Incidence, Disease Spectrum, and Outcomes of Tuberculous Meningitis in South African Children: The Initial Impact of COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population and Setting
2.2. Case Identification
2.3. Data Collection and Definitions
2.4. Statistical Analysis
2.5. Regulatory Approval
3. Results
3.1. Demographic Characteristics
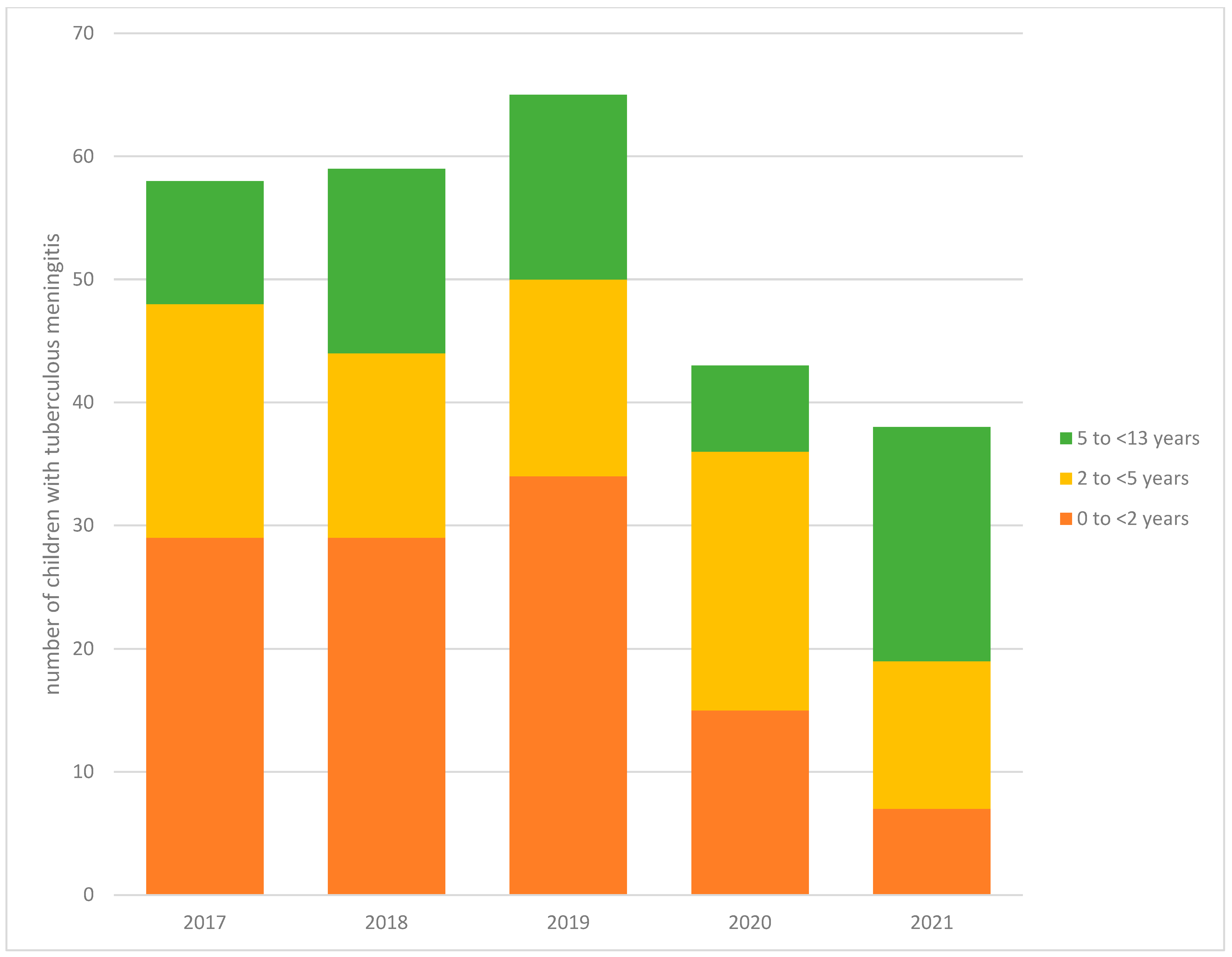
3.2. Trends in TBM Episodes
3.3. History and Clinical Characteristics
3.4. Early (In-Hospital) and Final TB Treatment Outcomes
3.5. Univariable and Multivariable Analysis
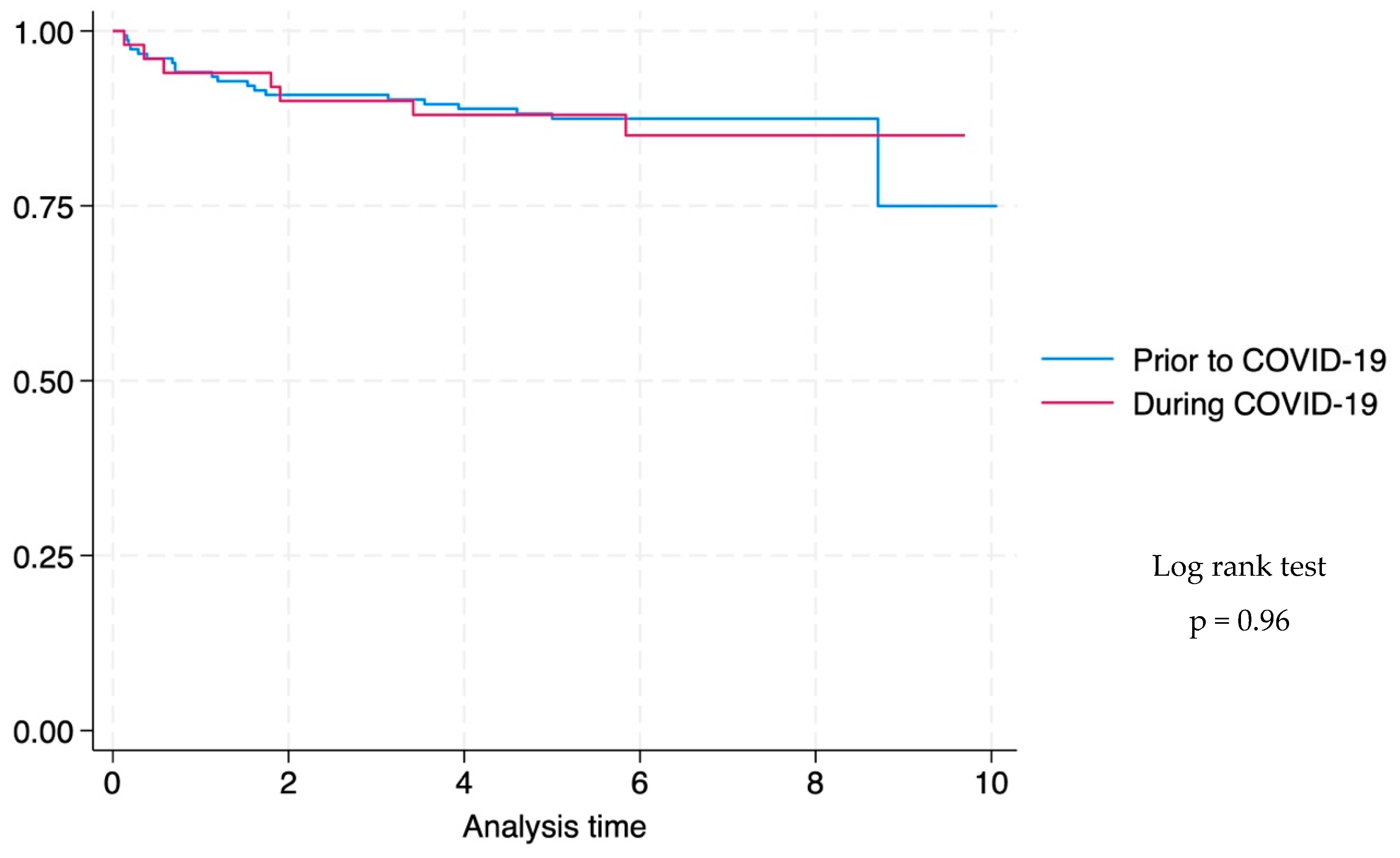
3.6. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, S.S.; Khan, F.A.; Milstein, M.B.; Tolman, A.W.; Benedetti, A.; Starke, J.R.; Becerra, M.C. Treatment outcomes of childhood tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Marais, B.J.; Gie, R.P.; Schaaf, H.S.; Hesseling, A.C.; Obihara, C.C.; Starke, J.R.; Enarson, D.A.; Donald, P.R.; Beyers, N. The natural history of childhood intra-thoracic tuberculosis: A critical review of literature from the pre-chemotherapy era. Int. J. Tuberc. Lung Dis. 2004, 8, 392–402. [Google Scholar]
- Schoeman, J.; Wait, J.; Burger, M.; van Zyl, F.; Fertig, G.; van Rensburg, A.J.; Springer, P.; Donald, P. Longterm follow up of childhood tuberculous meningitis. Dev. Med. Child Neurol. 2002, 44, 522–526. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2024. Available online: https://iris.who.int/bitstream/handle/10665/379339/9789240101531-eng.pdf?sequence=1 (accessed on 31 October 2024).
- Donald, P.R.; Cotton, M.F.; Hendricks, M.K.; Schaaf, H.S.; de Villiers, J.N.; Willemse, T.E. Pediatric meningitis in the Western Cape Province of South Africa. J. Trop. Pediatr. 1996, 42, 256–261. [Google Scholar] [CrossRef]
- Wolzak, N.K.; Cooke, M.L.; Orth, H.; van Toorn, R. The changing profile of pediatric meningitis at a referral centre in Cape Town, South Africa. J. Trop. Pediatr. 2012, 58, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, E.M. Tuberculous Meningitis in Children, With Special Reference to Serous Meningitis: Part I. Tuberculous Meningitis. Am. Rev. Tuberc. 1947, 56, 75–94. [Google Scholar]
- du Preez, K.; Schaaf, H.S.; Dunbar, R.; Walters, E.; Swartz, A.; Solomons, R.; Hesseling, A.C. Complementary surveillance strategies are needed to better characterise the epidemiology, care pathways and treatment outcomes of tuberculosis in children. BMC Public Health 2018, 18, 397. [Google Scholar] [CrossRef] [PubMed]
- du Preez, K.; Seddon, J.A.; Schaaf, H.S.; Hesseling, A.C.; Starke, J.R.; Osman, M.; Lombard, C.J.; Solomons, R. Global shortages of BCG vaccine and tuberculous meningitis in children. Lancet Glob. Health 2019, 7, e28–e29. [Google Scholar] [CrossRef]
- du Preez, K.; Jenkins, H.E.; Donald, P.R.; Solomons, R.S.; Graham, S.M.; Schaaf, H.S.; Starke, J.R.; Hesseling, A.C.; Seddon, J.A. Tuberculous Meningitis in Children: A Forgotten Public Health Emergency. Front. Neurol. 2022, 13, 751133. [Google Scholar] [CrossRef]
- Gunasekera, K.S.; Zelner, J.; Becerra, M.C.; Contreras, C.; Franke, M.F.; Lecca, L.; Murray, M.B.; Warren, J.L.; Cohen, T. Children as sentinels of tuberculosis transmission: Disease mapping of programmatic data. BMC Med. 2020, 18, 234. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Migliori, G.B.; Thong, P.M.; Alffenaar, J.W.; Denholm, J.; Tadolini, M.; Alyaquobi, F.; Blanc, F.X.; Buonsenso, D.; Cho, J.G.; Codecasa, L.R.; et al. Gauging the impact of the COVID-19 pandemic on tuberculosis services: A global study. Eur. Respir. J. 2021, 58, 2101786. [Google Scholar] [CrossRef]
- Ranasinghe, L.; Achar, J.; Groschel, M.I.; Whittaker, E.; Dodd, P.J.; Seddon, J.A. Global impact of COVID-19 on childhood tuberculosis: An analysis of notification data. Lancet Glob. Health 2022, 10, e1774–e1781. [Google Scholar] [CrossRef]
- Department of Co-Operative Governance and Traditional Affairs. South Africa to Move from Level 5 Lockdown to Level 4. Available online: https://www.cogta.gov.za/index.php/2020/04/24/south-africa-to-move-from-level-5-lockdown-to-level-4/ (accessed on 29 September 2024).
- South African Government. About Alert System. Available online: https://www.gov.za/covid-19/about/about-alert-system (accessed on 29 September 2024).
- Moonasar, D.; Pillay, A.; Leonard, E.; Naidoo, R.; Mngemane, S.; Ramkrishna, W.; Jamaloodien, K.; Lebese, L.; Chetty, K.; Bamford, L.; et al. COVID-19: Lessons and experiences from South Africa’s first surge. BMJ Glob. Health 2021, 6, e004393. [Google Scholar] [CrossRef] [PubMed]
- Moultrie, H.; Kachingwe, E.; Ismail, F. Impact of COVID-19 and Public Health Restrictions on TB Testing and Case Detection in South Africa. Available online: https://www.nicd.ac.za/wp-content/uploads/2020/09/COVID-19-Special-Public-Health-Surveillance-Bulletin-_Issue-4.pdf (accessed on 29 September 2024).
- Jennings, K.; Lembani, M.; Hesseling, A.C.; Mbula, N.; Mohr-Holland, E.; Mudaly, V.; Smith, M.; Osman, M.; Meehan, S.A. A decline in tuberculosis diagnosis, treatment initiation and success during the COVID-19 pandemic, using routine health data in Cape Town, South Africa. PLoS ONE 2024, 19, e0310383. [Google Scholar] [CrossRef]
- Statistics South Africa. Census 2022. Available online: https://census.statssa.gov.za/#/province/1/2 (accessed on 18 November 2024).
- Boulle, A.; Heekes, A.; Tiffin, N.; Smith, M.; Mutemaringa, T.; Zinyakatira, N.; Phelanyane, F.; Pienaar, C.; Buddiga, K.; Coetzee, E.; et al. Data Centre Profile: The Provincial Health Data Centre of the Western Cape Province, South Africa. Int. J. Popul. Data Sci. 2019, 4, 1143. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Available online: https://www.who.int/publications/i/item/924154693X (accessed on 2 May 2025).
- World Health Organization. Growth Reference Data for 5–19 Years. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years (accessed on 2 May 2025).
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- van Toorn, R.; Springer, P.; Laubscher, J.A.; Schoeman, J.F. Value of different staging systems for predicting neurological outcome in childhood tuberculous meningitis. Int. J. Tuberc. Lung Dis. 2012, 16, 628–632. [Google Scholar] [CrossRef]
- Department of Health Republic of South Africa. Guidelines for the Management of Tuberculosis in Children. Available online: https://www.nicd.ac.za/assets/files/Guidelines%20for%20management%20of%20TB%20in%20children%202013.pdf (accessed on 2 September 2024).
- Lebina, L.; Dube, M.; Hlongwane, K.; Brahmbatt, H.; G Lala, S.; Reubenson, G.; Martinson, N. Trends in paediatric tuberculosis diagnoses in two South African hospitals early in the COVID-19 pandemic. SAMJ S. Afr. Med. J. 2020, 110, 1149–1150. [Google Scholar] [CrossRef]
- Elmi, N.; Smit, L.; Wessels, T.; Zunza, M.; Rabie, H. COVID-19 lockdown effect on healthcare utilization and in-hospital mortality in children under 5 years in Cape Town, South Africa: A cross-sectional study. J. Trop. Pediatr. 2023, 69, fmad035. [Google Scholar] [CrossRef]
- Kehoe, K.; Morden, E.; Zinyakatira, N.; Heekes, A.; Jones, H.E.; Walter, S.R.; Jacobs, T.; Murray, J.; Buys, H.; Redaniel, M.T.; et al. Lower respiratory tract infection admissions and deaths among children under 5 years in public sector facilities in the Western Cape Province, South Africa, before and during the COVID-19 pandemic (2019–2021). S. Afr. Med. J. 2024, 114, e1560. [Google Scholar] [CrossRef]
- Dheda, K.; Perumal, T.; Moultrie, H.; Perumal, R.; Esmail, A.; Scott, A.J.; Udwadia, Z.; Chang, K.C.; Peter, J.; Pooran, A.; et al. The intersecting pandemics of tuberculosis and COVID-19: Population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 2022, 10, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.; Mazanderani, A.H.; Sherman, G.; Feucht, U. How paediatric HIV services weathered the COVID-19 storm in Tshwane District, South Africa. S. Afr. J. HIV Med. 2024, 25, 1557. [Google Scholar] [CrossRef] [PubMed]
- Dorward, J.; Khubone, T.; Gate, K.; Ngobese, H.; Sookrajh, Y.; Mkhize, S.; Jeewa, A.; Bottomley, C.; Lewis, L.; Baisley, K.; et al. The impact of the COVID-19 lockdown on HIV care in 65 South African primary care clinics: An interrupted time series analysis. Lancet HIV 2021, 8, e158–e165. [Google Scholar] [CrossRef] [PubMed]
- Jardim, C.G.R.; Zamani, R.; Akrami, M. Evaluating the Impact of the COVID-19 Pandemic on Accessing HIV Services in South Africa: A Systematic Review. Int. J. Env. Res. Public Health 2022, 19, 11899. [Google Scholar] [CrossRef]
- WHO/UNICEF. Estimates of National Immunization Coverage. Available online: https://worldhealthorg.shinyapps.io/wuenic-trends/ (accessed on 4 October 2024).
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.C.; Croda, J.; Hill, P.C.; et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet Glob. Health 2022, 10, e1307–e1316. [Google Scholar] [CrossRef]
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar] [CrossRef]
- Cardoso Pinto, A.M.; Ranasinghe, L.; Dodd, P.J.; Budhathoki, S.S.; Seddon, J.A.; Whittaker, E. Disruptions to routine childhood vaccinations in low- and middle-income countries during the COVID-19 pandemic: A systematic review. Front. Pediatr. 2022, 10, 979769. [Google Scholar] [CrossRef]
- Szkwarko, D.; Hirsch-Moverman, Y.; Du Plessis, L.; Du Preez, K.; Carr, C.; Mandalakas, A.M. Child contact management in high tuberculosis burden countries: A mixed-methods systematic review. PLoS ONE 2017, 12, e0182185. [Google Scholar] [CrossRef]
- Ayieko, A.; Abuogi, L.; Simchowitz, B.; Bukusi, E.A.; Smith, A.H.; Reingold, A. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: A meta-analysis. BMC Infect. Dis. 2014, 14, 91. [Google Scholar] [CrossRef]
- Thee, S.; Basu Roy, R.; Blazquez-Gamero, D.; Falcon-Neyra, L.; Neth, O.; Noguera-Julian, A.; Lillo, C.; Galli, L.; Venturini, E.; Buonsenso, D.; et al. Treatment and Outcome in Children With Tuberculous Meningitis: A Multicenter Pediatric Tuberculosis Network European Trials Group Study. Clin. Infect. Dis. 2022, 75, 372–381. [Google Scholar] [CrossRef]
- van Toorn, R.; Schaaf, H.S.; Laubscher, J.A.; van Elsland, S.L.; Donald, P.R.; Schoeman, J.F. Short Intensified Treatment in Children with Drug-susceptible Tuberculous Meningitis. Pediatr. Infect. Dis. J. 2014, 33, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Baloyi, D.P.; Myburgh, H.; Bester, D.; Anthony, M.G.; Switala, J.; Schaaf, H.S.; Naidoo, L.; Solomons, R.; Nuttall, J.; Murray, J.; et al. Navigating complex care pathways-healthcare workers’ perspectives on health system barriers for children with tuberculous meningitis in Cape Town, South Africa. PLOS Glob. Public Health 2024, 4, e0003518. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, J.; Malan, G.; van Toorn, R.; Springer, P.; Parker, F.; Booysen, J. Home-based treatment of childhood neurotuberculosis. J. Trop. Pediatr. 2009, 55, 149–154. [Google Scholar] [CrossRef]
- van Elsland, S.L.; van Dongen, S.I.; Bosmans, J.E.; Schaaf, H.S.; van Toorn, R.; van Furth, A.M. Cost-effectiveness of home-based vs. in-hospital treatment of paediatric tuberculous meningitis. Int. J. Tuberc. Lung Dis. 2018, 22, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
| Prior to COVID-19 Pandemic b | During COVID-19 Pandemic c | Total | p-Value a | |
|---|---|---|---|---|
| n (%) | n (%) | N (%) | ||
| Number (%) | 198 (75.3) | 65 (24.7) | 263 (100.0) | |
| Age bands | ||||
| 0 to <2 y | 98 (49.5) | 16 (24.6) | 114 (43.3) | 0.002 |
| 2 to <5 y | 57 (28.8) | 26 (40.0) | 83 (31.6) | |
| 5 to <13 y | 43 (21.7) | 23 (35.4) | 66 (25.1) | |
| Sex | ||||
| Male | 96 (48.7) | 40 (60.6) | 136 (51.7) | 0.095 |
| Female | 101 (51.3) | 26 (39.4) | 127 (48.3) | |
| Nutrition status (weight for age z-score) d | ||||
| Severely underweight | 38 (24.2) | 13 (23.6) | 51 (24.1) | 0.978 |
| Underweight | 33 (21.0) | 11 (20.0) | 44 (20.8) | |
| Normal weight | 86 (54.8) | 31 (56.4) | 117 (55.2) | |
| BCG vaccinated | ||||
| Yes | 104 (52.5) | 33 (50.8) | 137 (52.1) | 0.267 |
| No | 35 (17.7) | 7 (10.8) | 42 (16.0) | |
| Vaccination status recorded as unknown/not recorded | 59 (29.8) | 25 (38.5) | 84 (31.9) | |
| TB contact history in the last 12 months | ||||
| Yes | 79 (39.9) | 21 (32.3) | 100 (38.0) | 0.307 |
| No | 98 (49.5) | 33 (50.8) | 131 (49.8) | |
| No data on contact history | 21 (10.6) | 11 (16.9) | 32 (12.2) | |
| Eligible for TPT e (n = 99) | ||||
| Yes | 68 (87.2) | 19 (90.5) | 87 (87.9) | 0.681 |
| No | 10(12.8) | 2(9.5) | 12 (12.1) | |
| TPT initiated f (n = 87) | ||||
| Yes | 9 (13.2) | 1 (5.3) | 10 (11.5) | 0.147 |
| No | 26 (38.2) | 4 (21.1) | 30 (34.5) | |
| Unknown | 33 (48.5) | 14 (73.7) | 47 (54.0) | |
| TBM stage at admission | ||||
| Stage 1 | 39 (19.7) | 15 (23.1) | 54 (20.5) | 0.832 |
| Stage 2a | 50 (25.3) | 16 (24.6) | 66 (25.1) | |
| Stage 2b | 41 (20.7) | 13 (20.0) | 54 (20.5) | |
| Stage 3 | 59 (29.8) | 20 (30.8) | 79 (30.0) | |
| Not recorded | 9 (4.5) | 1 (1.5) | 10 (3.8) | |
| HIV status | ||||
| Positive | 7 (3.5) | 10 (15.4) | 17 (6.5) | 0.001 |
| Exposed uninfected | 18 (9.1) | 9 (13.8) | 27 (10.3) | |
| Negative | 168 (84.8) | 45 (69.2) | 213 (81.0) | |
| HIV status unknown | 5 (2.0) | 1 (1.5) | 6 (2.3) | |
| If living with HIV g (n = 16) | ||||
| Newly diagnosed | 2 (33.3) | 4 (40.0) | 6 (37.5) | 0.411 |
| On ART preceding TBM diagnosis | 3 (50.0) | 2 (20.0) | 5 (31.2) | |
| Not on ART at time of TBM diagnosis | 1 (16.7) | 4 (40.0) | 5 (31.2) | |
| CSF Xpert (n = 192) h | ||||
| Positive | 40 (28.8) | 14 (26.4) | 54 (28.1) | 0.510 |
| Trace positive i | 15 (10.8) | 9 (17.0) | 24 (12.5) | |
| Negative | 84 (60.4) | 30 (56.6) | 114 (59.4) | |
| CSF culture (n = 77) j | ||||
| Positive | 13 (22.0) | 5 (27.8) | 18 (23.4) | 0.614 |
| Negative | 46 (78.0) | 13 (72.2) | 59 (76.6) | |
| Microbiological confirmation by CSF or another specimen k | ||||
| Xpert Ultra and/or culture positive | 128 (64.6) | 37 (56.9) | 165 (62.7) | 0.520 |
| Xpert Ultra and/or culture negative | 64 (32.3) | 26 (40.0) | 90 (34.2) | |
| Xpert Ultra and culture missing | 6 (3.0) | 2 (3.1) | 8 (3.0) | |
| Treatment received | ||||
| Drug-susceptible TBM | 185 (93.4) | 61 (93.8) | 246 (93.5) | 0.907 |
| Drug-resistant TBM l | 13 (6.6) | 4 (6.2) | 17 (6.5) |
| IRR a | 95% CI | p-Value | |
|---|---|---|---|
| Overall | 0.57 | 0.39–0.84 | 0.004 |
| Age stratified | |||
| 0 to <2 years | 0.31 | 0.15–0.62 | 0.001 |
| 2 to <5 years | 0.92 | 0.51–1.67 | 0.793 |
| 5 to <13 years | 0.70 | 0.34–1.44 | 0.333 |
| Prior to COVID-19 Pandemic | During COVID-19 Pandemic | Total | p-Value c | |
|---|---|---|---|---|
| n (%) | n (%) | N (%) | ||
| Number (%) | 198 (75.3) | 65 (24.7) | 263 (100) | |
| Outcome at hospital discharge (n = 263) | ||||
| Alive | 187 (94.4) | 63 (96.9) | 250 (95.1) | 0.424 |
| Died | 11 (5.6) | 2 (3.1) | 13 (4.9) | |
| Healthcare facility referred to after discharge (n = 250) | ||||
| Home-based care | 93 (49.7) | 39 (61.9) | 132 (52.8) | 0.002 a |
| Medium-term facility | 0 (0.0) | 1 (1.6) | 1 (0.4) | |
| TB hospital | 88 (47.1) | 16 (25.4) | 104 (41.6) | |
| Other | 6 (3.2) | 6 (9.5) | 12 (4.8) | |
| Not recorded | 0 (0.0) | 1 (1.6) | 1 (0.4) | |
| TBM Treatment final outcomes a (n = 263) | ||||
| Cured/Treatment completed | 155 (78.3) | 49 (75.4) | 204 (77.6) | 0.772 |
| Lost to follow-up | 19 (9.6) | 8 (12.3) | 27 (10.3) | |
| Died | 22 (11.1) | 8 (12.3) | 30 (11.4) | |
| Treatment failure | 2 (1.0) | 0 (0.0) | 2 (0.8) | |
| Binary treatment outcomes b (n = 263) | ||||
| Favourable | 155 (78.3) | 49 (75.4) | 204 (77.6) | 0.627 |
| Unfavourable | 43 (21.7) | 16 (24.6) | 59 (22.4) |
| Predictor Variable | OR a | 95% CI | p-Value | aOR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Age band (n = 263) | ||||||
| 0 to <2 years | Reference | |||||
| 2 to <5 years | 2.80 | 1.38–5.64 | 0.004 | 2.62 | 1.28–5.39 | 0.009 |
| 5 to <13 years | 3.28 | 1.58–6.81 | 0.001 | 2.91 | 1.35–6.28 | 0.006 |
| Sex (n = 263) | ||||||
| Male | ||||||
| Female | 0.64 | 0.36–1.13 | 0.125 | 0.74 | 0.40–1.35 | 0.323 |
| HIV (n = 257) | ||||||
| Negative | Reference | |||||
| Exposed uninfected | 1.87 | 0.79–4.43 | 0.157 | 1.91 | 0.79–4.63 | 0.152 |
| Positive | 5.19 | 1.92–14.80 | 0.001 | 4.01 | 1.39–11.62 | 0.010 |
| BCG vaccination (n = 179) | ||||||
| No | Reference | |||||
| Yes | 1.59 | 0.64–3.91 | 0.315 | – | – | – |
| TPT initiation (n = 40) | – | – | – | |||
| No | Reference | |||||
| Yes | 0.72 | 0.07–7.34 | 0.783 | – | – | – |
| TBM staging (n = 253) | – | – | – | |||
| Stage 1 | Reference | |||||
| Stage 2a | 0.83 | 0.37–1.89 | 0.660 | |||
| Stage 2b | 0.82 | 0.35–1.95 | 0.661 | |||
| Stage 3 | 0.88 | 0.40–1.93 | 0.752 | |||
| Nutrition status (n = 212) | ||||||
| Severely underweight | Reference | |||||
| Underweight | 0.97 | 0.38–2.47 | 0.956 | |||
| Normal weight | 1.05 | 0.50–2.23 | 0.892 | |||
| Outcome at discharge (n = 263) | – | – | – | |||
| Alive | Reference | |||||
| Died | 0.54 | 0.12–2.50 | 0.431 | |||
| Binary Treatment outcomes b (n = 263) | – | – | – | |||
| Unfavourable | Reference | |||||
| Favourable | 0.85 | 0.44–1.64 | 0.627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namukuta, V.E.; Smith, M.; Bester, D.; van Niekerk, M.; Solomons, R.; van Toorn, R.; Schaaf, H.S.; Seddon, J.A.; Rabie, H.; Davies, M.-A.; et al. Incidence, Disease Spectrum, and Outcomes of Tuberculous Meningitis in South African Children: The Initial Impact of COVID-19. Trop. Med. Infect. Dis. 2025, 10, 127. https://doi.org/10.3390/tropicalmed10050127
Namukuta VE, Smith M, Bester D, van Niekerk M, Solomons R, van Toorn R, Schaaf HS, Seddon JA, Rabie H, Davies M-A, et al. Incidence, Disease Spectrum, and Outcomes of Tuberculous Meningitis in South African Children: The Initial Impact of COVID-19. Tropical Medicine and Infectious Disease. 2025; 10(5):127. https://doi.org/10.3390/tropicalmed10050127
Chicago/Turabian StyleNamukuta, Victoria E., Mariette Smith, Danite Bester, Magriet van Niekerk, Regan Solomons, Ronald van Toorn, Hendrik Simon Schaaf, James A. Seddon, Helena Rabie, Mary-Ann Davies, and et al. 2025. "Incidence, Disease Spectrum, and Outcomes of Tuberculous Meningitis in South African Children: The Initial Impact of COVID-19" Tropical Medicine and Infectious Disease 10, no. 5: 127. https://doi.org/10.3390/tropicalmed10050127
APA StyleNamukuta, V. E., Smith, M., Bester, D., van Niekerk, M., Solomons, R., van Toorn, R., Schaaf, H. S., Seddon, J. A., Rabie, H., Davies, M.-A., Hesseling, A. C., & du Preez, K. (2025). Incidence, Disease Spectrum, and Outcomes of Tuberculous Meningitis in South African Children: The Initial Impact of COVID-19. Tropical Medicine and Infectious Disease, 10(5), 127. https://doi.org/10.3390/tropicalmed10050127







