A.; Kemperman, M.; Neitzel, D.; Wee, S.-B.; White, J.L.; Mead, P.S.; Meek, J.I. Testing practices and volume of non-Lyme tickborne diseases in the United States. Ticks Tick-Borne Dis. 2016, 7, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.J.; Puri, B.K. Commercial test kits for detection of Lyme borreliosis: A meta-analysis of test accuracy. Int. J. Gen. Med. 2016, 9, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Chiao, J.W.; Pavia, C.; Riley, M.; Altmann-Lasekan, W.; Abolhassani, M.; Liegner, K.; Mittelman, A. Antigens of Lyme disease of spirochaete Borrelia burgdorferi inhibits antigen or mitogen-induced lymphocyte proliferation. FEMS Immunol. Med. Mic. 1994, 8, 151–155. [Google Scholar] [CrossRef]
- Aberer, E.; Koszik, F.; Silberer, M. Why is chronic Lyme borreliosis chronic? Clin. Infect. Dis. 1997, 25, S64–S70. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Zhang, N.; Wooten, R.M. Borrelia burgdorferi elicited-IL-10 suppresses the production of inflammatory mediators, phagocytosis, and expression of co-stimulatory receptors by murine macrophages and/or dendritic cells. PLoS ONE 2013, 8, e84980. [Google Scholar] [CrossRef] [PubMed]
- Jarefors, S.; Janefjord, C.K.; Forsberg, P.; Jenmalm, M.C.; Ekerfelt, C. Decreased up-regulation of the interleukin-12Rbeta2-chain and interferon-gamma secretion and increased number of forkhead box P3-expressing cells in patients with a history of chronic Lyme borreliosis compared with asymptomatic Borrelia-exposed individuals. Clin. Exp. Immunol. 2007, 147, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Elsner, R.A.; Hastey, C.J.; Baumgarth, N. CD4+ T cells promote antibody production but not sustained affinity maturation during Borrelia burgdorferi infection. Infect Immun. 2015, 83, 48–56. [Google Scholar] [CrossRef]
- Elsner, R.A.; Hastey, C.J.; Olsen, K.J.; Baumgarth, N. Suppression of Long-Lived Humoral Immunity Following Borrelia burgdorferi Infection. PLoS Pathog. 2015, 11, e1004976. [Google Scholar] [CrossRef]
- Tunev, S.S.; Hastey, C.J.; Hodzic, E.; Feng, S.; Barthold, S.W.; Baumgarth, N. Lymphoadenopathy during lyme borreliosis is caused by spirochete migration-induced specific B cell activation. PLoS Pathog. 2011, 7, e1002066. [Google Scholar] [CrossRef] [PubMed]
- Hastey, C.J.; Elsner, R.A.; Barthold, S.W.; Baumgarth, N. Delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J. Immunol. 2012, 188, 5612–5622. [Google Scholar] [CrossRef] [PubMed]
- Aberer, E.; Schwantzer, G. Course of Antibody Response in Lyme Borreliosis Patients before and after Therapy. ISRN Immunol 2012, 1–4. [Google Scholar] [CrossRef][Green Version]
- Lomholt, H.; Lebech, A.M.; Hansen, K.; Brandrup, F.; Halkier-Sørensen, L. Long-term serological follow-up of patients treated for chronic cutaneous borreliosis or culture-positive erythema migrans. Acta Derm. Venereol. 2000, 80, 362–366. [Google Scholar]
- Tracy, K.E.; Baumgarth, N. Borrelia burgdorferi Manipulates Innate and Adaptive Immunity to Establish Persistence in Rodent Reservoir Hosts. Front Immunol. 2017, 8, 116. [Google Scholar] [CrossRef]
- Vayssier-Taussat, M.; Kazimirova, M.; Hubalek, Z.; Hornok, S.; Farkas, R.; Cosson, J.-F.; Bonnet, S.; Vourch, G.; Gasqui, P.; Mihalca, A.D.; et al. Emerging horizons for tick-borne pathogens: From the “one pathogen-one disease” vision to the pathobiome paradigm. Future Microbiol. 2015, 10, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Aucott, J.; Morrison, C.; Munoz, B.; Rowe, P.C.; Schwarzwalder, A.; West, S.K. Diagnostic challenges of early Lyme disease: Lessons from a community case series. BMC Infect Dis. 2009, 9, 79. [Google Scholar] [CrossRef]
- Aucott, J.N.; Seifter, A. Misdiagnosis of early Lyme disease as the summer flu. Orthop. Rev. 2011, 3, e14. [Google Scholar] [CrossRef]
- Johnson, L.; Wilcox, S.; Mankoff, J.; Stricker, R.B. Severity of chronic Lyme disease compared to other chronic conditions: A quality of life survey. PeerJ 2014, 2, e322. [Google Scholar] [CrossRef]
- Berghoff, W. Chronic Lyme Disease and Co-infections: Differential Diagnosis. Open Neurol. J. 2012, 6, 158–178. [Google Scholar] [CrossRef]
- Maggi, R.G.; Mozayeni, B.R.; Pultorak, E.L.; Hegarty, B.C.; Bradley, J.M.; Correa, M.; Breitschwerdt, E.B. Bartonella spp. bacteremia and rheumatic symptoms in patients from Lyme disease-endemic region. Emerg. Infect Dis. 2012, 18, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, M.; Haier, J.; Nicolson, G. Multiple Mycoplasmal Infections Detected in Blood of Patients with Chronic Fatigue Syndrome and/or Fibromyalgia Syndrome. Eur. J. Clin. Microbiol. Infect Dis. 1999, 18, 859–865. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Gan, R.; Haier, J. Multiple co-infections (Mycoplasma, Chlamydia, human herpes virus-6) in blood of chronic fatigue syndrome patients: Association with signs and symptoms. Apmis 2003, 111, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Staunton, C.; Slokenberga, S.; Mascalzoni, D. The GDPR and the research exemption: Considerations on the necessary safeguards for research biobanks. Eur. J. Hum. Genet. 2019, 27, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Powers, M.L.; Gronowski, K.S.; Gronowski, A.M. Human Tissue Ownership and Use in Research: What Laboratorians and Researchers Should Know. Clin. Chem. 2010, 56, 1675–1682. [Google Scholar] [CrossRef]
- van Diest, P.J.; Savulescu, J. For and against: No consent should be needed for using leftover body material for scientific purposes For Against. BMJ 2002, 325, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Health, M. of S.A. and Act on the Medical Use of Human Organs, Tissues and Cells. Available online: https://www.finlex.fi/fi/laki/ajantasa/2001/20010101 (accessed on 20 February 2019).
- Health, M. of S.A. and Medical Research Act. Available online: https://www.finlex.fi/en/laki/kaannokset/1999/en19990488 (accessed on 20 February 2019).
- Standardization, I.O. for ISO 15189:2012 Medical Laboratories—Requirements for Quality and Competence. Available online: https://www.iso.org/standard/56115.html (accessed on 20 February 2019).
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F.; Petrie, A. Method agreement analysis: A review of correct methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent. Endod. 2017, 42, 152. [Google Scholar] [CrossRef]
- Kodym, P.; Kurzová, Z.; Berenová, D.; Pícha, D.; Smíšková, D.; Moravcová, L.; Malý, M. Serological Diagnostics of Lyme Borreliosis: Comparison of Universal and Borrelia Species-Specific Tests Based on Whole-Cell and Recombinant Antigens. J. Clin. Microbiol. 2018, 56, e00601–e006018. [Google Scholar] [CrossRef]
- Krause, P.J.; Telford, S.R.; Spielman, A.; Sikand, V.; Ryan, R.; Christianson, D.; Burke, G.; Brassard, P.; Pollack, R.; Peck, J.; et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 1996, 275, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Loebermann, M.; Fingerle, V.; Lademann, M.; Fritzsche, C.; Reisinger, E.C. Borrelia burgdorferi and Anaplasma phagocytophilum Coinfection. Emerg. Infect Dis. 2006, 12, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Bjöersdorff, A.; Wittesjö, B.; Berglund, J.; Massung, R.F.; Eliasson, I. Human Granulocytic Ehrlichiosis as a Common Cause of Tick-associated Fever in Southeast Sweden: Report from a Prospective Clinical Study. Scand. J. Infect Dis. 2009, 34, 187–191. [Google Scholar] [CrossRef]
- Koetsveld, J.; Tijsse-Klasen, E.; Herremans, T.; Hovius, J.W.; Sprong, H. Serological and molecular evidence for spotted fever group Rickettsia and Borrelia burgdorferi sensu lato co-infections in The Netherlands. Ticks Tick-Borne Dis. 2016, 7, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Nakao, R.; Ohnuma, A.; Kawamori, F.; Sugimoto, C. Microbial Population Analysis of the Salivary Glands of Ticks; A Possible Strategy for the Surveillance of Bacterial Pathogens. PLoS ONE 2014, 9, e103961. [Google Scholar] [CrossRef] [PubMed]
- Klemola, T.; Sormunen, J.J.; Mojzer, J.; Mäkelä, S.; Vesterinen, E.J. High tick abundance and diversity of tick-borne pathogens in a Finnish city. Urban Ecosyst. 2019, 22, 817–826. [Google Scholar] [CrossRef]
- Junttila, J.; Tanskanen, R.; Tuomi, J. Prevalence of Borrelia burgdorferi in selected tick populations in Finland. Scand J. Infect Dis 1994, 26, 349–355. [Google Scholar] [CrossRef]
- Haapasalo, K.; Suomalainen, P.; Sukura, A.; Siikamaki, H.; Jokiranta, T.S. Fatal babesiosis in man, Finland, 2004. Emerg. Infect Dis. 2010, 16, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Dhar, A.; Hernandez, J.; Fischer, P.A.; Sikand, V.K.; Schoen, R.T.; Nowakowski, J.; McHugh, G.; Persing, D.H. Systemic symptoms without erythema migrans as the presenting picture of early Lyme disease. Am. J. Med. 2003, 114, 58–62. [Google Scholar] [CrossRef]
- Nowakowski, J.; Schwartz, I.; Liveris, D.; Wang, G.; Aguero-Rosenfeld, M.E.; Girao, G.; McKenna, D.; Nadelman, R.B.; Cavaliere, L.F.; Wormser, G.P.; et al. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: A comparison of different techniques. Clin. Infect. Dis. 2001, 33, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Reed, K.D.; Mitchell, P.D.; Mueller-Rizner, N.; Vandermause, M.; Finkel, M.F.; Kazmierczak, J.J. Tickborne Infections as a Cause of Nonspecific Febrile Illness in Wisconsin. Clin. Infect. Dis. 2001, 32, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, U.; Perret-Liaudet, A.; van Doorn, L.J.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, B.H.; et al. A Practical Guide to Immunoassay Method Validation. Front. Neurol. 2015, 6, 179. [Google Scholar] [CrossRef]
- Crowther, J.R. The ELISA Guidebook; Humana Press Inc.: Totowa, NJ, USA, 2001; Volume 149. [Google Scholar]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Meriläinen, L.; Brander, H.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Pleomorphic forms of Borrelia burgdorferi induce distinct immune responses. Microb Infect 2016, 18, 484–495. [Google Scholar] [CrossRef]
- Diterich, I.; Rauter, C.; Kirschning, C.J.; Hartung, T. Borrelia burgdorferi-induced tolerance as a model of persistence via immunosuppression. Infect Immun. 2003, 71, 3979–3987. [Google Scholar] [CrossRef]
- Tuuminen, T.; Hedman, K.; Söderlund-Venermo, M.; Seppälä, I. Acute Parvovirus B19 Infection Causes Nonspecificity Frequently in Borrelia and Less Often in Salmonella and Campylobacter Serology, Posing a Problem in Diagnosis of Infectious Arthropathy. Clin. Vaccine Immunol. 2011, 18, 167–172. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, K.; Jokiranta, T.S.; Filén, S.; Gilbert, L. Assessing the Need for Multiplex and Multifunctional Tick-Borne Disease Test in Routine Clinical Laboratory Samples from Lyme Disease and Febrile Patients with a History of a Tick Bite. Trop. Med. Infect. Dis. 2021, 6, 38. https://doi.org/10.3390/tropicalmed6010038
Garg K, Jokiranta TS, Filén S, Gilbert L. Assessing the Need for Multiplex and Multifunctional Tick-Borne Disease Test in Routine Clinical Laboratory Samples from Lyme Disease and Febrile Patients with a History of a Tick Bite. Tropical Medicine and Infectious Disease. 2021; 6(1):38. https://doi.org/10.3390/tropicalmed6010038
Chicago/Turabian StyleGarg, Kunal, T. Sakari Jokiranta, Sanna Filén, and Leona Gilbert. 2021. "Assessing the Need for Multiplex and Multifunctional Tick-Borne Disease Test in Routine Clinical Laboratory Samples from Lyme Disease and Febrile Patients with a History of a Tick Bite" Tropical Medicine and Infectious Disease 6, no. 1: 38. https://doi.org/10.3390/tropicalmed6010038
APA StyleGarg, K., Jokiranta, T. S., Filén, S., & Gilbert, L. (2021). Assessing the Need for Multiplex and Multifunctional Tick-Borne Disease Test in Routine Clinical Laboratory Samples from Lyme Disease and Febrile Patients with a History of a Tick Bite. Tropical Medicine and Infectious Disease, 6(1), 38. https://doi.org/10.3390/tropicalmed6010038







