Use of Geospatial Surveillance and Response Systems for Vector-Borne Diseases in the Elimination Phase
Abstract
1. Introduction
2. Data Collection
3. GEOHealth: Part of the Global Earth Observation System of Systems (GEOSS)
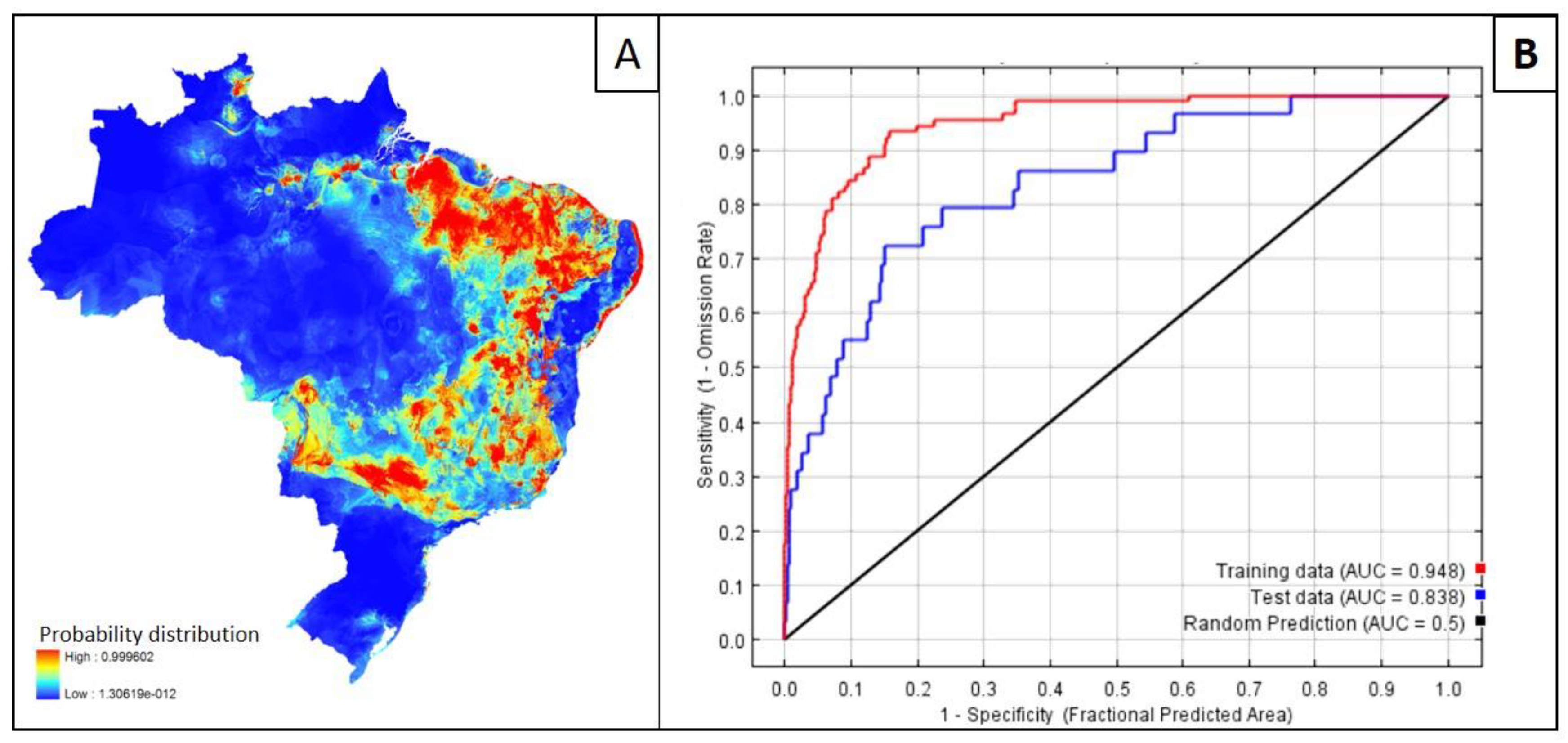
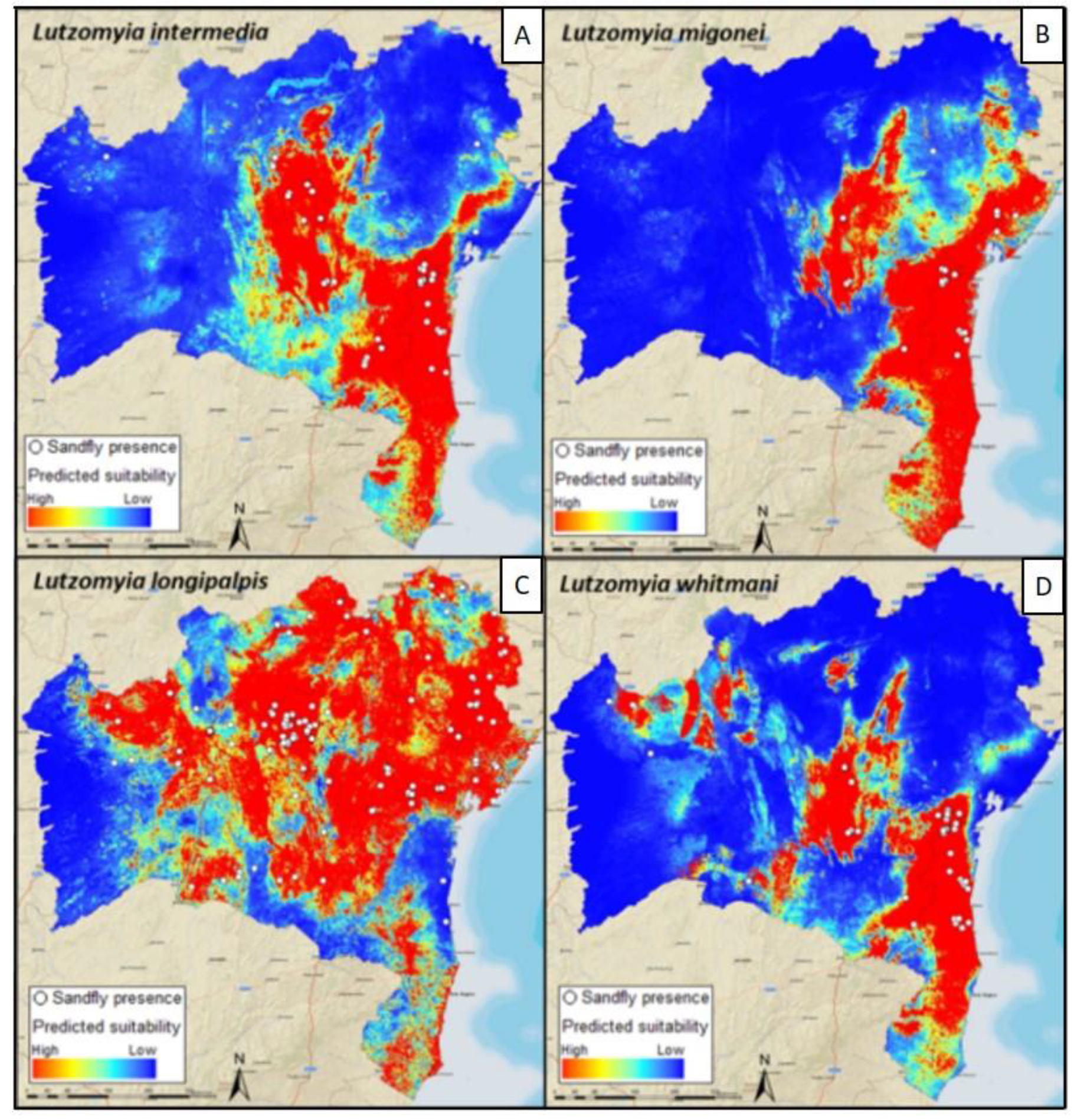
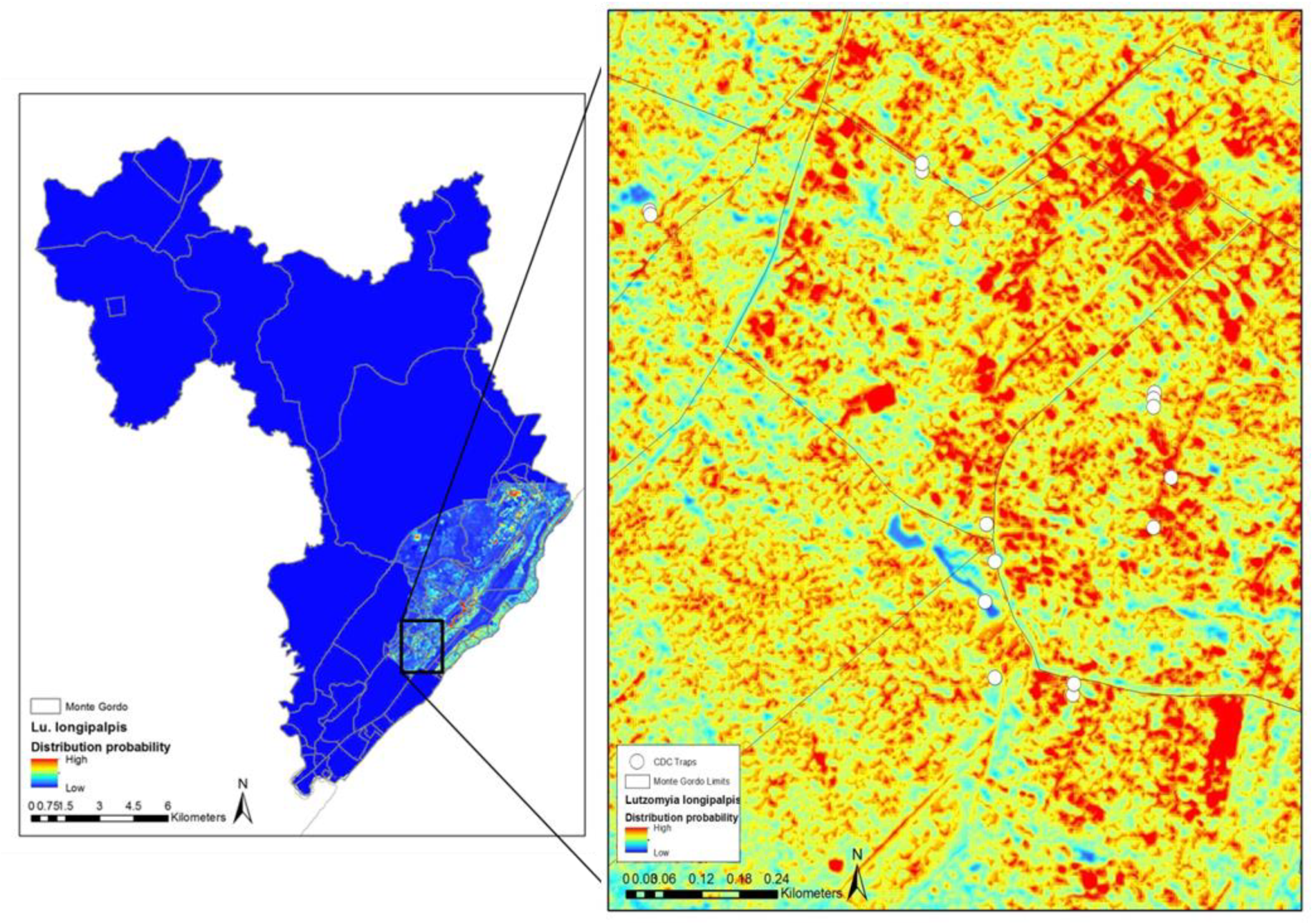
4. Mapping and Modelling NTDs in the Americas
5. Schistosomiasis
5.1. Africa
5.2. China and Southeast Asia
5.3. Latin America
6. Healthy Futures
7. Future Potential
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bergquist, R. New tools for epidemiology: A space odyssey. Mem. Inst. Oswaldo Cruz 2011, 106, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, R.; Tanner, M. Visual approaches for strengthening research, science communication and public health impact. Geospat. Health 2012, 6, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.B.; Tourre, Y.M.; Faruque, F.; Luvall, J.C.; Bergquist, R. Towards establishment of GeoHealth, an open-data portal for health mapping and modelling based on Earth observations by remote sensing. Geospat. Health 2014, 8, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, R.; Rinaldi, L. Health research based on geospatial tools: A timely approach in a changing environment. J. Helminthol. 2010, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Randolph, S.E. Climate change and vector-borne diseases. Adv. Parasitol. 2006, 62, 345–381. [Google Scholar] [PubMed]
- Foley, D.H.; Wilkerson, R.C.; Dornak, L.L.; Pecor, D.B.; Nyari, A.S.; Rueda, L.M.; Long, L.S.; Richardson, J.H. SandflyMap: Leveraging spatial data on sand fly vector distribution for disease risk assessments. Geospat. Health 2012, 6, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, R. Climate and the distribution of vector-borne diseases: What’s in store? Geospat. Health 2017, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.S.; Torr, S.J.; Auty, H.K.; Brock, P.M.; Byamungu, M.; Hargrove, J.W.; Morrison, L.J.; Mramba, F.; Vale, G.A.; Stanton, MC. Geostatistical models using remotely-sensed data predict savanna tsetse decline across the interface between protected and unprotected areas in Serengeti, Tanzania. J. Appl. Ecol. 2018, 4, 1997–2007. [Google Scholar] [CrossRef]
- Misslin, R.; Vaguet, Y.; Vaguet, A.; Daudé, É. Estimating air temperature using MODIS surface temperature images for assessing Aedes aegypti thermal niche in Bangkok, Thailand. Environ. Monit. Assess. 2018, 190, 537. [Google Scholar] [CrossRef]
- Randolph, S.E.; Rogers, D.J. Tick-borne disease systems: Mapping geographic and phylogenetic space. Adv. Parasitol. 2006, 62, 263–291. [Google Scholar]
- Genchi, C.; Mortarino, M.; Rinaldi, L.; Cringoli, G.; Traldi, G.; Genchi, M. Changing climate and changing vector-borne disease distribution: The example of Dirofilaria in Europe. Vet. Parasitol. 2011, 176, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Vounatsou, P.; Zhou, X.N.; Tanner, M.; Utzinger, J. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia 2005, 47, 127–134. [Google Scholar] [PubMed]
- Zhou, X.N.; Yang, G.J.; Yang, K.; Wang, X.H.; Hong, Q.B.; Sun, L.P.; Malone, J.B.; Kristensen, T.K.; Bergquist, N.R.; Utzinger, J. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008, 78, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Harrus, S.; Baneth, G. Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. Int. J. Parasitol. 2005, 35, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, R.; Stensgaard, A.S.; Rinaldi, L. Vector-borne diseases in a warmer world: Will they stay or will they go? Geospat. Health 2018, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Pavlovskii, E.N. The ecological parasitology. J. Gen. Biol. 1945, 6, 65–92. [Google Scholar]
- Rinaldi, L.; Hendrickx, G.; Cringoli, G.; Biggeri, A.; Ducheyne, E.; Catelan, D.; Morgan, E.; Williams, D.; Charlier, J.; von Samson-Himmelstjerna, G.; et al. Mapping and modelling helminth infections in ruminants in Europe: Experience from GLOWORM. Geospat. Health 2015, 19, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sun, LP.; Huang, Y.X.; Yang, G.J.; Wu, F.; Hang, D.R.; Li, W.; Zhang, J.F.; Liang, Y.S.; Zhou, X.N. A real-time platform for monitoring schistosomiasis transmission supported by Google Earth and a web-based geographical information system. Geospat. Health 2012, 6, 195–203. [Google Scholar] [CrossRef]
- Rinaldi, L.; Musella, V.; Biggeri, A.; Cringoli, G. New insights into the application of geographical information systems and remote sensing in veterinary parasitology. Geospat. Health 2006, 1, 33–47. [Google Scholar] [CrossRef]
- Kistemann, T.; Dangendorf, F.; Schweikart, J. New perspectives on the use of Geographical Information Systems (GIS) in environmental health sciences. Int. J. Hyg. Environ. Health 2002, 205, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.B. Biology-based mapping of vector-borne parasites by geographic information systems and remote sensing. Parassitologia 2005, 47, 27–50. [Google Scholar] [PubMed]
- Luvall, J.C. The power of the pixel—A thermodynamic paradigm for studying disease vector’s habitats & life cycles using NASA’s Remote sensing data. Presented at the 8th International Symposium on Geospatial Health, New Orleans, LA, USA, 1–2 November 2014. [Google Scholar]
- Marechal, F.; Ribeiro, N.; Lafaye, M.; Guell, A. Satellite imaging and vector-borne diseases: The approach of the French National Space Agency (CNES). Geospat. Health 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Kuze, A.; Sobue, S.; Yamamoto, A.; Yamamoto, K.; Oyoshi, K.; Imaoka, K.; Fukuda, T. Japan’s efforts to promote global health using satellite remote sensing data from the Japanese aerospace exploration agency (JAXA) for prediction of infectious diseases and air quality. Geospat. Health 2014, 8, S603–S610. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; Rinaldi, L.; Malone, J.B.; Krauth, S.J.; Kristensen, T.K.; Cringoli, G.; Bergquist, R. Geospatial health: The first five years. Geospat. Health 2011, 6, 137–154. [Google Scholar] [CrossRef] [PubMed]
- De Roeck, E.; Van Coillie, F.; De Wulf, R.; Soenen, K.; Charlier, J.; Vercruysse, J.; Hantson, W.; Ducheyne, E.; Hendrickx, G. Fine-scale mapping of vector habitats using very high resolution satellite imagery: A case-study on liver fluke. Geospat. Health 2014, 8, S671–S683. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, A.; Pindozzi, S.; Okello, C.; Boccia, L. Indirect technology for detecting areas object of illegal spills, harmful to human health, by applying drones, photogrammetry and hydrological models. Geospat. Health 2014, 8, S699–S707. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Mischler, P.; Kearney, M.; McCarroll, J.C.; Scholte, R.G.; Vounatsou, P.; Malone, J.B. Environmental and socio-economic risk modelling for Chagas disease in Bolivia. Geospat. Health 2012, 6, S59–S66. [Google Scholar] [CrossRef]
- Martins, M. The Use of Geographic Information Systems and Ecological Niche Modeling to Map Transmission Risk for Visceral Leishmaniasis in Salvador, Bahia, Brazil. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2015. [Google Scholar]
- Cross, E.R.; Perrine, R.; Sheffield, C. Predicting areas endemic for schistosomiasis using weather variables and a Landsat database. Mil. Med. 1984, 149, 542–544. [Google Scholar] [CrossRef]
- Malone, J.B.; Huh, O.K.; Fehler, D.P.; Wilson, P.A.; Wilensky, D.E.; Holmes, R.A.; Elmagdoub, A.I. Temperature data from satellite imagery and the distribution of schistosomiasis in Egypt. Am. J. Trop. Med. Hyg. 1994, 50, 714–722. [Google Scholar] [CrossRef]
- Abdel Rahman, M.S.; El Bahy, M.M.; El Bahy, N.M.; Malone, J.B. Development and validation of a satellite based Geographic information system (GIS) model for epidemiology of Schistosoma risk assessment at snail level in Kafr El Sheikh governorate. J. Egypt. Soc. Parasitol. 1997, 27, 299–316. [Google Scholar] [PubMed]
- Geospatial Surveillance and Response Systems for Schistosomiasis; Malone, J.B., Bergquist, R., Rinaldi, L., Eds.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 28; p. 523. [Google Scholar]
- Yang, G.J.; Bergquist, R. Potential Impact of Climate Change on Schistosomiasis: A Global Assessment Attempt. Trop. Med. Infect. Dis. 2018, 3, 117. [Google Scholar] [CrossRef]
- Clements, A.C.; Deville, MA.; Ndayishimiye, O.; Brooker, S.; Fenwick, A. Spatial co-distribution of neglected tropical diseases in the East African Great Lakes region: Revisiting the justification for integrated control. Trop. Med. Int. Health 2010, 15, 198–207. [Google Scholar] [CrossRef]
- Bergquist, R. Closing in on ‘perhaps the most dreadful of the remaining plagues’: An independent view of the multidisciplinary alliance to optimize schistosomiasis control in Africa. Acta Trop. 2013, 128, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Stensgaard, A.S.; Saarnak, C.F.L.; Utzinger, J.; Vounatsou, P.; Simoonga, C.; Mushinge, G.; Rahbek, C.; Møhlenberg, F.; Kristensen, T.K. Virtual globes and geospatial health: The potential of new tools in the management and control of vector-borne diseases. Geospat. Health 2009, 3, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuente, L.A.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013, 128, 423–440. [Google Scholar] [CrossRef]
- WHO. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation. 2012. Available online: http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf (accessed on 17 January 2012).
- Mayangadze, T.; Chimbari, M.J.; Gebreslasie, M.; Mukaratirwa, S. Application of geo-spatial technology in schistosomiasis modeling in Africa: A review. Geospat. Health 2015, 10, 326. [Google Scholar] [CrossRef]
- Zhou, X.N.; Chen, M.G.; McManus, D.; Bergquist, R. Schistosomiasis control in the 21st century. Proceedings of the International Symposium on Schistosomiasis, Shanghai, 4–6 July 2001. Acta Trop. 2001, 82, 95–114. [Google Scholar]
- Zhu, H.R.; Liu, L.; Zhou, X.N.; Yang, G.J. Ecological Model to Predict Potential Habitats of Oncomelania hupensis, the Intermediate Host of Schistosoma japonicum in the Mountainous Regions, China. PLoS Negl. Trop. Dis. 2015, 9, e0004028. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, D.A. Rapid Monitoring and Evaluation Method of Schistosomiasis Based on Spatial Information Technology. Int. J. Environ. Res. Publ. Health 2015, 12, 15843–15859. [Google Scholar] [CrossRef]
- Yang, G.J.; Vounatsou, P.; Zhou, X.N.; Tanner, M.; Utzinger, J.A. Bayesian-based approach for spatio-temporal modeling of county level prevalence of Schistosoma japonicum infection in Jiangsu province. China Int. J. Parasitol. 2005, 35, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Vounatsou, P.; Tanner, M.; Zhou, X.N.; Utzinger, J. Remote sensing for predicting potential habitats of Oncomelania hupensis in Hongze, Baima and Gaoyou lakes in Jiangsu province, China. Geospat. Health 2006, 1, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.G.; Vounatsou, P.; Cao, C.L.; Utzinger, J.; Zhu, H.Q.; Anderegg, D.; Zhu, R.; He, Z.Y.; Li, D.; Hu, F. A geographic information and remote sensing based model for prediction of Oncomelania hupensis habitats in the Poyang Lake area, China. Acta Trop. 2005, 96, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Zhu, R.; Zhang, Z.J.; Yao, B.D.; Zhang, L.J.; Gao, J.; Jiang, Q.W. Identification of snail habitats in the Poyang Lake region, based on the application of indices on joint normalized difference vegetation and water. Zhonghua Liu Xing Bing Xue Za Zhi 2012, 33, 823–827. (In Chinese) [Google Scholar]
- Malone, J.B.; Yang, G.J.; Leonardo, L.; Zhou, X.N. Implementing a geospatial health data infrastructure for control of Asian schistosomiasis in the People’s Republic of China and the Philippines. Adv. Parasitol. 2010, 73, 1–100. [Google Scholar] [CrossRef]
- Leonardo, L.R.; Crisostomo, B.A.; Solon, J.A.; Rivera, P.T.; Marcelo, A.B.; Villasper, J.M. Geographical information systems in health research and services delivery in the Philippines. Geospat. Health 2007, 1, 147–155. [Google Scholar] [CrossRef]
- Leonardo, L.R.; Rivera, P.T.; Crisostomo, B.A.; Sarol, J.N.; Bantayan, N.C.; Tiu, W.U.; Bergquist, N.R. A study of the environmental determinants of malaria and schistosomiasis in the Philippines using Remote Sensing and Geographic Information Systems. Parassitologia 2005, 47, 105–114. [Google Scholar]
- Bavia, M.E.; Hale, L.T.; Malone, J.B.; Braud, D.H.; Shane, S. Geographic information systems and the environmental risk of schistosomiasis in Bahia, Brazil. Am. J. Trop. Med. Hyg. 1999, 60, 566–572. [Google Scholar] [CrossRef]
- Bavia, M.E.; Malone, J.B.; Hale, L.; Dantes, A.; Marroni, L.; Reis, R. Use of thermal and vegetation index data from earth observing satellites to evaluate the risk of schistosomiasis in Bahia, Brazil. Acta Trop. 2001, 79, 79–85. [Google Scholar] [CrossRef]
- Gazzinelli, A.; Kloos, H. The use of spatial tools in the study of Schistosoma mansoni and its intermediate host snails in Brazil: A brief review. Geospat. Health 2007, 2, 51–58. [Google Scholar] [CrossRef]
- Guimarães, R.J.; Freitas, C.C.; Dutra, L.V.; Moura, A.C.; Amaral, R.S.; Drummond, S.C.; Scholte, R.G.; Carvalho, O.S. Schistosomiasis risk estimation in Minas Gerais State, Brazil, using environmental data and GIS techniques. Acta Trop. 2008, 108, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.J.; Freitas, C.C.; Dutra, L.V.; Scholte, R.G.; Martins-Bedé, F.T.; Fonseca, F.R.; Amaral, R.S.; Drummond, S.C.; Felgueiras, C.A.; Oliveira, G.C.; et al. A geoprocessing approach for studying and controlling schistosomiasis in the state of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2010, 105, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.B.; Bergquist, N.R. Mapping and modelling neglected tropical diseases and poverty in Latin America and the Caribbean. Geospat. Health 2012, 6, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Barboza, D.M.; Zhang, C.; Santos, N.C.; Silva, M.M.; Rollemberg, C.V.; de Amorim, F.J.; Ueta, M.T.; de Melo, C.M.; de Almeida, J.A.; Jeraldo, V.; et al. Biomphalaria species distribution and its effect on human Schistosoma mansoni infection in an irrigated area used for rice cultivation in northeast Brazil. Geospat. Health 2012, 6, S103–S109. [Google Scholar] [CrossRef] [PubMed]
- Scholte, R.G.C.; Carvlho, O.S.; Malone, J.B.; Utzinger, J.; Vounatsou, P. Spatial distribution of Biomphalaria spp., the intermediate host snails of Schistosoma mansoni, in Brazil. Geospat. Health 2012, 6, S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Stensgaard, A.S.; Utzinger, J.; Vounatsou, P.; Hürlimann, E.; Saarnak, C.F.L.; Mubita, P.; Simoonga, C.; Kabatereine, N.B.; Tchuem Tchuenté, L.A.; Simoonga, C.; et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: Does climate matter? Acta Trop. 2013, 128, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, A.; Caporaso, L. Assessment of malaria transmission changes in Africa, due to the climate impact of land use change using Coupled Model Intercomparison Project Phase 5 earth system models. Geospat. Health 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Bizimana, J.P.; Kienberger, S.; Hagenlocher, M.; Twarabamenye, E. Modelling homogeneous regions of social vulnerability to malaria in Rwanda. Geospat. Health 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- McCreech, N.; Nikulin, G.; Booth, M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasites Vectors 2015, 8, 4. [Google Scholar] [CrossRef]
- Taylor, D.; Hagenlocher, M.; Jones, A.; Kienberger, S.; Leedale, J.; Morse, A. Environmental change and Rift Valley fever in eastern Africa: Projecting beyond Healthy futures. Geospat. Health 2016, 11. [Google Scholar] [CrossRef]
- Hagenlocher, M.; Castro, M.C. Mapping malaria risk and vulnerability in the United Republic of Tanzania: A spatial explicit model. Popul. Health Metr. 2015, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H. NASA Health and Air Quality (HAQ) Newsletter, Volume 16, June–September 2018. Available online: https://appliedsciences.nasa.gov/system/files/sites/default/files/HAQ%20Newsletter%20Jun-Sept18.pdf (accessed on 11 October 2018).
- Boulos, M.N. Web GIS in practice III: Creating a simple interactive map of England’s Strategic Health Authorities using Google Maps API, Google Earth KML, and MSN Virtual Earth Map Control. Int. J. Health Geogr. 2005, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Elvidge, C.D.; Tuttle, B.T. How virtual globes are revolutionizing Earth observation data access and integration. Int. Arch. Photogram. Rem. Sens. Spat. Inf. Sci. 2008, 37, 137–139. [Google Scholar]
- Sabeson, S.; Raju, K.; Subramanian, S.; Srivastava, P.; Jambulingam, P. Lymphatic filariasis transmission risk map of India based on a geo-environmental risk model. Vector Borne Zoonotic Dis. 2013, 13, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Yaro, C.A.; Kogi, E.; Luka, S.A. Spatial distribution and modeling of soil transmitted helminthes infection in Nigeria. Research J. Parasitol. 2018, 13, 19–35. [Google Scholar] [CrossRef]
| Satellite Platform | Frequency | Swath | Sensor | Spatial Resolution | Applications/Comments |
|---|---|---|---|---|---|
| GPM a Launched Feb. 2014 | Integrated multi-satellite retrievals (IMERGE) 0.5 hours | Dual-frequency Precipitation Radar (DPR) 125–245 km; Global Microwave Imager (GMI) 885 km | Core Observatory radar/radiometer system | 1 km | Measures precipitation using a reference standard to unify measurements from a constellation of related research and operational satellites. Extends Tropical Rainfall Measuring Mission (TRMM) records |
| GOES b 16 Launched Nov. 2014 | 5–15 min | Full disk image of the Earth consisting of 22 swaths | Advanced Baseline Imager (ABI) with 16 bands | 0.5–1 km–2 km | Meteorology; Geostationary orbit over the western hemisphere |
| Suomi-NPP c Launched Oct. 2011 | Daily | 3000 km | Visible-Infrared Imaging Radiometer Suite (VIIRS) | 1 km | 8-day Land Surface Temperature (LST) measurements for day and night. Extends MODIS d, AVHRR e |
| Soil Moisture Active Passive (SMAP) | 3 h | L band Radar and Microwave Imager | 3–10 km | Measures water content in the top 5 cm of the soil | |
| Landsat 8 Launched Jan. 2013 | 16 days | 185 km | Operational Land Imager (OLI), Thermal Infrared Sensor (TIRS) | OLI: Panchrom. = 15 m VIS-NIR-SWIR f = 30 m TIRS: thermal bands = 100 m | OLI and TIRS replace the Thematic Mapper (TM) and the enhanced Thematic Mapper Plus (ETM+) on previous Landsat satellites (Landsat legacy data has a continuous record since 1972 |
| Sentinel 1 (A&B) A launched 2014 B launched 2015 | 12 days | 250 km | C-band Synthetic Aperture Radar (C-SAR) Multi-spectral instrument | 5 and 20 m | EU contribution to GEOSS with applications related to land, coastal water with respect to natural disasters, resources, environment, weather, seasonal forecasting and climate. Monitors plant growth and forests, changes in land cover marine and ecosystems through leaf chlorophyll and water content indexes |
| Sentinel 2 A launched 2015 B launched 2016 | 10 days | 290 km | (MSI) with 13 channels in VIS-NIR-SWIR f Radar altimeter, micro wave | 10, 20 and 60 m | |
| Sentinel 3 A launched 2015 B launched 2016 | 27 days | 1270 km | radiometer, sea and land surface temperature radiometer | 300 m | |
| Worldview 3 Aug. 2013 Worldview 4 Nov. 2016 | <1 day | 13.1 km | Pan, 8 Multi-spectral, 8 SWIR | Panchromatic = 31 cm Multispectral = 1.24 m | Optical data collection at the habitat-household level |
| International Space Station (ISS) | 3 days | 385–415 km | ECOSTRESS g Launched July 2018 | 38 × 57 m | Measures plant evapotranspiration (ET) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malone, J.B.; Bergquist, R.; Martins, M.; Luvall, J.C. Use of Geospatial Surveillance and Response Systems for Vector-Borne Diseases in the Elimination Phase. Trop. Med. Infect. Dis. 2019, 4, 15. https://doi.org/10.3390/tropicalmed4010015
Malone JB, Bergquist R, Martins M, Luvall JC. Use of Geospatial Surveillance and Response Systems for Vector-Borne Diseases in the Elimination Phase. Tropical Medicine and Infectious Disease. 2019; 4(1):15. https://doi.org/10.3390/tropicalmed4010015
Chicago/Turabian StyleMalone, John B., Robert Bergquist, Moara Martins, and Jeffrey C. Luvall. 2019. "Use of Geospatial Surveillance and Response Systems for Vector-Borne Diseases in the Elimination Phase" Tropical Medicine and Infectious Disease 4, no. 1: 15. https://doi.org/10.3390/tropicalmed4010015
APA StyleMalone, J. B., Bergquist, R., Martins, M., & Luvall, J. C. (2019). Use of Geospatial Surveillance and Response Systems for Vector-Borne Diseases in the Elimination Phase. Tropical Medicine and Infectious Disease, 4(1), 15. https://doi.org/10.3390/tropicalmed4010015




