New Foci of Spotted Fever Group Rickettsiae Including Rickettsia honei in Western Australia
Abstract
:1. Introduction
2. Clinical Records
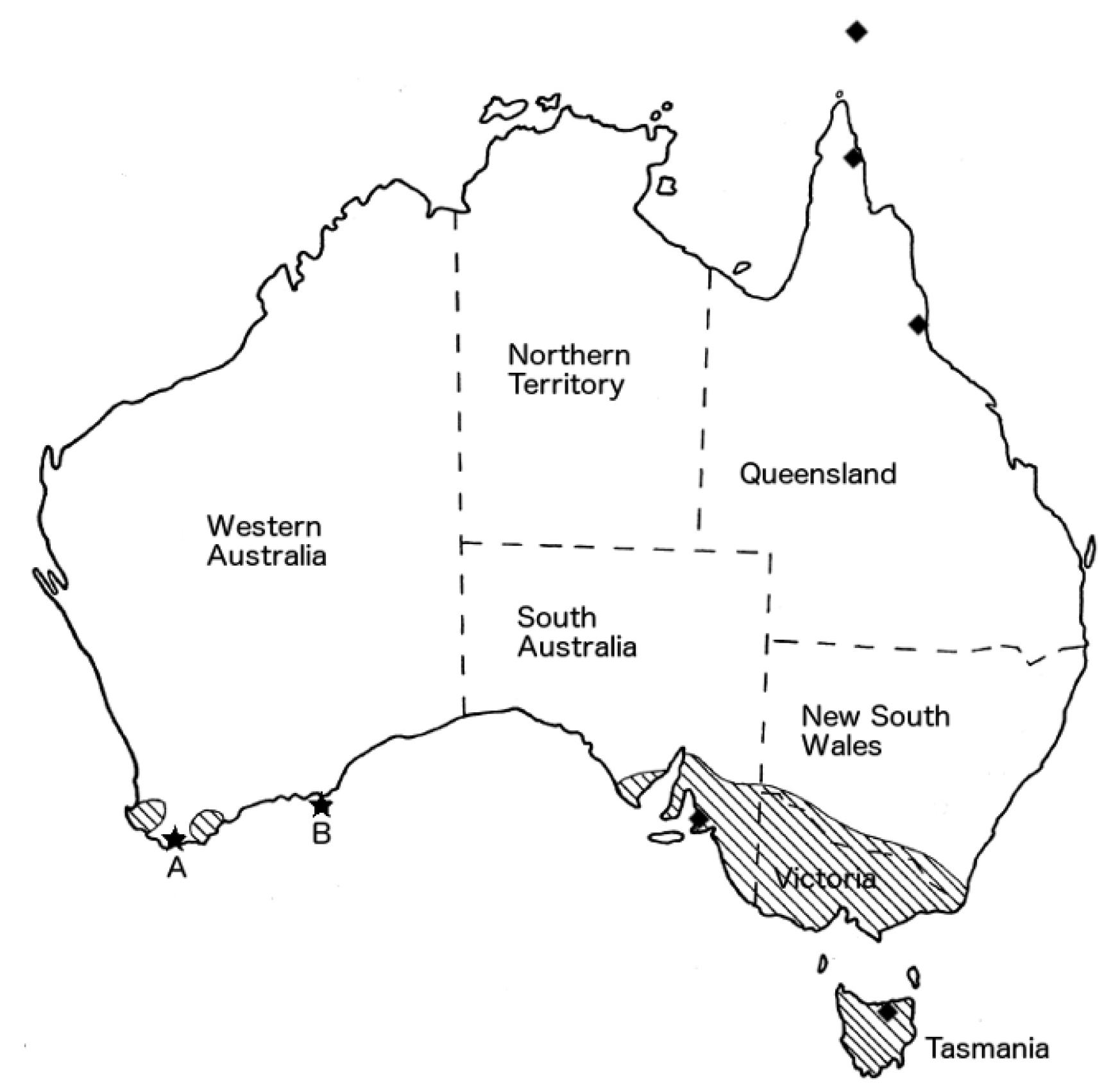
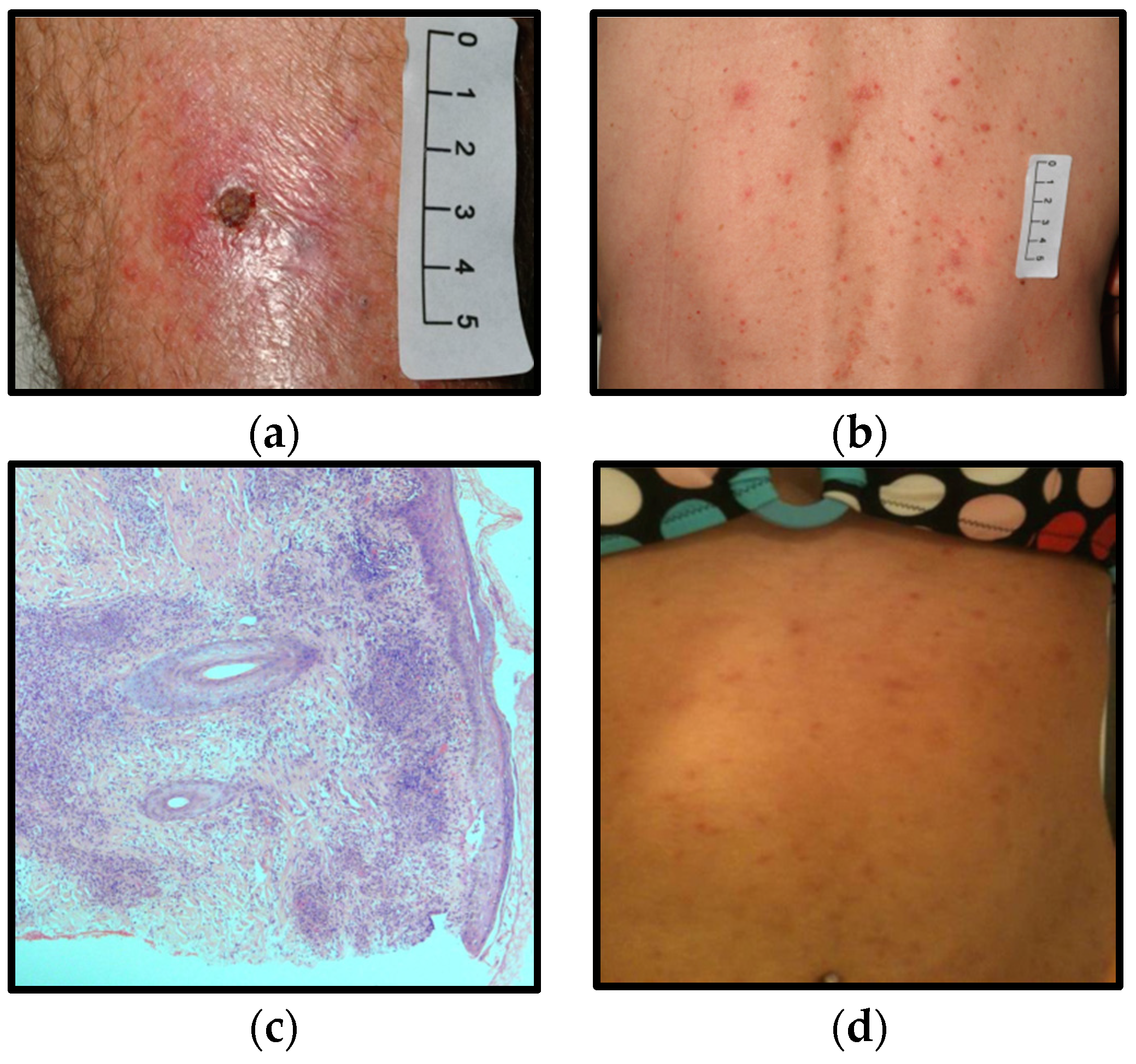
2.1. Case A
2.2. Case B
2.3. Case C
3. Discussion
Author Contributions
Conflicts of Interest
References
- Saint, E.G.; Drummond, A.F.; Thorburn, I.O. Murine typhus in Western Australia. Med. J. Aust. 1954, 2, 731–737. [Google Scholar] [PubMed]
- O’Connor, L.F.; Kelly, H.A.; Lubich, J.M.; Lindsey, R.J.; McComish, M.J. A cluster of murine typhus cases in Western Australia. Med. J. Aust. 1996, 165, 24–26. [Google Scholar] [PubMed]
- Graves, S.; Wang, L.; Nack, Z.; Jones, S. Rickettsia serosurvey in Kimberley, Western Australia. Am. J. Trop. Med. Hyg. 1999, 60, 786–789. [Google Scholar] [PubMed]
- Abdad, M.Y.; Cook, A.; Dyer, J.; Stenos, J.; Fenwick, S.G. Seroepidemiological study of outdoor recreationists’ exposure to spotted fever group Rickettsia in Western Australia. Am. J. Trop. Med. Hyg. 2014, 91, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Owen, H.; Clark, P.; Stenos, J.; Robertson, I.; Fenwick, S. Potentially pathogenic spotted fever group rickettsiae present in Western Australia. Aust. J. Rural Health 2006, 14, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Owen, H.; Unsworth, N.; Stenos, J.; Robertson, I.; Clark, P.; Fenwick, S. Detection and identification of a novel spotted fever group rickettsia in Western Australia. Ann. N. Y. Acad. Sci. 2006, 1078, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Schloderer, D.; Owen, H.; Clark, P.; Stenos, J.; Fenwick, S.G. Rickettsia felis in fleas, Western Australia. Emerg. Infect. Dis. 2006, 12, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, N.B.; Stenos, J.; Graves, S.R.; Faa, A.G.; Cox, G.E.; Dyer, J.R.; Boutlis, C.S.; Lane, A.M.; Shaw, M.D.; Robson, J.; et al. Flinders Island spotted fever rickettsioses caused by “marmionii” strain of Rickettsia honei, eastern Australia. Emerg. Infect. Dis. 2007, 13, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.C.; Walker, A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 2014, 1–144. [Google Scholar] [CrossRef] [PubMed]
- Stenos, J.; Graves, S.R.; Unsworth, N.B. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am. J. Trop. Med. Hyg. 2005, 73, 1083–1085. [Google Scholar] [PubMed]
- Webb, L.; Carl, M.; Malloy, D.C.; Dasch, G.A.; Azad, A.F. Detection of murine typhus infection in fleas by using the polymerase chain reaction. J. Clin. Microbiol. 1990, 28, 530–534. [Google Scholar] [PubMed]
- Marangou, A.; Guarneri, F.; Benvenga, S. Graves’ disease precipitated by rickettsial infection. Endocrine 2015, 50, 828–829. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, N.B.; Stenos, J.; Faa, A.G.; Graves, S.R. Three rickettsioses, Darnley Island, Australia. Emerg. Infect. Dis. 2007, 13, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Stenos, J.; Roux, V.; Walker, D.; Raoult, D. Rickettsia honei sp. nov., the aetiological agent of Flinders Island spotted fever in Australia. Int. J. Syst. Bacteriol. 1998, 48, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.S. Flinders Island spotted fever: A newly recognised endemic focus of tick typhus in Bass Strait. Part 1. Clinical and epidemiological features. Med. J. Aust. 1991, 154, 94–99. [Google Scholar] [PubMed]
- Dyer, J.R.; Einsiedel, L.; Ferguson, P.E.; Lee, A.S.; Unsworth, N.B.; Graves, S.R.; Gordon, D.L. A new focus of Rickettsia honei spotted fever in South Australia. Med. J. Aust. 2005, 182, 231–234. [Google Scholar] [PubMed]
- Murphy, H.; Renvoise, A.; Pandey, P.; Parola, P.; Raoult, D. Rickettsia honei infection in human, Nepal, 2009. Emerg. Infect. Dis. 2011, 17, 1865–1867. [Google Scholar] [CrossRef] [PubMed]
- Sentausa, E.; Abdad, M.Y.; Robert, C.; Stenos, J.; Raoult, D.; Fournier, P.E. Genome sequence of Rickettsia gravesii, isolated from Western Australian ticks. Genome Announc. 2013, 1, e00975-13. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.R.; (Australian Rickettsial Reference Laboratory, Geelong, Victoria, Australia); Barker, S.C.; (The University of Queensland, Brisbane, Australia); Vincent, G.A.; (Australian Rickettsial Reference Laboratory, Geelong, Victoria, Australia). Personal communication, 2016.
- Li, A.Y.; Adams, P.J.; Abdad, M.Y.; Fenwick, S.G. High prevalence of Rickettsia gravesii sp. nov. in Amblyomma triguttatum collected from feral pigs. Vet. Microbiol. 2010, 146, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Stenos, J.; Graves, S.; Popov, V.L.; Walker, D.H. Aponomma hydrosauri, the reptile-associated tick reservoir of Rickettsia honei on Flinders Island, Australia. Am. J. Trop. Med. Hyg. 2003, 69, 314–317. [Google Scholar] [PubMed]
| Day 7/8 | 1 Month | 2 Months | 3 Months | 2 Years | 7 Years | ||
|---|---|---|---|---|---|---|---|
| Case A | SFG | <128 | 128 | 16,384 | 128 | <128 | |
| TG | <128 | <128 | 256 | <128 | <128 | ||
| STG | <128 | <128 | <128 | <128 | <128 | ||
| Case B | SFG | <128 | 2048 | ||||
| TG | <128 | <128 | |||||
| STG | <128 | <128 | |||||
| Case C | SFG | 128 | 512 | ||||
| TG | 128 | 128 | |||||
| STG | <128 | <128 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raby, E.; Pearn, T.; Marangou, A.G.; Merritt, A.J.; Murray, R.J.; Dyer, J.R.; Graves, S.R. New Foci of Spotted Fever Group Rickettsiae Including Rickettsia honei in Western Australia. Trop. Med. Infect. Dis. 2016, 1, 5. https://doi.org/10.3390/tropicalmed1010005
Raby E, Pearn T, Marangou AG, Merritt AJ, Murray RJ, Dyer JR, Graves SR. New Foci of Spotted Fever Group Rickettsiae Including Rickettsia honei in Western Australia. Tropical Medicine and Infectious Disease. 2016; 1(1):5. https://doi.org/10.3390/tropicalmed1010005
Chicago/Turabian StyleRaby, Edward, Toby Pearn, Andreas G. Marangou, Adam J. Merritt, Ronan J. Murray, John R. Dyer, and Stephen R. Graves. 2016. "New Foci of Spotted Fever Group Rickettsiae Including Rickettsia honei in Western Australia" Tropical Medicine and Infectious Disease 1, no. 1: 5. https://doi.org/10.3390/tropicalmed1010005
APA StyleRaby, E., Pearn, T., Marangou, A. G., Merritt, A. J., Murray, R. J., Dyer, J. R., & Graves, S. R. (2016). New Foci of Spotted Fever Group Rickettsiae Including Rickettsia honei in Western Australia. Tropical Medicine and Infectious Disease, 1(1), 5. https://doi.org/10.3390/tropicalmed1010005





