A Scoping Review of AI-Driven mHealth Systems for Precision Hydration: Integrating Food and Beverage Water Content for Personalized Recommendations
Abstract
1. Introduction
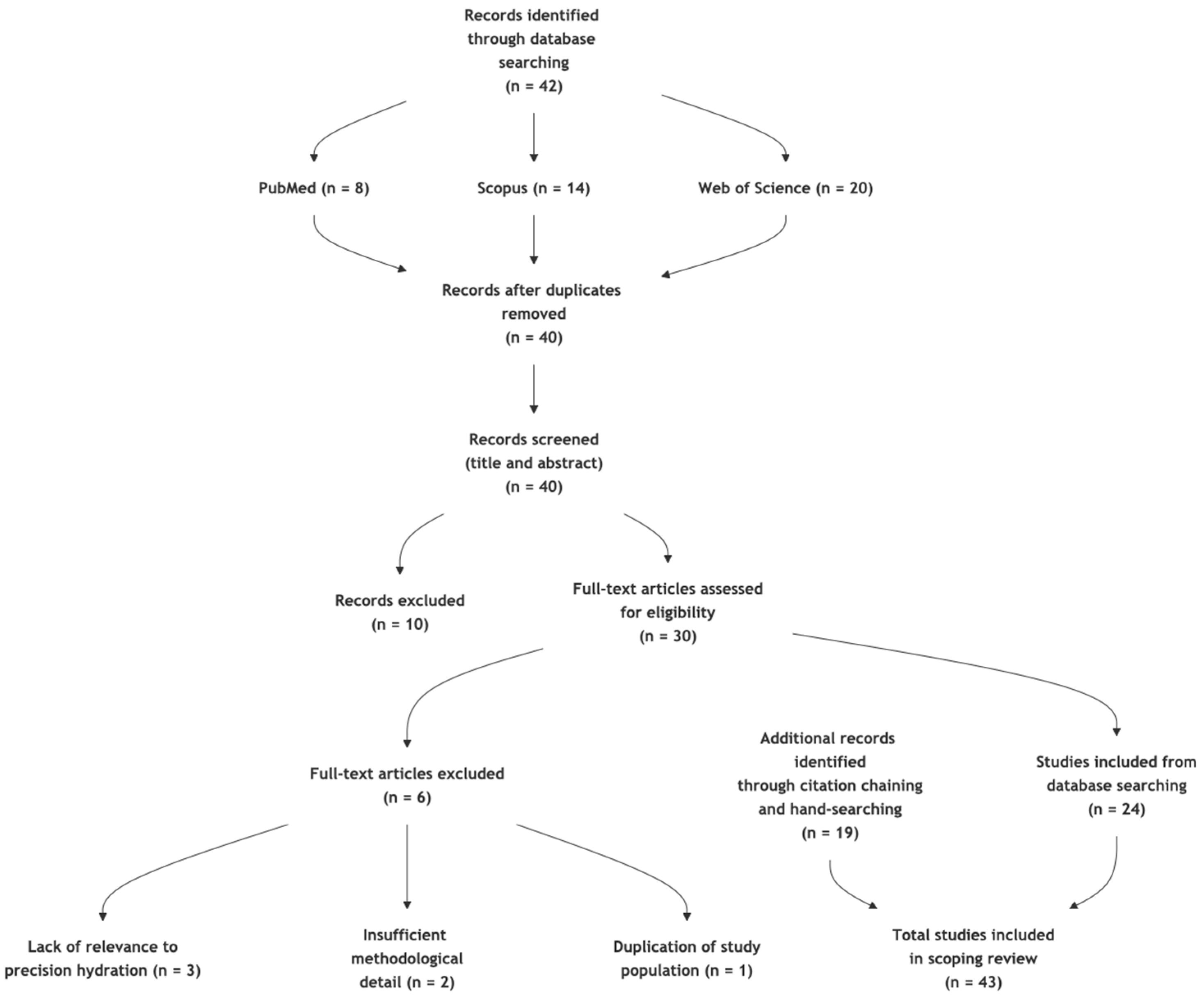
2. Materials and Methods
2.1. Eligibility Criteria
- -
- Studies involving the integration of hydration tracking with dietary patterns using multi-source (food + drink) water intake and AI or machine learning systems integrating both food and beverage intake for hydration.
- -
- mHealth applications that use AI for personalized hydration status assessment and guidance.
- -
- Behavioral, psychological, or environmental factors influencing hydration.
- -
- Food recommendation systems with hydration components.
- -
- The study was not available in English, to ensure accurate interpretation and consistency in data extraction.
- -
- The study focused solely on clinical outcomes without technological integration, as such studies fall outside the scope of our review on AI- and mHealth-based hydration systems.
- -
- The study lacked relevance to AI, mHealth, or hydration, in order to maintain a clear and focused synthesis of studies addressing the intersection of these domains.
2.2. Information Sources and Search Strategy
2.3. Selection and Data Charting Process
- -
- Study author(s), year of publication;
- -
- Study design, population characteristics;
- -
- ML/AI;
- -
- mHealth technologies used;
- -
- Wearable sensors;
- -
- Food + beverage hydration;
- -
- Personalized guidance;
- -
- Outcome measures (e.g., hydration status, performance metrics);
- -
- Data Fusion/Multimodal Input;
- -
- Behavioral and environmental integration;
- -
- Implementation barriers and policy considerations.
2.4. Synthesis of Results
3. Results
3.1. Food Water Content
3.2. Hydration Diet Recommenders
3.3. Psychological, Social, and Environmental Drivers of Fluid Intake Behavior
3.4. Integration of AI and mHealth Technologies in Hydration Diet Recommenders
3.5. Smart Applications Assessing Hydration
4. Discussion
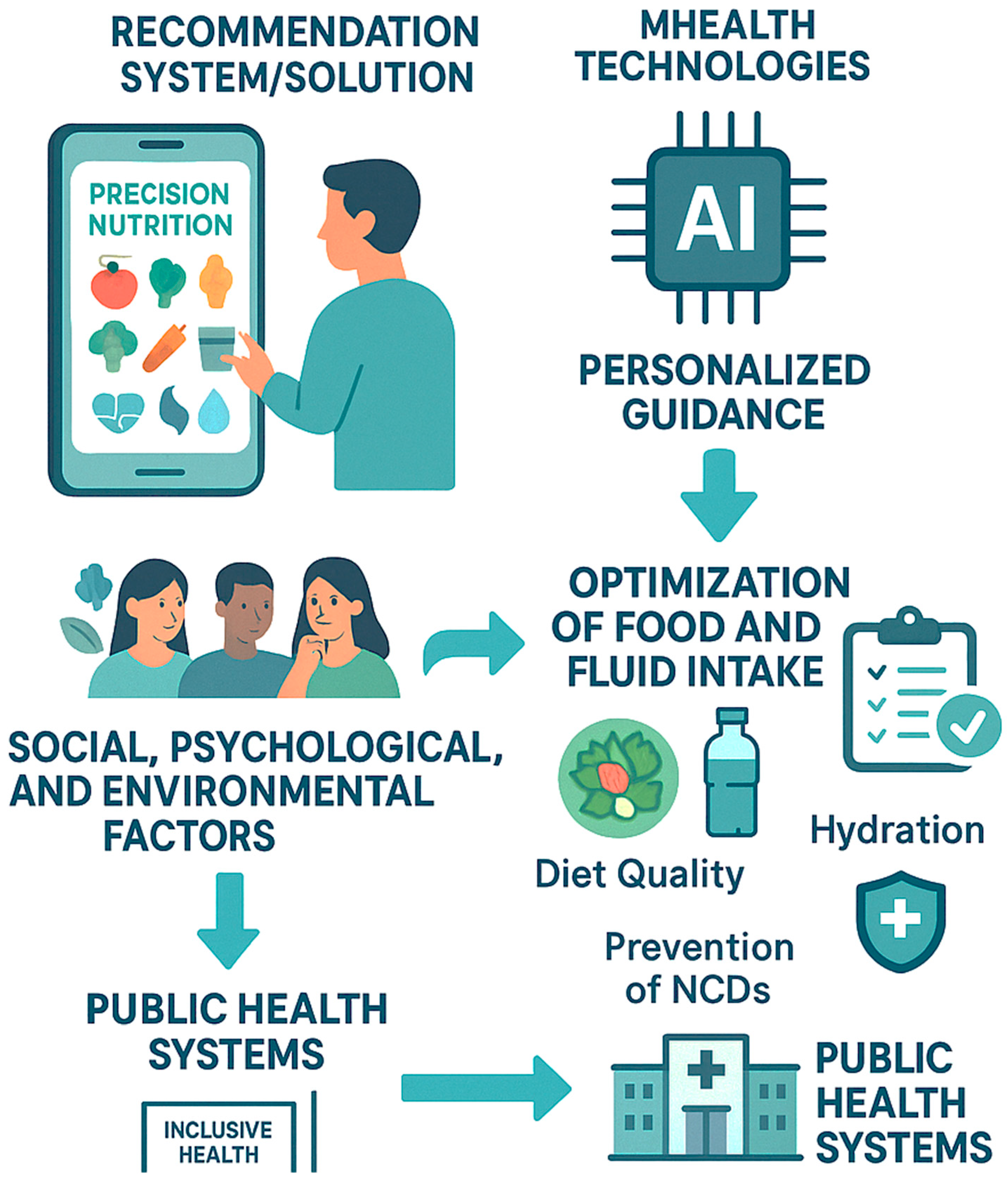
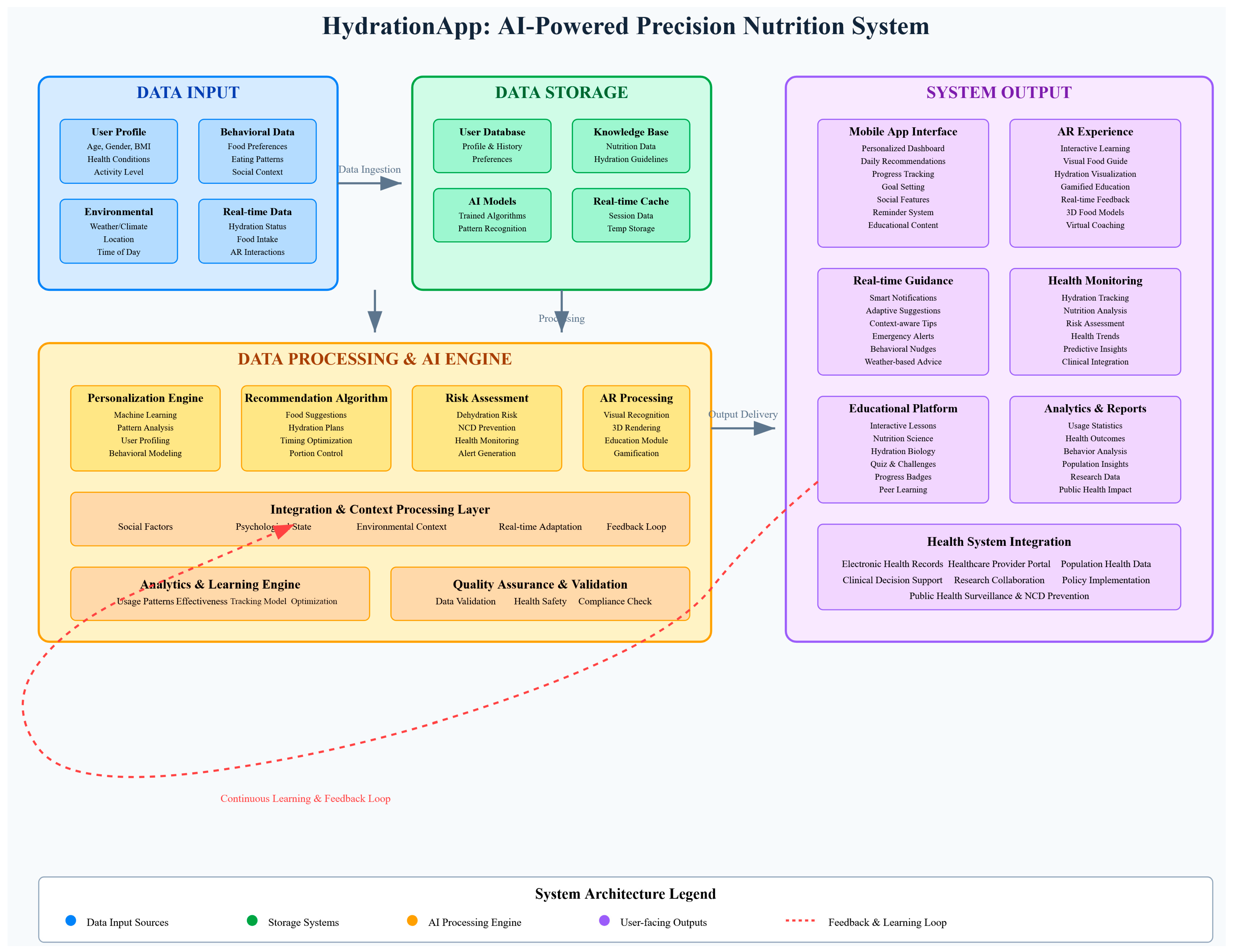
4.1. Proposal
4.2. Public Health Implications
4.3. Challenges and Proposed Solutions
4.4. Psychological and Social Dimensions in Future Hydration Systems
4.5. Strengths and Limitations
4.6. Figures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AR | Augmented Reality |
| CASP | Critical Appraisal Skills Programme |
| EFSA | European Food Safety Authority |
| IOM | Institute of Medicine |
| mHealth | Mobile Health |
| NCDs | Non-Communicable Chronic Diseases |
| ML | Machine Learning |
| MCDA | Multi-Criteria Decision Analysis |
| ROBIS | Risk Of Bias In Systematic reviews |
| SMART Goals | Specific, Measurable, Achievable, Realistic, and Timely Goals |
| TFI | Total Fluid Intake |
| TWI | Total Water Intake |
| WHO | World Health Organization |
Appendix A
- Artificial intelligence and machine learning
- 2.
- Digital and mobile health
- 3.
- Personalized nutrition
- 4.
- Hydration and fluid intake
- 5.
- Food and nutrition guidance
- ▪
- Database-Specific Search Strings:
- ▪
- PubMed
- ▪
- Scopus
- ▪
- Web of Science
References
- World Health Organization. Non-Communicable Diseases: Key Facts; WHO: Geneva, Switzerland, 2025. [Google Scholar]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-Communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Taylor, K.; Tripathi, A.K.; Jones, E.B. Adult Dehydration. In StatPearl; StatPearls Publishing: St. Petersburg, FL, USA, 2025. [Google Scholar]
- Liska, D.; Mah, E.; Brisbois, T.; Barrios, P.L.; Baker, L.B.; Spriet, L.L. Narrative Review of Hydration and Selected Health Outcomes in the General Population. Nutrients 2019, 11, 70. [Google Scholar] [CrossRef]
- Perrier, E.T. Hydration for Health: So What? Ten Advances in Recent Hydration History. Ann. Nutr. Metab. 2019, 74, 4–10. [Google Scholar] [CrossRef]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, Hydration, and Health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Kolasa, K.M.; Lackey, C.J.; Grandjean, A.C. Hydration and Health Promotion. Nutr. Today 2009, 44, 190–201. [Google Scholar] [CrossRef]
- El-Sharkawy, A.M.; Sahota, O.; Lobo, D.N. Acute and Chronic Effects of Hydration Status on Health. Nutr. Rev. 2015, 73, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.T. Shifting Focus: From Hydration for Performance to Hydration for Health. Ann. Nutr. Metab. 2017, 70, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.T.; Armstrong, L.E.; Bottin, J.H.; Clark, W.F.; Dolci, A.; Guelinckx, I.; Iroz, A.; Kavouras, S.A.; Lang, F.; Lieberman, H.R. Hydration for Health Hypothesis: A Narrative Review of Supporting Evidence. Eur. J. Nutr. 2021, 60, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Echouffo-Tcheugui, J.B.; Williamson, D.F. How Effective Were Lifestyle Interventions In Real-World Settings That Were Modeled On The Diabetes Prevention Program? Health Aff. 2012, 31, 67–75. [Google Scholar] [CrossRef]
- Betts, J.A.; Gonzalez, J.T. Personalised Nutrition: What Makes You so Special? Nutr. Bull. 2016, 41, 353–359. [Google Scholar] [CrossRef]
- Dolci, A.; Vanhaecke, T.; Qiu, J.; Ceccato, R.; Arboretti, R.; Salmaso, L. Personalized Prediction of Optimal Water Intake in Adult Population by Blended Use of Machine Learning and Clinical Data. Sci. Rep. 2022, 12, 19692. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.M.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of Personalized Nutrition on Health-Related Behaviour Change: Evidence from the Food4Me European Randomized Controlled Trial. Int. J. Epidemiol. 2017, 46, 578–588. [Google Scholar] [CrossRef]
- Michel, M.; Burbidge, A. Nutrition in the Digital Age—How Digital Tools Can Help to Solve the Personalized Nutrition Conundrum. Trends Food Sci. Technol. 2019, 90, 194–200. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Ruiz, L.A.D.; Ordovás, J.M. Personalized Nutrition and Cardiovascular Disease Prevention: From Framingham to PREDIMED. Adv. Nutr. 2014, 5, 368S–371S. [Google Scholar] [CrossRef]
- Tsolakidis, D.; Gymnopoulos, L.P.; Dimitropoulos, K. Artificial Intelligence and Machine Learning Technologies for Personalized Nutrition: A Review. Informatics 2024, 11, 62. [Google Scholar] [CrossRef]
- Mathers, J.C. Paving the Way to Better Population Health through Personalised Nutrition. EFSA J. 2019, 17, e170713. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Water. EFSA J. 2010, 8, 1459. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R. Social Determinants of Food Choice. Proc. Nutr. Soc. 1999, 58, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.L. Role of Whole Foods in Promoting Hydration after Exercise in Humans. J. Am. Coll. Nutr. 2007, 26, 592S–596S. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.J.; Nowson, C.A. Relationship between Stress, Eating Behavior, and Obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Le Bellego, L.; Jean, C.; Jiménez, L.; Magnani, C.; Tang, W.; Boutrolle, I. Understanding Fluid Consumption Patterns to Improve Healthy Hydration. Nutr. Today 2010, 45, S22–S26. [Google Scholar] [CrossRef]
- Thokala, P.; Duenas, A. Multiple Criteria Decision Analysis for Health Technology Assessment. Value Health 2012, 15, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, Y.; Pham, M.; Do, H.M.; Sheng, W. AutoHydrate: A Wearable Hydration Monitoring System. In Proceedings of the 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Daejeon, Republic of Korea, 9–14 October 2016; pp. 1857–1862. [Google Scholar]
- Järvelä-Reijonen, E.; Karhunen, L.; Sairanen, E.; Muotka, J.; Lindroos, S.; Laitinen, J.; Puttonen, S.; Peuhkuri, K.; Hallikainen, M.; Pihlajamäki, J.; et al. The Effects of Acceptance and Commitment Therapy on Eating Behavior and Diet Delivered through Face-to-Face Contact and a Mobile App: A Randomized Controlled Trial. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 22. [Google Scholar] [CrossRef]
- Beierle, F.; Probst, T.; Allemand, M.; Zimmermann, J.; Pryss, R.; Neff, P.; Schlee, W.; Stieger, S.; Budimir, S. Frequency and Duration of Daily Smartphone Usage in Relation to Personality Traits. Digit. Psychol. 2020, 1, 20–28. [Google Scholar] [CrossRef]
- Liaqat, S.; Dashtipour, K.; Arshad, K.; Ramzan, N. Non Invasive Skin Hydration Level Detection Using Machine Learning. Electronics 2020, 9, 1086. [Google Scholar] [CrossRef]
- Millard-Stafford, M.; Snow, T.K.; Jones, M.L.; Suh, H. The Beverage Hydration Index: Influence of Electrolytes, Carbohydrate and Protein. Nutrients 2021, 13, 2933. [Google Scholar] [CrossRef]
- Jo, S.; Sung, D.; Kim, S.; Koo, J. A Review of Wearable Biosensors for Sweat Analysis. Biomed. Eng. Lett. 2021, 11, 117–129. [Google Scholar] [CrossRef]
- Suppiah, H.T.; Ng, E.L.; Wee, J.; Taim, B.C.; Huynh, M.; Gastin, P.B.; Chia, M.; Low, C.Y.; Lee, J.K. Hydration Status and Fluid Replacement Strategies of High-Performance Adolescent Athletes: An Application of Machine Learning to Distinguish Hydration Characteristics. Nutrients 2021, 13, 4073. [Google Scholar] [CrossRef]
- Kulkarni, N.; Compton, C.; Luna, J.; Alam, M.A.U. A Non-Invasive Context-Aware Dehydration Alert System. In Proceedings of the 22nd International Workshop on Mobile Computing Systems and Applications, Virtual, 24–26 February 2021; pp. 157–159. [Google Scholar]
- Malik, V.S.; Hu, F.B. The Role of Sugar-Sweetened Beverages in the Global Epidemics of Obesity and Chronic Diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Al-Rayes, S.; Al Yaqoub, F.A.; Alfayez, A.; Alsalman, D.; Alanezi, F.; Alyousef, S.; AlNujaidi, H.; Al-Saif, A.K.; Attar, R.; Aljabri, D.; et al. Gaming Elements, Applications, and Challenges of Gamification in Healthcare. Inf. Med. Unlocked 2022, 31, 100974. [Google Scholar] [CrossRef]
- Oc, Y.; Plangger, K. GIST Do It! How Motivational Mechanisms Help Wearable Users Develop Healthy Habits. Comput. Hum. Behav. 2022, 128, 107089. [Google Scholar] [CrossRef]
- Rodin, D.; Shapiro, Y.; Pinhasov, A.; Kreinin, A.; Kirby, M. An Accurate Wearable Hydration Sensor: Real-World Evaluation of Practical Use. PLoS ONE 2022, 17, e0272646. [Google Scholar] [CrossRef]
- Sabry, F.; Eltaras, T.; Labda, W.; Hamza, F.; Alzoubi, K.; Malluhi, Q. Towards On-Device Dehydration Monitoring Using Machine Learning from Wearable Device’s Data. Sensors 2022, 22, 1887. [Google Scholar] [CrossRef]
- Wang, S.; Lafaye, C.; Saubade, M.; Besson, C.; Margarit-Taule, J.M.; Gremeaux, V.; Liu, S.-C. Predicting Hydration Status Using Machine Learning Models from Physiological and Sweat Biomarkers during Endurance Exercise: A Single Case Study. IEEE J. Biomed. Health Inf. 2022, 26, 4725–4732. [Google Scholar] [CrossRef]
- Hillesheim, E.; Brennan, L. Distinct Patterns of Personalised Dietary Advice Delivered by a Metabotype Framework Similarly Improve Dietary Quality and Metabolic Health Parameters: Secondary Analysis of a Randomised Controlled Trial. Front. Nutr. 2023, 10, 1282741. [Google Scholar] [CrossRef]
- Rod, M.H.; Rod, N.H.; Russo, F.; Klinker, C.D.; Reis, R.; Stronks, K. Promoting the Health of Vulnerable Populations: Three Steps towards a Systems-Based Re-Orientation of Public Health Intervention Research. Health Place 2023, 80, 102984. [Google Scholar] [CrossRef]
- de Castro, B.A.; Levens, S.M.; Sullivan, M.; Shaw, G. Recommender Systems Use in Weight Management MHealth Interventions: A Scoping Review. Obes. Rev. 2024, 26, e13863. [Google Scholar] [CrossRef] [PubMed]
- Dimou, V.; Styliaras, G.; Apergi, K.; Malisova, O. HydrationApp: Educating Young People on Hydration Through AR; Bastiaens, T., Ed.; Association for the Advancement of Computing in Education (AACE): Waynesville, NC, USA; Brussels, Belgium, 2024; pp. 558–566. [Google Scholar]
- Tonello, S.; Zacchini, A.; Galli, A.; Golparvar, A.; Meimandi, A.; Peruzzi, G.; Pozzebon, A.; Lago, N.; Cester, A.; Giorgi, G. Design and in Vitro Characterization of a Wearable Multisensing System for Hydration Monitoring. IEEE Trans. Instrum. Meas. 2024, 73, 1–11. [Google Scholar] [CrossRef]
- Chiao, J.-C.; Bing, S.; Chawang, K.; Crowe, B. Thirsty for a Noninvasive Wearable to Detect Dehydration: A Review. IEEE Antennas Propag. Mag. 2024, 66, 66–76. [Google Scholar] [CrossRef]
- Li, J.-H.; Yu, P.-W.; Wang, H.-C.; Lin, C.-Y.; Lin, Y.-C.; Liu, C.-P.; Hsieh, C.-Y.; Chan, C.-T. Multi-Sensor Fusion Approach to Drinking Activity Identification for Improving Fluid Intake Monitoring. Appl. Sci. 2024, 14, 4480. [Google Scholar] [CrossRef]
- Alaslani, R.; Perzhilla, L.; Rahman, M.M.U.; Laleg-Kirati, T.-M.; Al-Naffouri, T.Y. You Can Monitor Your Hydration Level Using Your Smartphone Camera. IEEE Trans. Instrum. Meas. 2025, 74, 1–14. [Google Scholar] [CrossRef]
- Sreeharsha, A.; McHale, S.; Nnamoko, N.; Pereira, E. Towards Data-Driven Hydration Monitoring: Insights from Wearable Sensors and Advanced Machine Learning Techniques. Electronics 2024, 13, 4960. [Google Scholar] [CrossRef]
- Waterllama. Available online: https://www.waterllama.com (accessed on 10 April 2025).
- Water Time Drink Tracker & Reminder. Available online: https://play.google.com/store/apps/details?id=com.victorsharov.mywaterapp&hl=en&gl=US (accessed on 10 April 2025).
- Plant Nanny Water Tracker. Available online: https://sparkful.app/plant-nanny (accessed on 10 April 2025).
- Institute of Medicine Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Armstrong, L.E.; Johnson, E.C. Water Intake, Water Balance, and the Elusive Daily Water Requirement. Nutrients 2018, 10, 1928. [Google Scholar] [CrossRef]
- Seal, A.D.; Suh, H.-G.; Jansen, L.T.; Summers, L.G.; Kavouras, S.A. Hydration and Health. In Analysis in Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–319. [Google Scholar]
- Stookey, J.D. The Diuretic Effects of Alcohol and Caffeine and Total Water Intake Misclassification. Eur. J. Epidemiol. 1999, 15, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Griffin, J. Caffeine Ingestion and Fluid Balance: A Review. J. Hum. Nutr. Diet. 2003, 16, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.L.; Funnell, M.P.; Reynolds, K.M.; Kenefick, R.W.; Cheuvront, S.N.; Mears, S.A.; James, L.J. Post-Exercise Rehydration: Comparing the Efficacy of Three Commercial Oral Rehydration Solutions. Front. Sports Act. Living 2023, 5, 1158167. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M. Post-Exercise Rehydration and Recovery. In Sports Drinks: Basic and Practical Aspects; Maughan, R.J., e Murray, R., Eds.; CRC Press: Boca Raton, FL, USA, 2000; p. 1. [Google Scholar]
- Laddu, D.; Hauser, M. Addressing the Nutritional Phenotype Through Personalized Nutrition for Chronic Disease Prevention and Management. Prog. Cardiovasc. Dis. 2019, 62, 9–14. [Google Scholar] [CrossRef]
- Russo, S.; Jongerius, C.; Faccio, F.; Pizzoli, S.F.M.; Pinto, C.A.; Veldwijk, J.; Janssens, R.; Simons, G.; Falahee, M.; de Bekker-Grob, E.; et al. Understanding Patients’ Preferences: A Systematic Review of Psychological Instruments Used in Patients’ Preference and Decision Studies. Value Health 2019, 22, 491–501. [Google Scholar] [CrossRef]
- Midoun, E.; Salem, Y.; Akelah, D.; Abed, H.; Darwish, R.; Kerdjani, M.; Ansari, S.H. Consumption of Sweets and Caffeine under Stress; a Cross-Sectional Study among Dental Students in Riyadh, Saudi Arabia. Donn J. Dent. Oral. Hyg. 2018, 4, 23–30. [Google Scholar]
- Mayén, A.-L.; Marques-Vidal, P.; Paccaud, F.; Bovet, P.; Stringhini, S. Socioeconomic Determinants of Dietary Patterns in Low- and Middle-Income Countries: A Systematic Review. Am. J. Clin. Nutr. 2014, 100, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Kenefick, R.W.; Sawka, M.N. Hydration at the Work Site. J. Am. Coll. Nutr. 2007, 26, 597S–603S. [Google Scholar] [CrossRef]
- Lewis, T.L.; Boissaud-Cooke, M.A.; Aungst, T.D.; Eysenbach, G. Consensus on Use of the Term “App” Versus “Application” for Reporting of MHealth Research. J. Med. Internet Res. 2014, 16, e174. [Google Scholar] [CrossRef]
- Martínez-Pérez, B.; de la Torre-Díez, I.; López-Coronado, M. Mobile Health Applications for the Most Prevalent Conditions by the World Health Organization: Review and Analysis. J. Med. Internet Res. 2013, 15, e120. [Google Scholar] [CrossRef]
- Abaza, H.; Marschollek, M. MHealth Application Areas and Technology Combinations. Methods Inf. Med. 2017, 56, e105–e122. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E. Toward a New Philosophy of Preventive Nutrition: From a Reductionist to a Holistic Paradigm to Improve Nutritional Recommendations. Adv. Nutr. 2014, 5, 430–446. [Google Scholar] [CrossRef] [PubMed]
- My Water—Daily Water Tracker. Available online: https://mywaterapp.me (accessed on 10 April 2025).
- WaterMinder. Available online: https://waterminder.com (accessed on 10 April 2025).
- Aqualert: Water Tracker Daily. Available online: https://www.aqualertapp.com (accessed on 10 April 2025).
- Hydro Coach. Available online: https://play.google.com/store/apps/details?id=com.codium.hydrocoach (accessed on 10 April 2025).
- Daily Water Tracker Reminder- Waterful. Available online: https://play.google.com/store/apps/details?id=com.drink.water.reminder.alarm.tracker (accessed on 10 April 2025).
- Aertsens, J.; Verbeke, W.; Mondelaers, K.; Van Huylenbroeck, G. Personal Determinants of Organic Food Consumption: A Review. Br. Food J. 2009, 111, 1140–1167. [Google Scholar] [CrossRef]
| No | Author(s)/Source | Year | Study Type/Source Type | Main Theme(s) |
|---|---|---|---|---|
| 1 | Shepherd [21] | 1999 | Review Article | Social determinants of food choice, providing behavioral context for AI-integrated food recommendation systems. |
| 2 | Sharp [22] | 2007 | Review Article | Role of whole foods in hydration, informing food-based hydration recommendation algorithms. |
| 3 | Torres and Nowson [23] | 2007 | Review Article | Stress-eating behavior relationships, informing psychological factors in personalized food recommendation algorithms. |
| 4 | Le Bellego et al. [24] | 2010 | Research Article | Fluid consumption pattern analysis supporting the development of personalized hydration recommendation systems. |
| 5 | Ali et al. [11] | 2012 | Review Article | Real-world effectiveness of lifestyle interventions modeled on diabetes prevention, validating clinical application potential. |
| 6 | Thokala and Duenas [25] | 2012 | Methodological Article | Multiple criteria decision analysis for health technology assessment, informing evaluation frameworks for AI nutrition systems. |
| 7 | Konstantinidou et al. [16] | 2014 | Review Article | Links personalized nutrition to cardiovascular disease prevention, demonstrating the clinical relevance of precision nutrition systems. |
| 8 | Betts and Gonzalez [12] | 2016 | Commentary | Theoretical foundation for personalized nutrition approaches, supporting individualized dietary guidance systems. |
| 9 | Mengistu, Y. et al. [26] | 2016 | Conference Paper | Development of AutoHydrate, a wearable hydration monitoring system using sensor data. |
| 10 | Celis-Morales et al. [14] | 2017 | Randomized Controlled Trial | Large-scale European RCT demonstrating the effectiveness of personalized nutrition interventions on health-related behavior change. |
| 11 | Järvelä-Reijonen et al. [27] | 2018 | Randomized Controlled Trial | RCT evidence of mobile app effectiveness in dietary behavior change, validating mHealth platforms for personalized nutrition interventions. |
| 12 | Michel and Burbidge [15] | 2019 | Review Article | Addresses digital tools for solving personalized nutrition challenges, providing a framework for AI-integrated recommendation systems. |
| 13 | Liska et al. [4] | 2019 | Narrative Review | Comprehensive review of hydration and health outcomes, providing a scientific foundation for hydration monitoring systems. |
| 14 | Perrier [5] | 2019 | Review Article | Historical perspective on hydration research advances, contextualizing current hydration monitoring technologies. |
| 15 | Mathers [18] | 2019 | Commentary | Population health through personalized nutrition, addressing the policy integration of AI-driven nutrition systems. |
| 16 | Budreviciute et al. [2] | 2020 | Review Article | NCD management and prevention strategies, providing health system context for AI-integrated nutrition platforms. |
| 17 | Beierle et al. [28] | 2020 | Cross-sectional Study | Smartphone usage patterns and personality traits, informing user engagement strategies for mHealth nutrition platforms. |
| 18 | Liaqat, S. et al. [29] | 2020 | Research Article | ML algorithms for non-invasive skin hydration level estimation. |
| 19 | Perrier et al. [10] | 2021 | Narrative Review | Evidence supporting hydration for health hypothesis, validating the importance of precision hydration guidance systems. |
| 20 | Millard-Stafford et al. [30] | 2021 | Research Article | Beverage hydration index research providing a scientific basis for fluid recommendation algorithms in AI systems. |
| 21 | Jo, S. et al., [31] | 2021 | Review Article | Wearable biosensors for sweat analysis and implications for real-time hydration tracking. |
| 22 | Suppiah, Haresh T. et al. [32] | 2021 | Research Article | Use of ML to classify hydration characteristics in adolescent athletes. |
| 23 | Kulkarni, N. et al. [33] | 2021 | Conference Paper | Development of a non-invasive, context-aware dehydration alert system using mobile and wearable inputs. |
| 24 | Dolci et al. [13] | 2022 | Research Article | Demonstrates machine learning application for personalized water intake prediction using clinical data, directly addressing AI-driven precision nutrition systems. |
| 25 | Malik and Hu [34] | 2022 | Review Article | Sugar-sweetened beverages and chronic disease risk, informing healthy food recommendation system parameters. |
| 26 | Al-Rayes et al. [35] | 2022 | Review Article | Gamification in healthcare applications, informing engagement strategies for AI-powered nutrition platforms. |
| 27 | Oc and Plangger [36] | 2022 | Research Article | Motivational mechanisms in wearable technology for healthy habits, supporting behavioral design in AI nutrition systems. |
| 28 | Rodin, D. et al. [37] | 2022 | Research Article | Validation study of a wearable hydration sensor under real-life conditions. |
| 29 | Sabry, F. et al. [38] | 2022 | Research Article | Machine learning applied to dehydration detection using data from wearable sensors. |
| 30 | Wang, S. et al. [39] | 2022 | Case Study | Personalized hydration prediction models using sweat biomarkers and physiological data. |
| 31 | Hillesheim and Brennan [40] | 2023 | Secondary Analysis RCT | Evidence of metabotype-based personalized dietary advice effectiveness, validating precision nutrition frameworks. |
| 32 | Rod et al. [41] | 2023 | Methodological Article | Systems-based public health intervention research, providing framework for implementing AI nutrition systems in healthcare. |
| 33 | Tsolakidis et al. [17] | 2024 | Review Article | Comprehensive review of AI and ML technologies in personalized nutrition, providing technological framework for intelligent food recommendation systems. |
| 34 | de Castro et al. [42] | 2024 | Scoping Review | Examines recommender systems in weight management mHealth interventions, bridging AI technology with mHealth applications. |
| 35 | Dimou et al. [43] | 2024 | Educational Tool Study | Augmented reality application for hydration education, showcasing innovative mHealth technologies for nutritional guidance. |
| 36 | Tonello, S. et al. [44] | 2024 | Research Article | Design and testing of a wearable multi-sensing hydration monitoring system. |
| 37 | Chiao, J.C. et al. [45] | 2024 | Review Article | Technical and clinical potential of noninvasive wearable devices for dehydration monitoring. |
| 38 | Li, J.H. et al. [46] | 2024 | Research Article | ML-based fluid intake recognition using multi-sensor fusion in fluid intake behavior monitoring. |
| 39 | Alaslani, R. et al. [47] | 2024 | Preprint/arXiv | Real-time hydration level estimation using a smartphone camera and computer vision. |
| 40 | Sreeharsha, A. et al. [48] | 2024 | Systematic Review | Review of wearable sensors and ML algorithms for hydration monitoring, highlighting sensor limitations and model performance. |
| 41 | Waterllama [49] | 2025 | Mobile Application | Commercial hydration tracking platform demonstrating current mHealth implementation for fluid intake monitoring. |
| 42 | Water Time Drink Tracker [50] | 2025 | Mobile Application | Exemplifies mobile reminder systems for fluid intake behavior modification and real-time fluid intake guidance. |
| 43 | Plant Nanny Water Tracker [51] | 2025 | Mobile Application | Demonstrates gamified mHealth approach to hydration tracking, illustrating behavioral engagement strategies in mobile platforms. |
| No | Author(s)/Source | Year | Wearable Sensors | Machine Learning/AI | Health/App-Based | Food + Beverage | Hydration | Personalized Guidance | Data Fusion/Multimodal Input | Behavioral and Environmental Integration | Implementation Barriers and Policy Considerations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shepherd [21] | 1999 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 2 | Sharp [22] | 2007 | X | Yes | Yes | Yes | Yes | Yes | X | Yes | Yes |
| 3 | Torres and Nowson [23] | 2007 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 4 | Le Bellego et al. [24] | 2010 | X | Yes | Yes | Yes | Yes | Yes | X | Yes | Yes |
| 5 | Ali et al. [11] | 2012 | X | Yes | Yes | X | X | Yes | X | Yes | Yes |
| 6 | Thokala and Duenas [25] | 2012 | X | Yes | Yes | X | X | Yes | X | Yes | Yes |
| 7 | Konstantinidou et al. [16] | 2014 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 8 | Betts and Gonzalez [12] | 2016 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 9 | Mengistu, Y. et al. [26] | 2016 | Yes | Yes | Yes | X | Yes | Yes | Yes | Yes | Yes |
| 10 | Celis-Morales et al. [14] | 2017 | X | Yes | Yes | X | X | Yes | X | Yes | Yes |
| 11 | Järvelä-Reijonen et al. [27] | 2018 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 12 | Michel and Burbidge [15] | 2019 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 13 | Liska et al. [4] | 2019 | X | X | Yes | X | Yes | X | X | Yes | X |
| 14 | Perrier [5] | 2019 | X | X | Yes | X | Yes | X | X | Yes | X |
| 15 | Mathers [18] | 2019 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 16 | Budreviciute et al. [2] | 2020 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 17 | Beierle et al. [28] | 2020 | X | Yes | Yes | X | X | Yes | X | Yes | X |
| 18 | Liaqat, S. et al. [29] | 2020 | Yes | Yes | Yes | X | Yes | Yes | Yes | Yes | Yes |
| 19 | Perrier et al. [10] | 2021 | X | X | Yes | X | Yes | X | X | Yes | X |
| 20 | Millard-Stafford et al. [30] | 2021 | X | Yes | Yes | Yes | Yes | Yes | X | Yes | X |
| 21 | Jo, S. et al. [31] | 2021 | Yes | X | X | X | Yes | X | X | Yes | X |
| 22 | Suppiah, H.T. et al. [32] | 2021 | Yes | Yes | Yes | X | Yes | Yes | X | Yes | X |
| 23 | Kulkarni, N. et al. [33] | 2021 | Yes | Yes | Yes | X | Yes | Yes | Yes | Yes | X |
| 24 | Dolci et al. [13] | 2022 | X | Yes | Yes | X | Yes | Yes | Yes | Yes | X |
| 25 | Malik and Hu [34] | 2022 | X | Yes | Yes | Yes | X | X | X | Yes | Yes |
| 26 | Al-Rayes et al. [35] | 2022 | X | Yes | Yes | Yes | X | X | X | Yes | X |
| 27 | Oc and Plangger [36] | 2022 | Yes | Yes | Yes | X | X | X | Yes | Yes | X |
| 28 | Rodin, D. et al. [37] | 2022 | Yes | X | X | X | Yes | X | X | X | X |
| 29 | Sabry, F. et al. [38] | 2022 | Yes | Yes | X | X | Yes | X | X | X | X |
| 30 | Wang, S. et al. [39] | 2022 | Yes | Yes | X | X | Yes | Yes | Yes | Yes | X |
| 31 | Hillesheim and Brennan [40] | 2023 | X | Yes | Yes | Yes | X | Yes | X | Yes | Yes |
| 32 | Rod et al. [41] | 2023 | X | Yes | Yes | X | X | Yes | X | Yes | Yes |
| 33 | Tsolakidis et al. [17] | 2024 | X | Yes | Yes | Yes | X | Yes | Yes | Yes | X |
| 34 | de Castro et al. [42] | 2024 | X | Yes | Yes | X | X | Yes | Yes | Yes | X |
| 35 | Dimou et al. [43] | 2024 | X | X | Yes | X | Yes | Yes | Yes | Yes | X |
| 36 | Tonello, S. et al. [44] | 2024 | Yes | Yes | Yes | X | Yes | Yes | Yes | Yes | X |
| 37 | Chiao, J.C. et al. [45] | 2024 | Yes | X | Yes | X | Yes | X | X | X | X |
| 38 | Li, J.H. et al. [46] | 2024 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | X |
| 39 | Alaslani, R. et al. [47] | 2024 | X | Yes | Yes | X | Yes | X | X | X | X |
| 40 | Sreeharsha, A. et al. [48] | 2024 | Yes | Yes | Yes | X | Yes | X | X | Yes | X |
| 41 | Waterllama [49] | 2025 | X | X | Yes | X | Yes | Yes | X | Yes | X |
| 42 | Water Time Drink Tracker [50] | 2025 | X | X | Yes | X | Yes | Yes | X | Yes | X |
| 43 | Plant Nanny Water Tracker [51] | 2025 | X | X | Yes | X | Yes | Yes | X | Yes | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apergi, K.; Styliaras, G.D.; Tsirogiannis, G.; Beligiannis, G.N.; Malisova, O. A Scoping Review of AI-Driven mHealth Systems for Precision Hydration: Integrating Food and Beverage Water Content for Personalized Recommendations. Multimodal Technol. Interact. 2025, 9, 112. https://doi.org/10.3390/mti9110112
Apergi K, Styliaras GD, Tsirogiannis G, Beligiannis GN, Malisova O. A Scoping Review of AI-Driven mHealth Systems for Precision Hydration: Integrating Food and Beverage Water Content for Personalized Recommendations. Multimodal Technologies and Interaction. 2025; 9(11):112. https://doi.org/10.3390/mti9110112
Chicago/Turabian StyleApergi, Kyriaki, Georgios D. Styliaras, George Tsirogiannis, Grigorios N. Beligiannis, and Olga Malisova. 2025. "A Scoping Review of AI-Driven mHealth Systems for Precision Hydration: Integrating Food and Beverage Water Content for Personalized Recommendations" Multimodal Technologies and Interaction 9, no. 11: 112. https://doi.org/10.3390/mti9110112
APA StyleApergi, K., Styliaras, G. D., Tsirogiannis, G., Beligiannis, G. N., & Malisova, O. (2025). A Scoping Review of AI-Driven mHealth Systems for Precision Hydration: Integrating Food and Beverage Water Content for Personalized Recommendations. Multimodal Technologies and Interaction, 9(11), 112. https://doi.org/10.3390/mti9110112








