Abstract
Schistosomiasis is a parasitic disease affecting more than 250 million people, primarily in sub-Saharan Africa. In Côte d’Ivoire both Schistosoma haematobium (causing urogenital schistosomiasis) and Schistosoma mansoni (causing intestinal schistosomiasis) co-exist. This study aimed to determine the prevalence of S. haematobium and S. mansoni and to identify risk factors among schoolchildren in the western and southern parts of Côte d’Ivoire. From January to April 2018, a cross-sectional study was carried out including 1187 schoolchildren aged 5–14 years. Urine samples were examined by a filtration method to identify and count S. haematobium eggs, while stool samples were subjected to duplicate Kato-Katz thick smears to quantify eggs of S. mansoni and soil-transmitted helminths. Data on sociodemographic, socioeconomic, and environmental factors were obtained using a pretested questionnaire. Multivariate logistic regression was employed to test for associations between variables. We found a prevalence of S. haematobium of 14.0% (166 of 1187 schoolchildren infected) and a prevalence of S. mansoni of 6.1% (66 of 1089 schoolchildren infected). In the southern part of Côte d’Ivoire, the prevalence of S. haematobium was 16.1% with a particularly high prevalence observed in Sikensi (35.6%), while S. mansoni was most prevalent in Agboville (11.2%). Swimming in open freshwater bodies was the main risk factor for S. haematobium infection (adjusted odds ratio (AOR) = 127.0, 95% confidence interval (CI): 25.0–634.0, p < 0.001). Fishing and washing clothes in open freshwater bodies were positively associated with S. haematobium and S. mansoni infection, respectively. Preventive chemotherapy using praziquantel should be combined with setting-specific information, education, and communication strategies in order to change children’s behavior, thus avoiding contact with unprotected open freshwater.
1. Introduction
Schistosomiasis is a water-based chronic parasitic disease caused by trematode worms of the genus Schistosoma. Considered as a neglected tropical disease by the World Health Organization (WHO), schistosomiasis affects more than 250 million people worldwide with an estimated global burden of 1.4 million disability-adjusted life years (DALYs) in 2017 [1,2,3]. Schistosomiasis remains a public health problem in countries of the tropics and subtropics with approximately 90% of cases concentrated in Africa [3,4]. Humans are the definitive host for adult parasites, while specific freshwater snails act as intermediate hosts [3,4]. Hence, the transmission of schistosomiasis is governed by social-ecological systems (e.g., conditions of poverty and living near open freshwater bodies) [5]. Schistosome eggs are excreted by humans with feces or urine. After hatching, miracidia infect specific snails to produce cercariae. Schistosome cercariae penetrate the unbroken skin of humans during domestic (e.g., washing clothes or dishes) and recreational activities (e.g., bathing and swimming in unprotected open freshwater bodies). Various factors have been shown to facilitate transmission of schistosomiasis in Africa, such as living in close proximity to freshwater bodies (e.g., rivers, small dams, irrigation schemes, and lakes), socioeconomic factors which influence occupational activities (e.g., poor people without running water at home are likely to contact freshwater bodies) and climate change [6,7,8]. The lack of access to improved sanitation contributes to open defecation, which results in environmental contamination that enhances the transmission of schistosomiasis [9].
In Côte d’Ivoire, snails of the genera Biomphalaria and Bulinus are the intermediate hosts for Schistosoma mansoni and Schistosoma haematobium, respectively [10]. While both S. mansoni and S. haematobium are endemic in Côte d’Ivoire [11], the former species is predominantly found in the western part of the country [12,13] and S. haematobium is mostly present in the central and southern parts [9,14]. In northern Côte d’Ivoire, a recent study reported low prevalence rates of 1.9% and 3.5% for S. haematobium and S. mansoni among school-aged children, respectively [15]. To enhance control efforts and shift the focus from morbidity control toward interruption of transmission, fine-grained information on the distribution of the disease is important, including underlying risk factors.
The purpose of this study was to determine the prevalence of schistosomiasis and risk factors among schoolchildren in the western and southern parts of Côte d’Ivoire where the disease is most prevalent. The results will assist public health authorities of Côte d’Ivoire to refine control measures and complement preventive chemotherapy with specific information about infection prevalence and intensity, and to enhance education and communication approaches that are readily tailored to specific social-ecological contexts.
2. Materials and Methods
2.1. Study Settings and Population
The study was carried out in two settings of Côte d’Ivoire, including four health districts: (i) Agboville (geographical coordinates: 5° 55′ 41″ N latitude, 4° 13′ 01″ W longitude); (ii) Adzopé (6° 06′ 25″ N, 3° 51′ 36″ W); and (iii) Sikensi (5° 40′ 34″ N, 4° 34′ 33″ W), all located in the southern part of Côte d‘Ivoire; and (iv) Duekoué (6° 44′ 00″ N, 7° 21′ 00″ W) in the western part (Figure 1). We included one health district from the western part of Côte d’Ivoire in order to contrast with the three health districts in the South, thus enriching ecological features (the western part of Côte d’Ivoire is hilly as opposed to mainly flat terrain in the South) and biological characteristics (setting-specific parasite-intermediate host systems). In view of limited financial and human resources, we were unable to include the same amount of health districts in the western compared to the southern setting of Côte d’Ivoire.
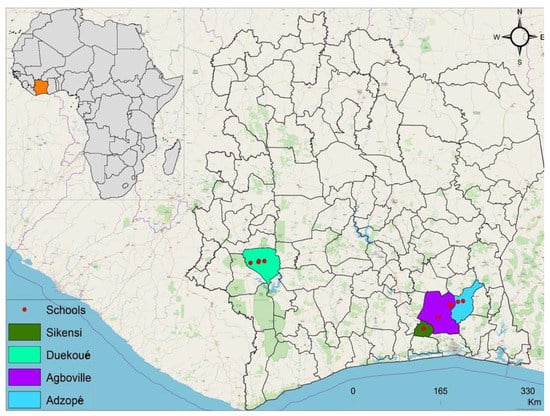
Figure 1.
Collection of maps displaying the study area in Côte d’Ivoire, West Africa: Adzopé, Agboville, and Sikensi in the southern setting; Duekoué in the western setting of Côte d’Ivoire. Primary schools (red dots) were selected in each health district on the basis of close proximity to open freshwater bodies (distance: < 10 km).
Subsistence farming is the main economic activity in all study settings, which are well known for their endemicity of S. haematobium [16] and S. mansoni [12,17]. In each of the health districts, there are rivers which act as main transmission sites for schistosomiasis [18]. Of note, Duekoué is additionally appreciated as tourist destination. The Guemon River is the predominant river running right through the city. Sikensi has several rivers that discharge in the Agnéby River. Water flowing through Agboville also discharges in the Agnéby River [19]. Adzopé shares rivers from different catchment areas [18].
The study population consisted of schoolchildren aged 5–14 years. Schools were chosen on the basis of their proximity to a stream, river, lake, or backwater used by children at a distance of less than 10 km.
2.2. Design and Sample Size
A cross-sectional study was carried out in the two study settings from January to April 2018. The sample size (n) was adjusted to 1187 children based on the following formula: n = (Z2 × p (1 − p) × C) / i2, where Z = 1.96, p = 40% is the prevalence expected based on a previous study [9], i is the precision or margin of the error (5%), and C is the correction coefficient (C = 2).
2.3. Data and Sample Collection
Only schoolchildren aged 5–14 years who had lived in the study area for at least one year prior to the survey were included. The number of children per school was proportionally allocated according to population size in each school. Children were randomly selected using readily available school lists and identified by unique codes. A questionnaire was administered to collect data about each child’s habits and behaviors, such as swimming/bathing in open freshwater bodies, washing clothes in rivers, and fishing. In total, 1187 urine and 1089 stool samples were collected from children in plastic containers and transferred to nearest health centres for parasitological examination. Urine samples were collected between 10 a.m. and 12 a.m. [20].
2.4. Parasitological Examination
A urine filtration method was employed to identify S. haematobium eggs [21]. In brief, 10 mL of urine was vigorously shaken and filtered through a Nytrel filter with a 40 µm mesh size and examined microscopically for the presence of S. haematobium eggs that were counted by experienced laboratory technicians. Stool samples were subjected to the Kato-Katz technique [22]. Two thick smears from each stool sample were microscopically examined to identify and quantify eggs of S. mansoni and soil-transmitted helminths.
After examination, all schoolchildren were treated with a single 40 mg/kg oral dose of praziquantel (600 mg; Biltricide, Bayer, Leverkusen, Germany) through the "Programme National de Lutte contre les Maladies Tropicales Negligées à Chimioprophylaxie Préventive" (PNLMTN-CP) of Côte d’Ivoire. Children with soil-transmitted helminths were treated with albendazole (400 mg).
2.5. Statistical Analysis
Statistical analyses were performed with STATA version 15.0 (Stata Corporation; College Station, TX, USA). Univariate analysis (χ2 and Fisher’s exact test, as appropriate) was used for comparison between groups. Children were stratified into three age groups (5–8, 9–11, and 12–14 years). Parasitic infections were defined as positive for S. haematobium or S. mansoni when at least one egg was identified in a urine or stool sample, respectively. Associations between parasitic infections and sociodemographic, socioeconomic, or environmental factors were assessed by mixed multivariable logistic regression models with random intercepts for schools and for classes nested within schools. The study area was used as a fixed factor. The risk factors investigated were occupation and educational attainment of parents/legal guardians, and swimming, fishing, and playing in freshwater by children. Associations and differences with a p-value below 0.05 were considered statistically significant.
2.6. Ethical Consideration
Ethical clearance was obtained from the Ministère de la Santé et de l’Hygiène Publique de Côte d’Ivoire (reference no. 003–18/MSHP/CNER-kp). School authorities, teachers, parents/guardians, and participants were informed about the objectives, procedures, and potential risks and benefits of the study. Written informed consent was obtained from children’s parents or legal guardians. Oral assent was obtained from children.
3. Results
3.1. Sociodemographic Characteristics of the Population
A total of 1187 schoolchildren were included in the study. There were considerably more boys than girls (61.2% vs. 38.8%) and the highest proportion of children was included in Agboville. The mean age was 9.9 years (standard deviation (SD) = 2.4 years) with a median age of 10 years. Children aged 9–11 years were the most common age class in Adzopé (43.8%), Duekoué (40.3%), and Sikensi (54.1%) but the least common age class in Agboville (31.6%). Table 1 shows the sociodemographic characteristics of the study population, stratified by setting.

Table 1.
Sociodemographic characteristics of the study population subjected to schistosomiasis diagnosis in different settings of Côte d’Ivoire in early 2018.
3.2. Urine and Stool Examination
3.2.1. Infection with S. haematobium
All 1187 schoolchildren included in the study provided a single urine sample (100%). S. haematobium eggs were found in 166 of the children, owing to an overall prevalence of 14.0% (95% confidence interval (CI): 12.1%–16.1%). The prevalence of S. haematobium was considerably higher in the three school locations of the southern compared with the western Côte d’Ivoire (16.1% vs. 9.4%). The highest prevalence was found in Sikensi (35.6%). Boys and girls showed similar S. haematobium prevalence (14.2% vs. 13.7%; p = 0.781). No statistically significant difference was observed in the prevalence between age groups (p = 0.337).
Two cases of co-infection with S. haematobium and S. mansoni were found in Agboville. In two children from Duekoué, eggs identified in urine samples were morphologically determined as S. mansoni (Table 2).

Table 2.
Prevalence rate of Schistosoma haematobium and Schistosoma mansoni infection, stratified by study settings, sex, and age group among schoolchildren from Côte d’Ivoire in early 2018.
3.2.2. Infection with S. mansoni
Stool samples were obtained from 1089 children (91.7%). The overall prevalence of S. mansoni infection was 6.1% (95% CI: 4.8%–7.6%). S. mansoni was most commonly found in Agboville (11.2%), while no infections were found in Sikensi. Age and sex were not associated with S. mansoni infection (p > 0.05) (Table 2). The arithmetic mean of S. mansoni eggs per gram of stool (EPG), including standard error (SE) from positive samples, was 91.1 EPG (SE: 11.2 EPG; 95% CI: 68.7–113.4 EPG) with a minimum and maximum of 20 and 400 EPG, respectively. The geometric mean of S. mansoni eggs from positive stool samples was 4.1 (SD: 0.9).
3.2.3. Other Helminths and Co-Infection
Three species of soil-transmitted helminths were identified in stool samples at very low rates: Trichuris trichiura (2.3%), Ascaris lumbricoides (1.7%), and hookworm (0.2%). In two school locations, children with concurrent Schistosoma and soil-transmitted helminth infections were identified; in Sikensi (S. haematobium-T. trichiura and S. haematobium-hookworm) and in Agboville (S. mansoni-A. lumbricoides, S. mansoni-T. trichiura, and triple species infection with S. mansoni, A. lumbricoides, and T. trichiura).
3.2.4. Multivariate Logistic Regression Models
Table 3 shows the association between Schistosoma infection and sociodemographic factors, socioeconomic status, and environmental factors. The key risk factors for S. haematobium were swimming (adjusted odds ratio (AOR): 127.0; 95% CI: 25.0–634.0) and playing in water (AOR: 74.0; 95% CI: 3.8–144.3). For S. mansoni, children who lacked tap water at home (AOR: 2.7; 95% CI: 1.2–5.8) and who washed their clothes in open freshwater bodies (AOR: 5.3; 95% CI: 2.3–12.1) were the most infected.

Table 3.
Multivariate logistic regression model analysis of variables associated with S. haematobium and S. mansoni infection among schoolchildren non-adjusted and adjusted for sociodemographic factors, socioeconomic status, and environmental factors.
The educational status as illiterate, of fathers (crude odds radio (COR): 3.3; 95% CI: 1.0–10.9) and mothers (COR: 11.5; 95% CI: 1.6–84.0) were also significantly associated with S. mansoni infection.
4. Discussion
This study was designed to determine the prevalence of the two known human Schistosoma species and to identify risk factors associated with infection among 5–14 year-old schoolchildren in southern and western parts of Côte d’Ivoire. We employed widely used diagnostic methods; namely a filtration method for detection and quantification of S. haematobium eggs in urine samples and the Kato-Katz technique for detection and quantification of S. mansoni (and soil-transmitted helminth) eggs in fecal samples. We found an overall prevalence of 14.0% for S. haematobium and 6.1% for S. mansoni, which classify our study settings as moderate and low endemic areas, respectively, for urogenital schistosomiasis and intestinal schistosomiasis, according to WHO guidelines [23]. The arithmetic mean of S. mansoni egg counts (91.9 EPG) recorded was low and would be classified as a light infection.
As expected, the current study reports a low prevalence of S. haematobium in Duekoué in the western part of Côte d’Ivoire, corroborating results from previous studies [11,17]. The highest prevalence of S. mansoni was found in Agboville, in one of the three school locations included in the southern part of Côte d’Ivoire. Similar studies showed the prevalence to be lower for S. haematobium (0.9%–4.4%), and higher for S. mansoni (17.5%–61.3%) in western Côte d’Ivoire [24]. Another study reported a high prevalence of S. mansoni (58.7%–68.4%) and low prevalence for S. haematobium (10.9%–18.4%) in southern Côte d’Ivoire [9]. The difference between the prevalence of the two schistosome species could be explained by the variation in ecological factors that influence the transmission dynamics. The low prevalence rate of S. haematobium and S. mansoni infections reported in our study is most likely the result of preventive chemotherapy campaigns pursued on an annual basis since several years by the PNLMTN-CP in Côte d’Ivoire[25].
We found similar prevalence rates for boys and girls, corroborating results from previous studies in Côte d’Ivoire [9]. However, it must be noted that the number of boys in our final study sample was considerably higher than that of girls (727 vs. 460), which was particularly pronounced in Sikensi in the southern part (142 vs. 63) and Duekoué in the western part of Côte d’Ivoire (240 vs. 132). This observation is in line with a large epidemiological study conducted in the late 1990s in the Man region of western Côte d’Ivoire. Among 12,227 children interviewed from 121 schools, there were 7489 boys and 4738 girls, pointing to a gender-bias in terms of school enrolment that tended to increase with age of children [26]. Interestingly, prior and current observations from Côte d’Ivoire are in contrast to a study from Senegal, where boys showed a higher prevalence of Schistosoma infection [27]. Results from other studies showed that when children are in contact with freshwater bodies for longer periods of time, they are more likely to be infected [9,28]. The risk of disease occurrence can increase because children are more often involved in recreational activities; hence, they are exposed to unprotected open surface freshwater for longer periods [29].
S. mansoni eggs, unmistakendly characterised by a lateral spine, were identified in two urine samples obtained from schoolchildren in Duekoué. The appearance of S. mansoni eggs in urine is unusual and has not been studied extensively [30]. In our investigation, Schistosoma species were determined by widely used methods based on egg morphology and light microscopy. Future studies should employ concurrent molecular approaches to improve diagnostic sensitivity.
In the current study, no differences in the prevalence of Schistosoma infection were observed among the three investigated age groups, which is in line with several other studies [20,31,32]. However, there are also studies reporting an increase in the prevalence with age of children [33,34]. Indeed, children aged 10–14 years can become more vulnerable for schistosomiasis during recreational activities, i.e., swimming and playing in water, or while fetching water for household use, or agriculture activities [35,36]. Most of the children in our study who did not have tap water at home were infected by S. mansoni, contrary to results reported from South Africa [34].
Socioeconomic factors were significantly associated with the occurrence of schistosomiasis. In particular, a significant relationship between illiteracy of the parents/guardians and S. mansoni infection was reported. Similar results were found in a previous study in Nigeria, which showed that better educated parents can understand the preventive campaigns more deeply and thus better explain them to their children [35].
The association between the occurrence of schistosomiasis and contact with freshwater bodies is well documented. In our study, swimming and fishing in freshwater by schoolchildren was strongly associated with infection with S. haematobium. This might be explained by the fact that, while swimming, infected children emit urine with S. haematobium eggs. The eggs hatch, infecting snails which produce furcocercariae that penetrate the skin of children exposed to the contaminated water. This corroborates the findings of other studies that reported high infection rates among children who swim in rivers [25,37]. Other researchers did not report a significant correlation between swimming and occurrence of schistosomiasis [20,38]. Our finding that washing clothes in water and lack of tap water at home were associated with the occurrence of S. mansoni infection is in line with results from other studies [9,34].
The low prevalence of any of the three common soil-transmitted helminths in our study confirms observations from previous surveys [39,40]. This observation is likely attributed to large-scale preventive chemotherapy campaigns by the PNLMTN-CP, coupled with systematic sensitization and deworming carried out by non-governmental organizations (NGOs) and improvements in sanitation in face of social and economic development.
The current study has several limitations, and hence, the findings should be interpreted with care. First, stool and urine samples were collected only on a single day, though duplicate Kato-Katz thick smears were examined from each stool sample to enhance diagnostic sensitivity. It is conceivable that a number of infections, particularly those of light intensity were missed, and hence, the overall prevalence of both S. haematobium and S. mansoni might be somewhat higher than reported here [41]. Second, data about sociodemographic, socioeconomic, and environmental factors were collected through a pretested questionnaire administered to children. There might be some kind of reporting bias. Third, no specific information was collected on water, sanitation, and hygiene behavior, although these are known risk factors for schistosomiasis [42,43].
5. Conclusions
Our study confirms that schistosomiasis remains prevalent in the southern and western parts of Côte d’Ivoire, although the overall prevalence in school-aged children was much lower than reported a few years earlier. Swimming, washing clothes, playing in unprotected open freshwater bodies, and low educational attainment of parents/guardians were identified as key risk factors of schistosomiasis in schoolchildren. Hence, preventive chemotherapy using praziquantel—which is the current mainstay of the national schistosomiasis control program—should be combined with targeted information and education campaigns to change children’s behaviors, with the goal of reducing the frequency of contact of children with open freshwater bodies.
Author Contributions
E.K.A., H.M., A.O.T., and J.T.C. conceived and validated the methodology of the study. K.E.A., J.B., O.R., and K.T. collected the data. K.E.A. performed statistical analysis. The manuscript was revised by H.M., A.O.T., J.T.C., J.U., and O.B. All authors read and approved the final manuscript prior to submission.
Funding
This work was funded by the Unité de Formation et de Recherche Sciences Pharmaceutiques et Biologiques, Université Félix Houphouët-Boigny (Abidjan, Côte d’Ivoire). Additional financial support was obtained by the Swiss National Science Foundation for E.K.A., J.U., and O.B. (grant no. 31003A_170113). K.E.A. is a recipient of a Swiss Government Excellence Scholarship (ESKAS, grant no. 2017–0746) for which he is deeply grateful.
Acknowledgments
We thank the children, parents, school communities, health workers, and the District Medical Officers in each location. The authors would like to thank the Institut Pasteur de Côte d’Ivoire and the Faculty of Pharmacy staff for technical advice during the study. Particular thanks go to Ms. Lisa Crump for assistance with manuscript improvement and to Professor Christian Schindler for statistical support. The authors thank the other team members for assistance during data collection and our colleagues at the Swiss Tropical and Public Health Institute (Basel, Switzerland) for logistical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hotez, P.J.; Alvarado, M.; Basáñez, M.-G.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M.; et al. The Global Burden of Disease Study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 DALYs and Hale Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primer 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Aagaard-Hansen, J.; Mwanga, J.R.; Bruun, B. Social science perspectives on schistosomiasis control in Africa: Past trends and future directions. Parasitology 2009, 136, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; N’Goran, E.K.; Caffrey, C.R.; Keiser, J. From innovation to application: Social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011, 120, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- McCreesh, N.; Nikulin, G.; Booth, M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit. Vectors 2015, 8, 4. [Google Scholar] [CrossRef]
- Coulibaly, J.T.; N’Gbesso, Y.K.; N’Guessan, N.A.; Winkler, M.S.; Utzinger, J.; N’Goran, E.K. Epidemiology of schistosomiasis in two high-risk communities of south Côte d’Ivoire with particular emphasis on pre-school–aged children. Am. J. Trop. Med. Hyg. 2013, 89, 32–41. [Google Scholar] [CrossRef]
- Tian-Bi, Y.-N.T.; Webster, B.; Konan, C.K.; Allan, F.; Diakité, N.R.; Ouattara, M.; Salia, D.; Koné, A.; Kakou, A.K.; Rabone, M.; et al. Molecular characterization and distribution of Schistosoma cercariae collected from naturally infected bulinid snails in northern and central Côte d’Ivoire. Parasit. Vectors 2019, 12, 117. [Google Scholar] [CrossRef]
- Chammartin, F.; Houngbedji, C.A.; Hürlimann, E.; Yapi, R.B.; Silué, K.D.; Soro, G.; Kouamé, F.N.; N’Goran, E.K.; Utzinger, J.; Raso, G.; et al. Bayesian risk mapping and model-based estimation of Schistosoma haematobium-Schistosoma mansoni co-distribution in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2014, 8, e3407. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; N’Goran, E.K.; Tanner, M.; Lengeler, C. Simple anamnestic questions and recalled water-contact patterns for self-diagnosis of Schistosoma mansoni infection among schoolchildren in western Côte d’Ivoire. Am. J. Trop. Med. Hyg. 2000, 62, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Assaré, R.K.; Lai, Y.-S.; Yapi, A.; Tian-Bi, Y.-N.T.; Ouattara, M.; Yao, P.K.; Knopp, S.; Vounatsou, P.; Utzinger, J.; N’Goran, E.K. The spatial distribution of Schistosoma mansoni infection in four regions of western Côte d’Ivoire. Geospat. Health 2015, 10, 345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soumahoro, M.; Bosson-Vanga, A.; Coulibaly, K.; Abbes, S.; Angora, E.; Kouadio, K.; N’Douba, A.K.; Sissoko, D.; Dosso, M. Investigation d’un foyer épidémique de bilharziose urinaire dans l’école primaire du village de Guébo 2, Abidjan, Côte d’Ivoire. Bull. Soc. Pathol. Exot. 2014, 107, 185–187. [Google Scholar] [CrossRef] [PubMed]
- M’Bra, R.K.; Kon_, B.; Yapi, Y.G.; Silué, K.D.; Sy, I.; Vienneau, D.; Soro, N.; Cissé, G.; Utzinger, J. Risk factors for schistosomiasis in an urban area in northern Côte d’Ivoire. Infect. Dis. Poverty 2018, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- N’Guessan, N.; Acka, C.A.; Utzinger, J.; N’Goran, E.K. Identification des régions à haut risque de schistosomoses en Côte d’lvoire. Bull. Soc. Pathol. Exot. 2007, 100, 119–123. [Google Scholar]
- Raso, G.; Matthys, B.; N’Goran, E.K.; Tanner, M.; Vounatsou, P.; Utzinger, J. Spatial risk prediction and mapping of Schistosoma mansoni infections among schoolchildren living in western Côte d’Ivoire. Parasitology 2005, 131, 97–108. [Google Scholar] [CrossRef]
- Nwaorgu, O.C.; Okeibunor, J.; Madu, E.; Amazigo, U.; Onyegegbu, N.; Evans, D. A school-based schistosomiasis and intestinal helminthiasis control programme in Nigeria: Acceptability to community members. Trop. Med. Int. Health 1998, 3, 842–849. [Google Scholar] [CrossRef]
- Diakité, N.R.; N’Zi, K.G.; Ouattara, M.; Coulibaly, J.T.; Saric, J.; Yao, P.K.; Hattendorf, J.; Utzinger, J.; N’Goran, E.K. Association of riverine prawns and intermediate host snails and correlation with human schistosomiasis in two river systems in south-eastern Côte d’Ivoire. Parasitology 2018, 145, 1792–1800. [Google Scholar] [CrossRef]
- Geleta, S.; Alemu, A.; Getie, S.; Mekonnen, Z.; Erko, B. Prevalence of urinary schistosomiasis and associated risk factors among Abobo primary school children in Gambella Regional State, southwestern Ethiopia: A cross sectional study. Parasit. Vectors 2015, 8, 215. [Google Scholar] [CrossRef]
- Mott, K.E.; Baltes, R.; Bambagha, J.; Baldassini, B. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Tropenmed. Parasitol. 1982, 33, 227–228. [Google Scholar] [PubMed]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 1972, 14, 397–400. [Google Scholar] [PubMed]
- WHO Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers; World Health Organization: Geneva, Switzerland, 2006; pp. 1–62.
- Yapi, Y.G.; Briët, O.J.T.; Diabate, S.; Vounatsou, P.; Akodo, E.; Tanner, M.; Teuscher, T. Rice irrigation and schistosomiasis in savannah and forest areas of Côte d’Ivoire. Acta Trop. 2005, 93, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Tian-Bi, Y.-N.T.; Ouattara, M.; Knopp, S.; Coulibaly, J.T.; Hürlimann, E.; Webster, B.; Allan, F.; Rollinson, D.; Meïté, A.; Diakité, N.R.; et al. Interrupting seasonal transmission of Schistosoma haematobium and control of soil-transmitted helminthiasis in northern and central Côte d’Ivoire: A SCORE study protocol. BMC Public Health 2018, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; N’Goran, E.K.; Ossey, Y.A.; Booth, M.; Traoré, M.; Lohourignon, K.L.; Allangba, A.; Ahiba, L.A.; Tanner, M.; Lengeler, C. Rapid screening for Schistosoma mansoni in western Côte d’Ivoire using a simple school questionnaire. Bull. World Health Organ. 2000, 78, 389–398. [Google Scholar] [PubMed]
- Sow, S.; de Vlas, S.J.; Stelma, F.; Vereecken, K.; Gryseels, B.; Polman, K. The contribution of water contact behavior to the high Schistosoma mansoni infection rates observed in the Senegal River Basin. BMC Infect. Dis. 2011, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Diakité, N.R.; Winkler, M.S.; Coulibaly, J.T.; Guindo-Coulibaly, N.; Utzinger, J.; N’Goran, E.K. Dynamics of freshwater snails and Schistosoma infection prevalence in schoolchildren during the construction and operation of a multipurpose dam in central Côte d’Ivoire. Infect. Dis. Poverty 2017, 6, 93. [Google Scholar] [CrossRef]
- Kazibwe, F.; Makanga, B.; Rubaire-Akiiki, C.; Ouma, J.; Kariuki, C.; Kabatereine, N.B.; Vennervald, B.J.; Rollinson, D.; Stothard, J.R. Transmission studies of intestinal schistosomiasis in Lake Albert, Uganda and experimental compatibility of local Biomphalaria spp. Parasitol. Int. 2010, 59, 49–53. [Google Scholar] [CrossRef]
- Ratard, R.; Ndamkou, C.; Kouemeni, L.; Ekani Bessala, M. Schistosoma mansoni eggs in urine. J. Trop. Med. Hyg. 1991, 94, 348–351. [Google Scholar]
- Abou-Zeid, A.H.; Abkar, T.A.; Mohamed, R.O. Schistosomiasis infection among primary school students in a war zone, Southern Kordofan State, Sudan: A cross-sectional study. BMC Public Health 2013, 13, 643. [Google Scholar] [CrossRef]
- Negussu, N.; Wali, M.; Ejigu, M.; Debebe, F.; Aden, S.; Abdi, R.; Mohamed, Y.; Deribew, A.; Deribe, K. Prevalence and distribution of schistosomiasis in Afder and Gode zone of Somali region, Ethiopia. J. Glob. Infect. Dis. 2013, 5, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Ivoke, N.; Ivoke, O.N.; Nwani, C.D.; Ekeh, F.N.; Asogwa, C.N.; Atama, C.I.; Eyo, J.E. Prevalence and transmission dynamics of Schistosoma haematobium infection in a rural community of southwestern Ebonyi State, Nigeria. Trop. Biomed. 2014, 31, 77–88. [Google Scholar] [PubMed]
- Kabuyaya, M.; Chimbari, M.J.; Mukaratirwa, S. Infection status and risk factors associated with urinary schistosomiasis among school-going children in the Ndumo area of Mkhanyakude district in KwaZulu-Natal, South Africa two years post-treatment. Int. J. Infect. Dis. 2018, 71, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ugbomoiko, U.S.; Ofoezie, I.E.; Okoye, I.C.; Heukelbach, J. Factors associated with urinary schistosomiasis in two peri-urban communities in south-western Nigeria. Ann. Trop. Med. Parasitol. 2010, 104, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Sady, H.; Al-Mekhlafi, H.M.; Mahdy, M.A.K.; Lim, Y.A.L.; Mahmud, R.; Surin, J. Prevalence and associated factors of schistosomiasis among children in Yemen: Implications for an effective control programme. PLoS Negl. Trop. Dis. 2013, 7, e2377. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, G.; Ouattara, M.; Dongo, K.; Hürlimann, E.; Bassa, F.K.; Koné, N.; Essé, C.; Yapi, R.B.; Bonfoh, B.; Utzinger, J.; et al. Epidemiology of intestinal parasite infections in three departments of south-central Côte d’Ivoire before the implementation of a cluster-randomised trial. Parasite Epidemiol. Control 2018, 3, 63–76. [Google Scholar] [CrossRef]
- Ayele, B.; Erko, B.; Legesse, M.; Hailu, A.; Medhin, G. Evaluation of circulating cathodic antigen (CCA) strip for diagnosis of urinary schistosomiasis in Hassoba school children, Afar, Ethiopia. Parasite 2008, 15, 69–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abossie, A.; Seid, M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health 2014, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Nundu Sabiti, S.; Aloni, M.-N.; Linsuke, S.-W.-L.; Ekila, M.-B.; Situakibanza, H.-T.; Polman, K.; Lutumba, P.-T. Prevalence of geohelminth infections in children living in Kinshasa. Arch. Pediatr. 2014, 21, 579–583. [Google Scholar] [CrossRef]
- Bärenbold, O.; Raso, G.; Coulibaly, J.T.; N’Goran, E.K.; Utzinger, J.; Vounatsou, P. Estimating sensitivity of the Kato-Katz technique for the diagnosis of Schistosoma mansoni and hookworm in relation to infection intensity. PLoS Negl. Trop. Dis. 2017, 11, e0005953. [Google Scholar] [CrossRef]
- Hilali, A.H.; Madsen, H.; Daffalla, A.A.; Wassila, M.; Christensen, N.O. Infection and transmission pattern of Schistosoma mansoni in the Managil irrigation scheme, Sudan. Ann. Trop. Med. Parasitol. 1995, 89, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.E.T.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The relationship between water, sanitation and schistosomiasis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014, 8, e3296-443. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).