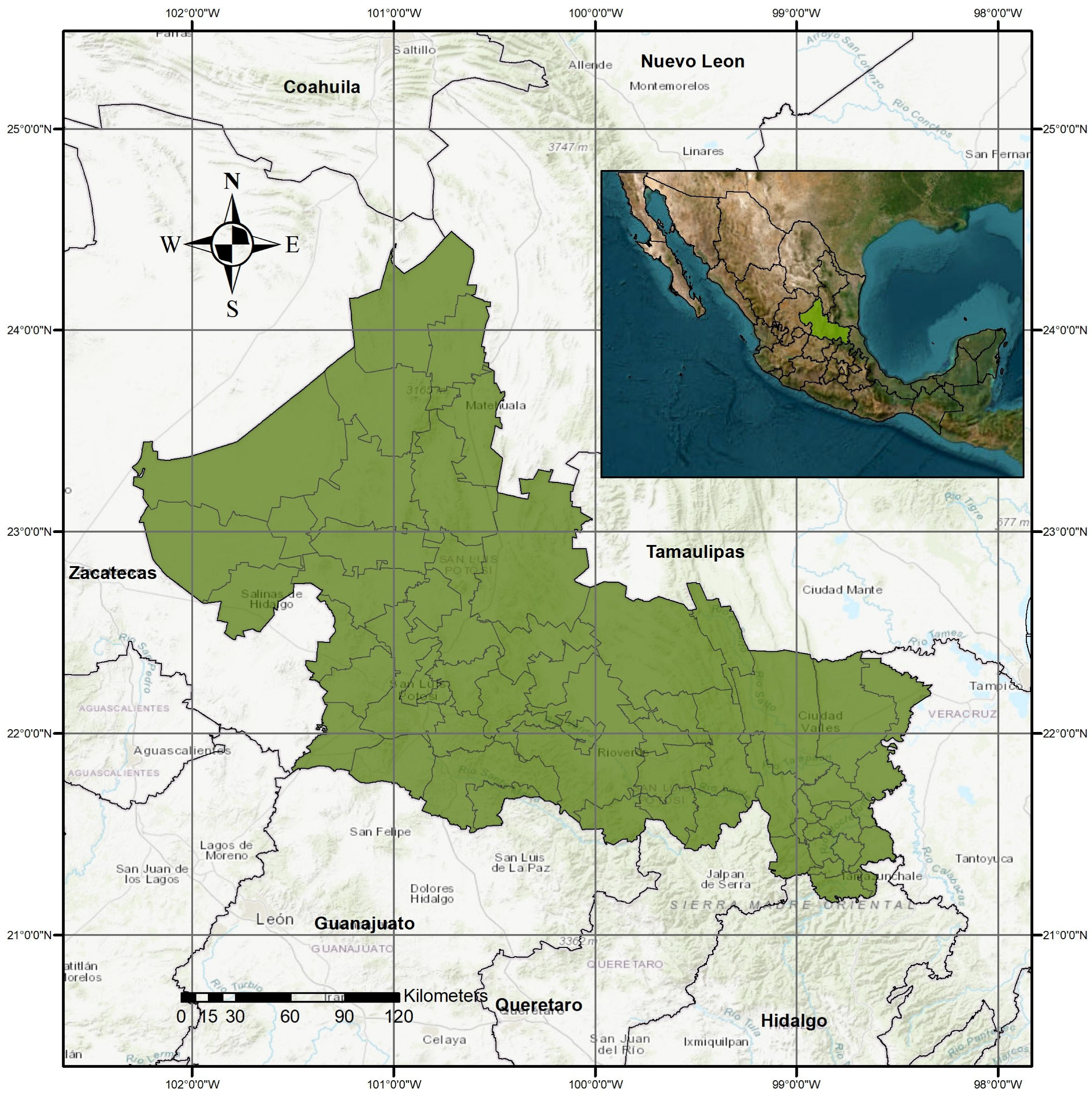
1. Introduction
Dengue is an infectious viral disease caused by the dengue virus, of which there are four serotypes, and is spread through mosquito bites [
1]. Consequently, individuals can be infected up to four times. The primary vectors for this disease are
Aedes aegypti mosquitoes, with
Aedes albopictus playing a secondary role in transmission.
Dengue is a global public health problem; in 2024, the World Health Organization (WHO) estimated that half of the world’s population is at risk of contracting dengue, with between 100 and 400 million infections occurring annually. Although most people who contract dengue are asymptomatic, the disease can sometimes worsen, requiring hospitalization; in severe cases, it may be fatal [
2]. Moreover, in 2023, it was reported that there were 2.8 million recorded cases of dengue in the Americas in the year 2022, more than double the 1.2 million cases reported in 2021 [
1].
In Mexico, the Secretary of Health in epidemiological week 52 of 2022 revealed that 6746 cases of dengue were reported in 2021; this number nearly doubled in 2022, reaching 12,671 cases [
3]. Similarly, in epidemiological week 52 of 2023 in the Mexican state of San Luis Potosí, it was revealed that 566 confirmed cases were reported in 2023, with 108 cases exhibiting severe to alarming symptoms [
4].
Dengue primarily occurs in tropical and subtropical regions worldwide, particularly in urban and semi-urban areas [
2]. However, Man et al. (2023) conducted a systematic search for articles that evaluated the prevalence or cumulative incidence of dengue and found that its incidence in rural areas can equal or exceed that in urban regions [
5]. To add to this problem, in 2023, the United Nations Organization (UNO) declared that global warming is expected to increase dengue cases worldwide [
6]. In a study carried out in Colombia, in three different ecosystems, using generalized linear models and generalized aditivis models to examine geographic data. The results showed that various factors such as migratory movements, inadequate sanitation, inappropriate water supply, among others, favor the development and spread of the vector [
7].
Relevant studies on dengue have used spatial and statistical modeling. such as the one developed to predict the geographical distribution of dengue cases in the metropolitan region of São Paulo, Brasil; MaxEnt distribution modeling was applied, incorporating sociodemographic and housing data [
8]. Recently, several high-resolution geospatial approaches have advanced the analysis of dengue risk. A study was done in an Amazon sub region of Colombia to develop dengue risk maps, based on the ordinary least squares regression technique and multicriteria analysis [
9]. Another investigation in Bhopal, India, combining Geographic Information Systems with machine-learning models to delineate risk areas from environmental, demographic, and infrastructural variables, demonstrating progress toward more precise predictive frameworks [
10]. In research conducted in Zhongshan, China, producing one-kilometer-scale risk maps that captured fine spatial variability in regions with limited entomological data [
11].
In Dhaka, Bangladesh, a study was developed, applying a multicriteria decision-making approach (MCDM + GIS) that integrated weighted environmental and demographic factors to estimate dengue susceptibility in densely populated urban settings. Collectively, these studies illustrate the consolidation of geospatial methodologies grounded in multivariable analysis and fine spatial resolution, designed to enhance the precision of vulnerability assessment and to clarify the influence of socio-environmental factors on dengue distribution [
12].
Dengue became endemic in Mexico during the 1980s, prompting governmental initiatives aimed at prevention and control through program implementation. However, these efforts were insufficient to curb the rise in cases, which spread to over 90% of the country’s states by 2000 [
13]. Dengue cases have increased significantly throughout the state of San Luis Potosí, even in municipalities where this disease did not occur, so it is important to know the conditions that favor the development and spread of the Aedes aegypti vector. Consequently, this study aims to develop geospatial and statistical models to estimate vulnerability to classic dengue and hemorrhagic dengue fever at the rural and urban basic geostatistical area (BGA) level in San Luis Potosí State, Mexico. It also seeks to assess these temporal evolution and spatial distribution of these dengue variants at the BGA level, advancing multivariate models to determine population vulnerability.
3. Results
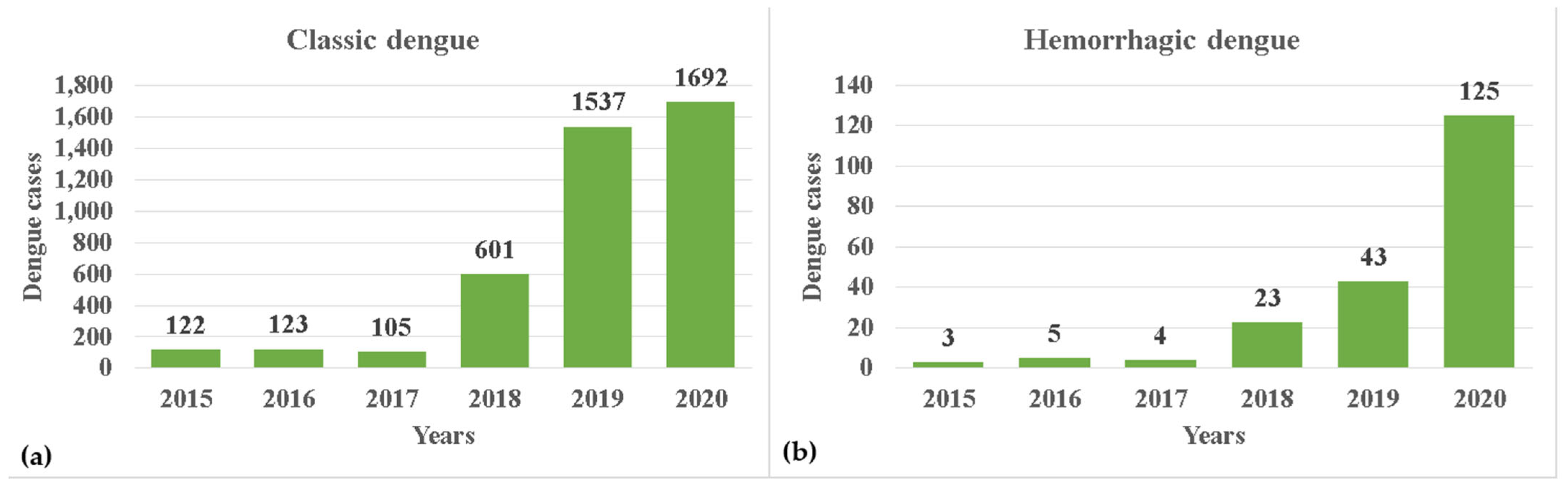
During the study period, 4383 cases of dengue were recorded. The evolution of classic dengue increased each year during the study period, except in 2017, the cumulative number reached 4180 (
Figure 4a). Meanwhile, the evolution of hemorrhagic dengue showed constant growth each year, reaching a cumulative total of 203. This means that for every case reported, 20.59 cases of classic dengue were reported (
Figure 4b).
Regarding the independent variables, more than 50% have a standard deviation greater than their respective means. Considering the mean and median, one can observe that, in the absence of dengue, the values are lower for the following variables: average annual temperature, average annual rainfall, and population. Conversely, the values are higher for the percentage of homes with piped water and those with drainage. For the remaining variables, a priori, it is not possible to specify any others. The reality is that the combination of these variable values can favor mosquito development (
Table 2).
3.1. Bivariate Logistic Regression Analysis
All independent variables (factors) demonstrated a statistically significant relationship with the two types of dengue, except for the variable ‘Elevation above sea level’ in hemorrhagic dengue. Aside from ‘Elevation above sea level,’ the beta coefficients for all environmental variables were positive, indicating that they are risk factors. In contrast, variables related to proximity had negative coefficients, suggesting that an increase in these variables leads to a decrease in risk. This is similarly observed for the percentage of homes with piped water and homes with drainage. Conversely, the variable ‘Population’ has a positive coefficient, indicating that as the population increases, so does the risk (
Table 3).
3.2. Preliminary Multivariate Logistic Regression Analysis
Table 4 reveals that the variable distance to agricultural areas was not statistically significant, whereas eight variables were not significant for dengue hemorrhagic fever. However, both models indicated multicollinearity among several factors, such as distance to roads for classic and hemorrhagic dengue fever.
3.3. Final Multivariate Logistic Regression Analysis
When variables that exhibited multicollinearity were excluded from the models, all variables were statistically significant in both types of dengue. In both models, the signs of the beta coefficients align with theoretical expectations; an increase in variables with negative beta coefficients indicates a decreased risk of contracting dengue, while those with positive coefficients increase it. Additionally, the omnibus tests for classic and hemorrhagic dengue showed p < 0.001, allowing us to interpret the logistic model. This suggests that at least one is significantly different from zero among all the model coefficients.
The models with standardized variables identified the same three risk factors for vulnerability to classic and hemorrhagic dengue. The most significant factor for hemorrhagic dengue was the mean annual temperature at the BGA level, whereas for classic dengue fever, it was the second most significant factor. Significant differences were observed in the means and medians, which were lower in BGAs without dengue, followed by BGAs with hemorrhagic dengue, and, finally, those with classic dengue fever. The mean and median temperatures of BGAs with dengue were 22.9 and 24.2 °C, respectively, with minimum and maximum temperatures ranging from 16.8 to 27.1 °C.
The population of the BGA is the primary factor for classic dengue fever and the third for hemorrhagic dengue. Increasing population density heightens vulnerability because an infected female mosquito is more likely to infect additional individuals. Similarly, rainfall was identified as the second most significant factor for hemorrhagic dengue and the third for classic dengue fever (
Table 5).
The Kappa test result, assessing the concordance between BGAs with registered cases and those without versus the presence or absence of dengue estimated by the models for the same BGAs, showed a Kappa coefficient of 0.791 (
p < 0.001) for classic dengue fever and a Kappa of 0.459 (
p < 0.001) for dengue hemorrhagic fever. Over 50% of the population was identified as having a high risk (
Table 6).
Figure 5 illustrates that the area under the curve demonstrates a strong ability to correctly distinguish whether a particular BGA has dengue, with values of 0.957 (
Figure 5a) for classic dengue fever, and 0.930 (
Figure 5c) for hemorrhagic dengue. In both instances, the confidence interval does not include 0.5, indicating a significant difference in the BGAs susceptible to dengue. The optimal point where sensitivity and specificity are maximized is 0.975 sensitivity and 0.816 specificity for classic dengue fever. For hemorrhagic dengue, these values are 0.926 and 0.768, respectively. Furthermore, the optimal decision threshold for BGA accurately identifying a BGA as having a true positive or true negative result was determined to be (0.184, 0.957) for classic dengue fever (
Figure 5b) and (0.232, 0.926) for hemorrhagic dengue
Figure 5d. At these thresholds, the maximum difference between sensitivity and 1-specificity was noted.
The dotted diagonal line, called non-discriminatory, indicates that the sensitivity and specificity values are equal to 0.5 (
Figure 5a,c). The dotted lines indicate the coordinates for identifying the optimal point (where they intersect) to accurately identify a true positive or negative result (
Figure 5b,c).
Classic dengue cases are located mainly in the Huasteca Zone and in the most populated municipalities such as San Luis Potosí and Soledad de Graciano Sánchez, and there are also cases in the north of the State (
Figure 6A). While cases of hemorrhagic dengue are less frequent, they follow a similar pattern to those of classic dengue. However, it is clear that in the north of the state, the problem is more serious than with classic dengue (
Figure 6B).
5. Discussion
In this study, 13.7% of classic dengue cases and 27.6% of hemorrhagic dengue cases were reported to occur in the desert and colder areas of the central and northern parts of the state, where dengue had not previously been reported. This shift is attributed to changes in infection patterns. As noted in [
22], these changes are driven by extreme meteorological phenomena, climate change, and the El Niño phenomenon (it is a natural and recurring weather pattern that affects the global climate. It warms the surface of the eastern and central Pacific Ocean), in conjunction with the adaptive capacity of mosquitoes [
41].
The Huasteca region features characteristics conducive to the proliferation of the Aedes aegypti mosquito, with climates ranging from warm humid and warm subhumid to temperate humid. Temperatures can fluctuate between 50 °C and −2 °C, and rainfall is abundant [
42]. Several factors contributing to this health problem are interrelated, such as rainfall and humidity [
1,
43], as well as sanitation, drainage, and population density [
2]. Therefore, multivariate logistic models developed for this study are robust in evaluating vulnerability to dengue as they consider the synergies between independent variables, where the simultaneous presence of several risk factors not only adds to vulnerability but also multiplies risk [
44]. Thus, the interpretation of each factor in assessing vulnerability assumes that other factors remain constant.
The mean annual temperature and median recorded in this study are lower in the BGAs without dengue (between 0 and 9.9 °C) and with dengue (between 22.2 and 24.2 °C), indicating a relationship between this variable and the disease and is within the ranges of several studies, such as [
8], which identified that mean temperatures between 18 and 25 °C create environments suitable for dengue development. Ref. [
45] confirmed that optimal temperature ranges vary between 23 and 29 °C. Ref. [
46] determined that thermal levels conducive to dengue propagation occur at 29 °C, whereas [
47] found that the mosquito lifespan shortens at 30 °C and extends significantly at 26 °C. Ref. [
48] noted that mosquito development and survival increase at 26–28 °C.
Applying the exponential to the beta coefficient of temperature (exp(0.198)), this study found that for each 1 °C increase in temperature in the BGA, and keeping the rest of the model variables fixed, the vulnerability risk increased by 21.9% for classic dengue fever; this risk was not significant for hemorrhagic dengue (
Table 4). Additionally, the mean and median population values were higher in BGAs with dengue presence. Of the examined cases, 47.1% of classic dengue fever and 57.3% of hemorrhagic dengue were reported in the ten municipalities with the highest population density per km
2 [
30]. Furthermore, vulnerability to dengue increased by 0.02% for classic dengue fever and 0.007% for classic dengue fever and hemorrhagic dengue, respectively, for each additional inhabitant in the BGA, keeping the rest of the model variables fixed (
Table 4). This finding is consistent with [
2], which states that population growth is a risk factor for mosquito development. Ref. [
49] also found that the incidence ratio of dengue increases with rising population density per km
2. Ref. [
50] supports the relationship between population density and dengue cases. Ref. [
51] showed that a population density of over 1000 inhabitants per km
2 in urban areas is associated with significant increases in dengue cases. In contrast, refs. [
52,
53] found no significant correlation between population density and dengue. Certain high-density urban sectors are inhabited predominantly by upper social classes, which have efficient infrastructure and heightened awareness of risk.
In this study, we found that for each millimeter increase in rainfall, and keeping the rest of the model variables fixed, the vulnerability of the BGA to classic and hemorrhagic dengue increased by 0.09% and 0.15%, respectively (
Table 4). Additionally, both the mean and the median are higher in BGAs with recorded dengue cases. Ref. [
54] found a positive correlation between the number of dengue cases and rainfall. Ref. [
55] observed an r = 0.6214 between the magnitude of rainfall and the recurrence of dengue cases in Paraguay. Furthermore, ref. [
56] found a correlation between dengue cases and rainfall ranging from 83 to 15 mm; each rainy day increased the dengue incidence rate. Refs. [
49,
57] confirmed that rainfall is related to the oviposition of female mosquitoes.
The mean and median humidity levels were higher in BGAs with dengue cases. Moreover, in the bivariate analysis (
Table 3), humidity was identified as a risk factor for classic and hemorrhagic dengue vulnerability, consistent with [
54], who found that a 1% increase in humidity corresponded to a linear increase in dengue cases. Humidity was also used as a variable factor to estimate the incidence of various diseases, including dengue [
58]. Ref. [
59] reported that female mosquitoes survived twice as long between 25 °C and 80% humidity. This result indirectly aligns with the present findings, as the medians of these two independent variables are close to the published values. Nevertheless, humidity emerged as a protective factor in the multivariate models (
Table 4 and
Table 5). This contradictory relationship could be partially explained by considering all analyzed factors’ simultaneous effects.
Land use and cover changes play a crucial role in the spread of vector-borne diseases. The results indicate that the farther a BGA is from agricultural areas, the lower the risk for vulnerability to classic dengue fever. The mean is higher in BGAs with dengue cases. These findings are consistent with those of [
60], who confirmed the recurrence of dengue cases close to agricultural areas. This study found that individuals living one meter farther from agricultural areas were 0.04% less likely to contract classic dengue and 0.05% less likely to contract hemorrhagic dengue (
Table 3), similar to the findings of [
19], who located vector breeding sites in plantations in Sri Lanka. Ref. [
61] concluded that farmers near plantations have an almost eightfold increased risk of dengue virus infection (relative risk 7.94, 95% CI 2.29–27.5).
Ref. [
62] argued that the global expansion of agriculture intensifies the spread of vector-borne diseases because vegetation and bodies of water provide ideal habitats for mosquitoes. Additionally, fumigations eliminate mosquitoes’ natural predators, leading to an overpopulation of vectors, including
Aedes aegypti [
63]. The median elevation above sea level is lower in the BGAs where dengue was not present. However, elevation proved to be a protective factor against vulnerability in the final multivariate model for classic dengue fever, although no general consensus exists regarding the altitude at which Aedes aegypti mosquitoes can survive. According to [
64], elevations above 1000 m are unfavorable for mosquito survival.
Another study reported that these mosquitoes can survive at altitudes lower than 700 m. This result aligns with findings in the present study, where the median elevation is 153.0 m for classic dengue fever and 201.0 m for hemorrhagic dengue, with survival recorded even at 1700 m [
65]. Similarly, ref. [
24] suggests that low-elevation areas favor dengue transmission. Additionally, ref. [
66] confirmed that the flight ceiling of
Ae. aegypti in Mexico from 1700 m to 2130 m. Ref. [
67] detected
Ae. aegypti vectors infected with dengue in Bellos, Colombia, at altitudes between 1900 and 2300 m. Conversely, it is stated that vectors inhabit below 1982 m [
68]. Ref. [
69] found a significant difference in mosquito percentages by elevation: 85.24% below 500 m, 13.06% below 1000 m, and 1.7% at 1000 m and above. These discrepancies in altitude parameters are attributed to mosquito adaptation and the interaction of environmental and population conditions. An inverse relationship was observed regarding the percentage of homes with piped water: as this percentage increased, vulnerability to dengue decreased. Without piped water, water must be stored in exposed containers, creating favorable conditions for mosquito breeding [
70]. Similarly, the drainage index showed an inverse relationship: as the percentage of homes with drainage increased, vulnerability to dengue decreased.
Dengue not only spreads in clean water but also turbid water [
71]. The methodology used in this study is relevant as decision-making in health has been supported by geographic information systems [
72].
6. Conclusions
The findings can offer valuable insights for designing and implementing specific strategies by the State Health Services. It is also the responsibility of state and municipal authorities and society to address this complex issue holistically. The identified factors, such as population, the percentage of homes with piped water, and drainage index, can be modified to a certain extent. Since these factors are interrelated, altering one may simultaneously affect others.
Despite its contributions, this study has several limitations. Firstly, the analysis relies on aggregated data at the BGA level, which may mask fine-scale, household-level risk factors. Secondly, the model’s predictive power is constrained by the resolution and accuracy of the input data, such as the land use classification and climatic interpolations. Variables potentially related to the issue, such as schooling, education, and health promotion, were not explored. Furthermore, the cross-sectional nature of our data establishes associations but not causal relationships. Future research would benefit from longitudinal data and the inclusion of additional socioeconomic variables, such as mobility patterns and public health intervention data, to provide a more dynamic understanding of dengue vulnerability.