Prognostic Significance of CRP/Albumin, D-Dimer/Albumin, D-Dimer/Fibrinogen Ratios and Triglyceride-Glucose Index in Crimean–Congo Hemorrhagic Fever: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawman, D.W.; Feldmann, H. Crimean-Congo haemorrhagic fever virus. Nat. Rev. Microbiol. 2023, 21, 463–477. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Alkhovsky, S.V.; Avšič-Županc, T.; Bergeron, É.; Burt, F.; Ergünay, K.; Garrison, A.R.; Marklewitz, M.; Mirazimi, A.; Papa, A.; et al. ICTV Virus Taxonomy Profile: Nairoviridae 2024. J. Gen. Virol. 2024, 105, 001974. [Google Scholar] [CrossRef]
- Fillâtre, P.; Revest, M.; Tattevin, P. Crimean-Congo hemorrhagic fever: An update. Med. Mal. Infect. 2019, 49, 574–585. [Google Scholar] [CrossRef]
- Frank, M.G.; Weaver, G.; Raabe, V. Crimean Congo Hemorrhagic Fever Virus for Clinicians-Virology, Pathogenesis, and Pathology. Emerg. Infect. Dis. 2024, 30, 847–853. [Google Scholar] [CrossRef]
- Akıncı, E.; Bodur, H.; Leblebicioglu, H. Pathogenesis of Crimean-Congo hemorrhagic fever. Vector Borne Zoonotic Dis. 2013, 13, 429–437. [Google Scholar] [CrossRef]
- Karaşahin, Ö.; İnan Sarıkaya, R. The Role of Blood Urea Nitrogen and C-Reactive Protein and Their Ratios to Albumin in Predicting Mortality in Crimean-Congo Hemorrhagic Fever. Mikrobiyol. Bul. 2023, 57, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, O.; Avci, O. Relationship between Systemic Immune-inflammation Index and Mortality in Intensive Care Patients Diagnosed with Crimean-Congo Hemorrhagic Fever. J. Coll. Physicians Surg. Pak. 2022, 32, 1538–1543. [Google Scholar] [PubMed]
- Vitiello, R.; Smimmo, A.; Matteini, E.; Micheli, G.; Fantoni, M.; Ziranu, A.; Maccauro, G.; Taccari, F. Systemic Inflammation Response Index (SIRI) and Monocyte-to-Lymphocyte Ratio (MLR) Are Predictors of Good Outcomes in Surgical Treatment of Periprosthetic Joint Infections of Lower Limbs: A Single-Center Retrospective Analysis. Healthcare 2024, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Sun, M.; Wang, M.; Wang, T.; Yu, X.; Wu, D.; Guo, Z.; Li, H.; Liu, Y.; Cao, J.; et al. Triglyceride-glucose index-A novel metabolism disorder biomarker as a promising indicator for predicting postoperative 30-day infections in elderly patients undergoing gastrointestinal-related abdominal and pelvic surgery. Surgery 2024, 176, 1433–1441. [Google Scholar] [CrossRef]
- Lu, J.; Fang, W.; Lei, Y.; Yang, J. Association between D-dimer-to-albumin ratio and 28-days all-cause mortality in patients with sepsis. Sci. Rep. 2024, 14, 28361. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, Y.-Y.; Tang, J.-N.; Yang, X.-M.; Guo, Q.-Q.; Zhang, J.-C.; Cheng, M.-D.; Song, F.-H.; Wang, K.; Zhang, Z.-L.; et al. D-Dimer to Fibrinogen Ratio as a Novel Prognostic Marker in Patients After Undergoing Percutaneous Coronary Intervention: A Retrospective Cohort Study. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620948586. [Google Scholar] [CrossRef]
- Hung, K.-C.; Huang, Y.-T.; Chang, Y.-J.; Yu, C.-H.; Wang, L.-K.; Wu, C.-Y.; Liu, P.-H.; Chiu, S.-F.; Sun, C.-K. Association between Fibrinogen-to-Albumin Ratio and Prognosis of Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1678. [Google Scholar] [CrossRef]
- Swanepoel, R.; Gill, D.E.; Shepherd, A.J.; Leman, P.A.; Mynhardt, J.H.; Harvey, S. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 1989, 11 (Suppl. 4), 794–800. [Google Scholar] [CrossRef] [PubMed]
- Ergonul, O.; Celikbas, A.; Baykam, N.; Eren, S.; Dokuzoguz, B. Analysis of risk-factors among patients with Crimean-Congo hae morrhagic fever virus infection: Severity criteria revisited. Clin. Microbiol. Infect. 2006, 12, 551–554. [Google Scholar] [CrossRef]
- Al Shaqri, E.J.; Balkhair, A. Relationship of C-reactive Protein/Serum Albumin Ratio and qPitt Bacteremia Score with An All-Cause In-Hospital Mortality in Patients with Bloodstream Infections. Cureus 2024, 16, e66584. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, S.; Altunok, İ. Comparison of the Predictive Ability of the Blood Urea Nitrogen/Albumin, C-Reactive Protein/Albumin, and Lactate/Albumin Ratios for Short-Term Mortality in SARS-CoV-2-Infected Patients. Avicenna J. Med. 2023, 13, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jin, Y.X.; Liu, Y.M.; Niu, Q.; Jiang, H. Clinical Significance of Coagulation Indicators and their Correlation with Inflammatory Factors in Critical Patients with Thromboembolism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017, 25, 1776–1780. [Google Scholar]
- Nemec, H.M.; Ferenczy, A.; Christie, B.D.; Ashley, D.W., 3rd; Montgomery, A. Correlation of D-dimer and Outcomes in COVID-19 Patients. Am. Surg. 2022, 88, 2115–2118. [Google Scholar] [CrossRef]
- Küçükceran, K.; Ayranci, M.K.; Girişgin, A.S.; Koçak, S. Predictive value of D-dimer/albumin ratio and fibrinogen/albumin ratio for in-hospital mortality in patients with COVID-19. Int. J. Clin. Pract. 2021, 75, e14263. [Google Scholar] [CrossRef]
- Murat, S.; Murat, B.; Dural, M.; Mert, G.O.; Cavusoglu, Y. Prognostic value of D-dimer/fibrinogen ratio on in-hospital outcomes of patients with heart failure and COVID-19. Biomark. Med. 2021, 15, 1519–1528. [Google Scholar] [CrossRef]
- Tayal, D.; Jain, P.; Goswami, B. D-dimer—A multifaceted molecule. Horm. Mol. Biol. Clin. Investig. 2024, 45, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.M.; McKinney, M.; McDonagh, J. Diagnosis of disseminated intravascular coagulation: Role of D-dimer. Am. J. Clin. Pathol. 1989, 91, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Devaraj, S.; Jialal, I. The role of the triglyceride-glucose index as a biomarker of cardio-metabolic syndromes. Lipids Health Dis. 2024, 23, 416. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Yang, Y.; Wang, F.; Yan, Y.; Shi, X.; Dong, K.; Yu, X.; Zhang, S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc. Diabetol. 2020, 19, 58. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, B.; Shang, X.; Yang, F.; Yin, Y.; Sun, Z.; Wu, X.; Zhang, J.; Liu, Y. Triglyceride-glucose index predicts sepsis-associated acute kidney injury and length of stay in sepsis: A MIMIC-IV cohort study. Heliyon 2024, 10, e29257. [Google Scholar] [CrossRef]
- Adams-Huet, B.; Jialal, I. An Increasing Triglyceride-Glucose Index Is Associated with a Pro-Inflammatory and Pro-Oxidant Phenotype. J. Clin. Med. 2024, 13, 3941. [Google Scholar] [CrossRef]
- Gangadharan, C.; Ahluwalia, R.; Sigamani, A. Diabetes and COVID-19: Role of insulin resistance as a risk factor for COVID-19 severity. World J. Diabetes 2021, 12, 1550–1562. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Amin, A.; Ferreira, D.V.; Figueiredo, L.M. How pathogens drive adipose tissue loss in the host. Curr. Opin. Microbiol. 2025, 85, 102597. [Google Scholar] [CrossRef]
- Barreto, E.A.; Cruz, A.S.; Veras, F.P.; Martins, R.; Bernardelli, R.S.; Paiva, I.M.; Lima, T.M.; Singh, Y.; Guimarães, R.C.; Damasceno, S.; et al. COVID-19-related hyperglycemia is associated with infection of hepatocytes and stimulation of gluconeogenesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2217119120. [Google Scholar] [CrossRef]
- Onguru, P.; Dagdas, S.; Bodur, H.; Yilmaz, M.; Akinci, E.; Eren, S.; Ozet, G. Coagulopathy parameters in patients with Crimean-Congo hemorrhagic fever and its relation with mortality. J. Clin. Lab. Anal. 2010, 24, 163–166. [Google Scholar] [CrossRef]
- Ergönül, O. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006, 6, 203–214. [Google Scholar] [CrossRef]
| Variables | Patients (n = 76) | Controls (n = 38) | p-Value |
|---|---|---|---|
| Age (Years) ± SD | 52.5 ± 15.2 | 51.1 ± 14.9 | 0.603 |
| Gender Male, n (%) Female, n (%) | 33 (43.4%) 43 (56.6%) | 16 (42.1%) 22 (57.9%) | 1.000 |
| Comorbidities | |||
| Hypertension | 14 (18.4%) | 0 (0%) | |
| Diabetes mellitus | 8 (10.5%) | 0 (0%) | |
| Cardiovascular disease | 6 (7.9%) | 0 (0%) | |
| Chronic obstructive lung disease | 1 (1.3%) | 0 (0%) | |
| Laboratory findings | |||
| CAR | 0.68 ± 0.80 | 0.06 ± 0.02 | <0.001 |
| DAR | 299.76 ± 390.92 | 5.14 ± 1.17 | <0.001 |
| DFR | 48.38 ± 71.49 | 0.81 ± 0.23 | <0.001 |
| TGI | 11.44 ± 15.28 | 3.01 ± 0.97 | <0.001 |
| Laboratory Findings | Survivor (n = 71) | Non-Survivor (n = 5) | p-Value |
|---|---|---|---|
| CAR | 0.59 ± 0.66 | 1.95 ± 1.52 | 0.005 |
| DAR | 235.59 ± 358.16 | 872.25 ± 350.07 | 0.004 |
| DFR | 37.13 ± 55.36 | 185.64 ± 107.65 | 0.001 |
| TGI | 10.61 ± 15.23 | 23.22 ± 11.50 | 0.003 |
| Laboratory Findings | Non-Severe (n = 44) | Severe (n = 32) | p-Value |
|---|---|---|---|
| CAR | 0.47 ± 0.48 | 0.97 ± 1.05 | 0.052 |
| DAR | 92.27 ± 143.49 | 571.63 ± 444.99 | <0.001 |
| DFR | 11.30 ± 16.02 | 98.71 ± 85.96 | <0.001 |
| TGI | 7.31 ± 4.88 | 17.11 ± 21.78 | 0.001 |
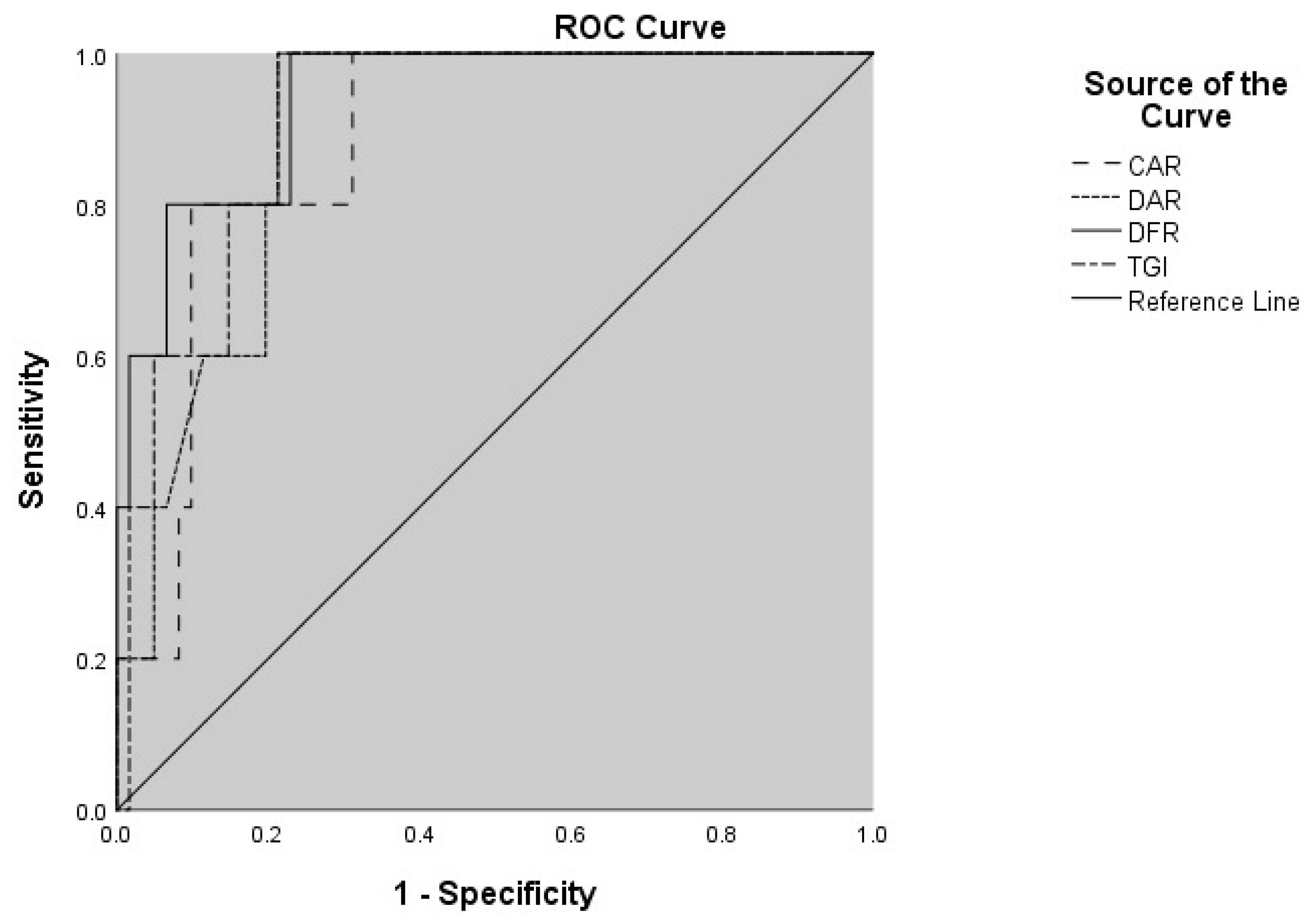
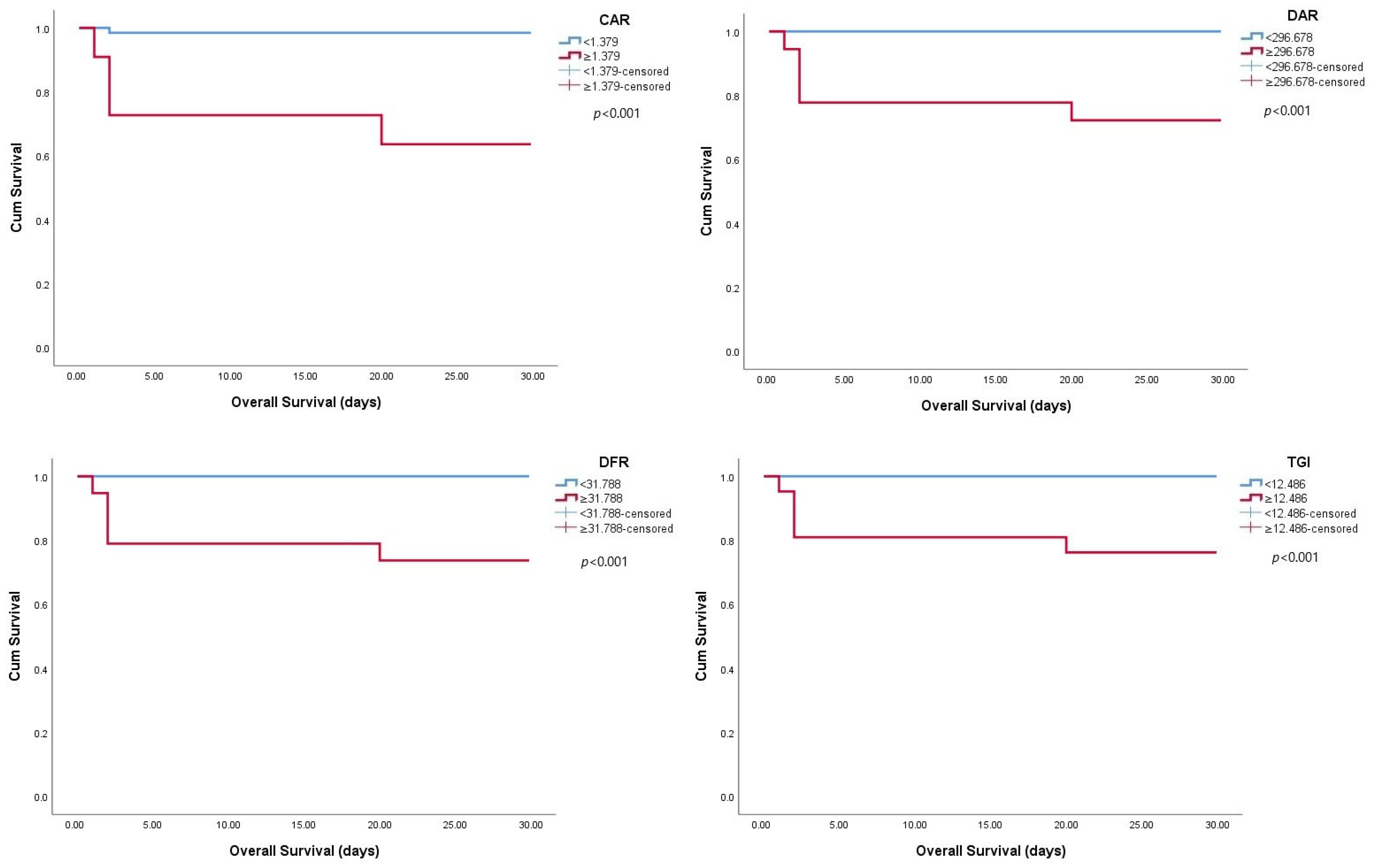
| Variables | AUC (95% CI) | Cut-Off Value | p-Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| CAR | 0.876 (0.761–0.991) | 1.379 | 0.005 | 80.0 | 90.1 |
| DAR | 0.892 (0.798–0.986) | 296.678 | 0.004 | 100.0 | 79.0 |
| DFR | 0.938 (0.852–1.000) | 31.788 | 0.001 | 100.0 | 77.0 |
| TGI | 0.899 (0.809–0.988) | 12.486 | 0.003 | 100.0 | 77.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydın, N.N.; Aydın, M. Prognostic Significance of CRP/Albumin, D-Dimer/Albumin, D-Dimer/Fibrinogen Ratios and Triglyceride-Glucose Index in Crimean–Congo Hemorrhagic Fever: A Prospective Observational Study. Trop. Med. Infect. Dis. 2025, 10, 287. https://doi.org/10.3390/tropicalmed10100287
Aydın NN, Aydın M. Prognostic Significance of CRP/Albumin, D-Dimer/Albumin, D-Dimer/Fibrinogen Ratios and Triglyceride-Glucose Index in Crimean–Congo Hemorrhagic Fever: A Prospective Observational Study. Tropical Medicine and Infectious Disease. 2025; 10(10):287. https://doi.org/10.3390/tropicalmed10100287
Chicago/Turabian StyleAydın, Nurten Nur, and Murat Aydın. 2025. "Prognostic Significance of CRP/Albumin, D-Dimer/Albumin, D-Dimer/Fibrinogen Ratios and Triglyceride-Glucose Index in Crimean–Congo Hemorrhagic Fever: A Prospective Observational Study" Tropical Medicine and Infectious Disease 10, no. 10: 287. https://doi.org/10.3390/tropicalmed10100287
APA StyleAydın, N. N., & Aydın, M. (2025). Prognostic Significance of CRP/Albumin, D-Dimer/Albumin, D-Dimer/Fibrinogen Ratios and Triglyceride-Glucose Index in Crimean–Congo Hemorrhagic Fever: A Prospective Observational Study. Tropical Medicine and Infectious Disease, 10(10), 287. https://doi.org/10.3390/tropicalmed10100287







