Testing a New Approach to Monitor Mild Cognitive Impairment and Cognition in Older Adults at the Community Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. In-Person Assessments Overview
2.3. In-Person Baseline Assessment—Month 0
2.4. In-Person Assessments—Month 3 and Month 6
2.5. Final Interview Overview
2.6. Data Analysis
3. Results
3.1. In-Person Assessments
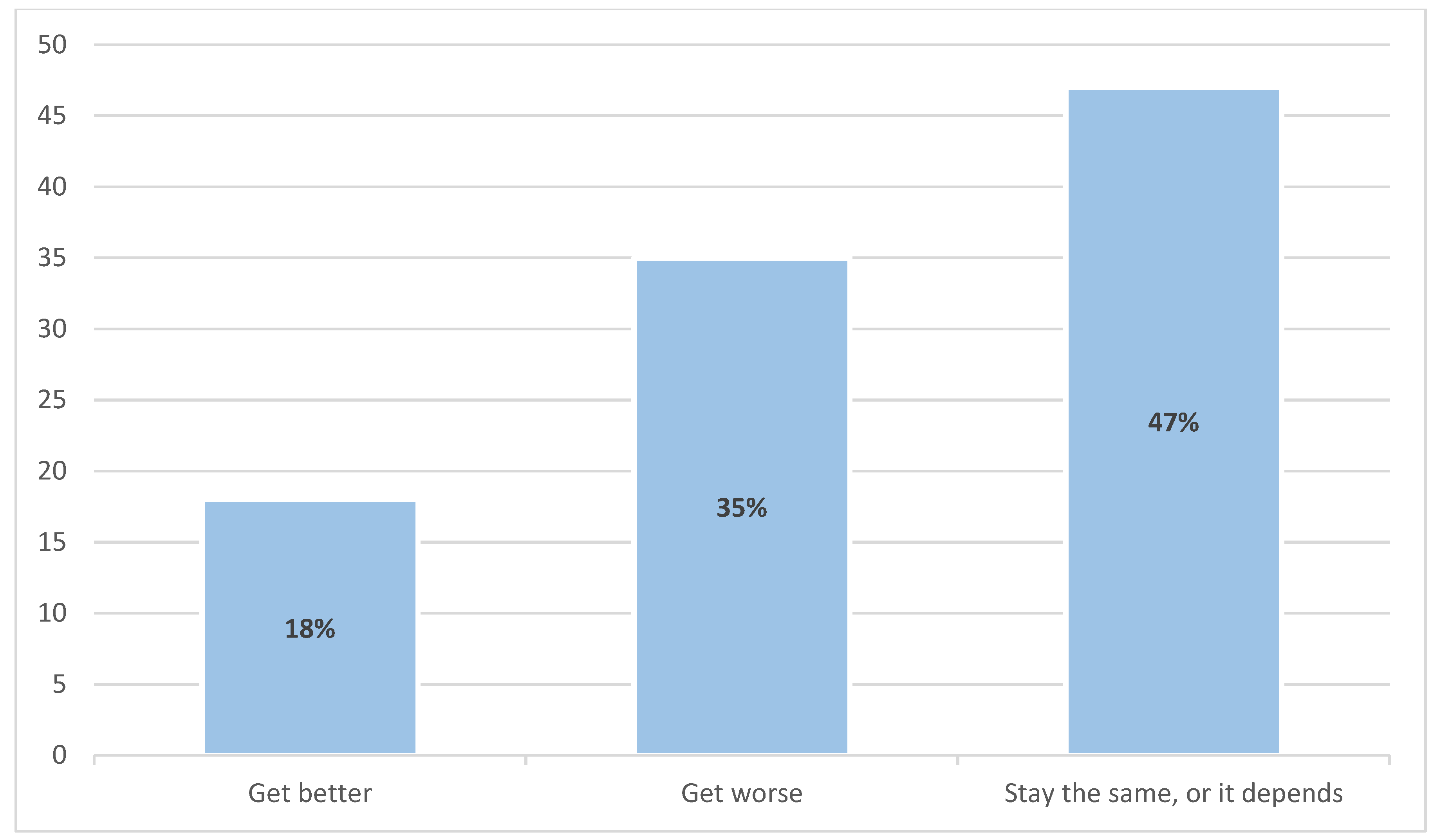
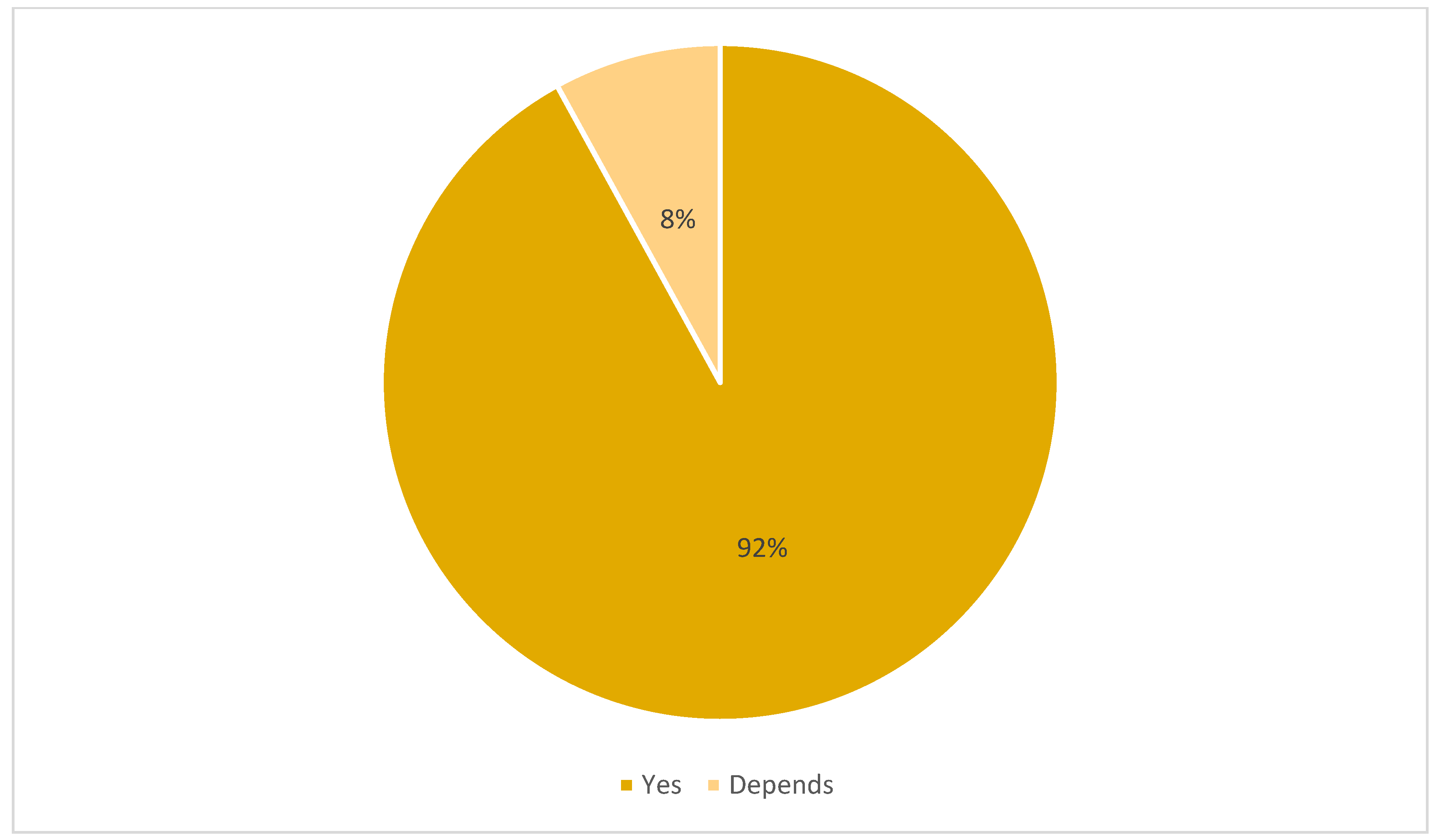
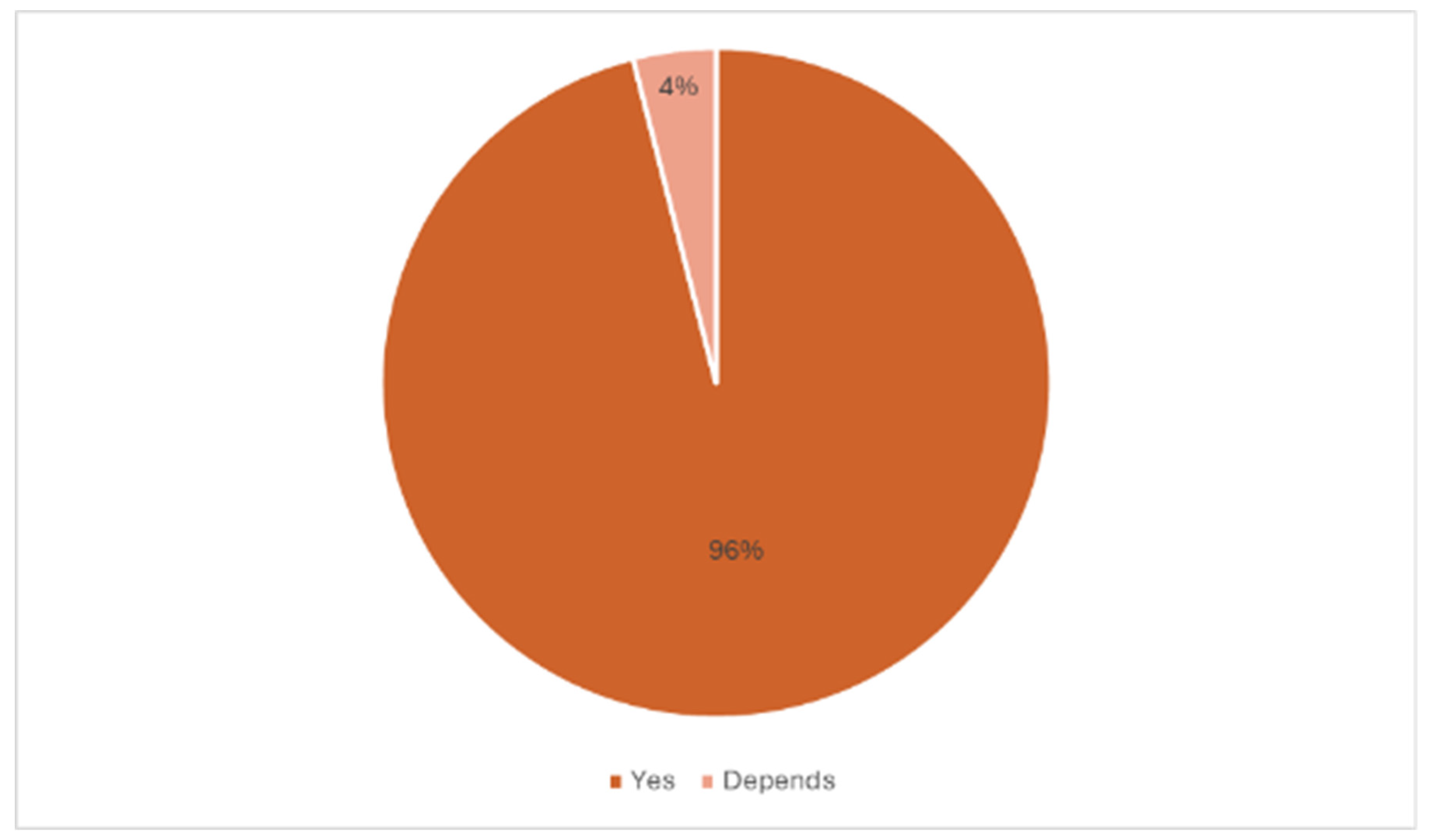
3.2. Final Interview Outcomes
3.3. Study Termination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dementia Numbers in Canada. Available online: https://alzheimer.ca/en/about-dementia/what-dementia/dementia-numbers-canada (accessed on 5 November 2024).
- Global Dementia Observatory (GDO). Available online: https://www.who.int/data/gho/data/themes/global-dementia-observatory-gdo (accessed on 30 October 2024).
- Cognitive Impairment—Clinician Summary. Available online: https://canadiantaskforce.ca/cognitive-impairment-clinician-summary/ (accessed on 29 October 2024).
- Dementia: Overview. Available online: https://www.canada.ca/en/public-health/services/diseases/dementia.html (accessed on 23 October 2024).
- Projected patterns of illness in Ontario. In Dalla Lana School of Public Health. Available online: https://pophealthanalytics.com/wp-content/uploads/2024/10/Study-Projected-Patterns-of-Illness-in-Ontario-Released-October-16-Final.pdf (accessed on 7 November 2024).
- Aging and Chronic Diseases: A Profile of Canadian Seniors. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/aging-chronic-diseases-profile-canadian-seniors-report.html (accessed on 26 February 2024).
- Strengthening the Health Care System in Canada. Available online: https://www.canada.ca/en/health-canada/news/2024/07/strengthening-the-health-care-system-in-canada.html (accessed on 30 October 2024).
- Rathbone, A.L.; Prescott, J. The use of mobile apps and SMS messaging as physical and mental health interventions: Systematic review. J. Med. Internet Res. 2017, 19, e295. [Google Scholar] [CrossRef]
- Pearson, A.L.; Mack, E.; Namanya, J. Mobile phones and mental well-being: Initial evidence suggesting the importance of staying connected to family in rural, remote communities in Uganda. PLoS ONE 2017, 12, e0169819. [Google Scholar] [CrossRef]
- Serrano, L.P.; Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Garcia, J.P.; Eldaly, A.S.; Haider, C.R.; Felton, C.L.; Paulson, M.R.; Maniaci, M.J.; et al. Benefits and challenges of remote patient monitoring as perceived by health care practitioners: A systematic review. Perm. J. 2023, 27, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.R.; Chung, M.K.; Deering, T.F.; Gopinathannair, R.; Albert, C.M.; Epstein, L.M.; Harding, C.V.; Hurwitz, J.L.; Jeffery, C.C.; Krahn, A.D.; et al. Guidance for rebooting electrophysiology through the COVID-19 pandemic. Heart Rhythm 2020, 17, e242–e254. [Google Scholar] [CrossRef]
- Wootton, R. Twenty years of telemedicine in chronic disease management—An evidence synthesis. J. Telemed. Telecare 2012, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M. Wearable sensors for remote health monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Deen, M.J. Information and communications technologies for elderly ubiquitous healthcare in a smart home. Pers. Ubiquitous Comput. 2015, 19, 573–599. [Google Scholar] [CrossRef]
- Becker, S.; Miron-Shatz, T.; Schumacher, N.; Krocza, J.; Diamantidis, C.; Albrecht, U. MHealth 2.0: Experiences, possibilities, and perspectives. JMIR Mhealth Uhealth 2014, 2, e24. [Google Scholar] [CrossRef]
- Martín, J.A.C.; Martínez-Pérez, B.; De La Torre-Díez, I.; López-Coronado, M. Economic impact assessment from the use of a mobile app for the self-management of heart diseases by patients with heart failure in a Spanish region. J. Med. Syst. 2014, 38, 96. [Google Scholar] [CrossRef]
- Luxton, D.D.; Hansen, R.N.; Stanfill, K. Mobile app self-care versus in-office care for stress reduction: A cost minimization analysis. J. Telemed. Telecare 2014, 20, 431–435. [Google Scholar] [CrossRef]
- Nghiem, N.; Leung, W.; Cleghorn, C.; Blakely, T.; Wilson, N. Mass media promotion of a smartphone smoking cessation app: Modelled health and cost-saving impacts. BMC Public Health 2019, 19, 283. [Google Scholar] [CrossRef]
- He, W.; Goodkind, D.; Kowal, P. An Aging World: 2015; International Population Reports; U.S. Government Publishing Office: Washington, DC, USA, 2016.
- Kaur, A.; Chen, W. Exploring AI literacy among older adults. Stud. Health Technol. Inform. 2023, 306, 9–16. [Google Scholar] [CrossRef]
- Chan, A.; Cohen, R.; Robinson, K.; Bhardwaj, D.; Gregson, G.; Jutai, J.W.; Millar, J.; Rincón, A.R.; Fekr, A.R. Evidence and user considerations of home health monitoring for older adults: Scoping review. JMIR Aging 2022, 5, e40079. [Google Scholar] [CrossRef]
- Feldberg, R. Understanding Older Adults’ Perceptions Towards Use of Remote Health Monitoring Through Smart Health Devices. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2022. [Google Scholar]
- Huang, J.C. Remote health monitoring adoption model based on artificial neural networks. Expert Syst. Appl. 2010, 37, 307–314. [Google Scholar] [CrossRef]
- Linkous, L.; Zohrabi, N.; Abdelwahed, S. Health monitoring in smart homes utilizing Internet of Things. In Proceedings of the 2019 IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Arlington, VA, USA, 25–27 September 2019; pp. 29–34. [Google Scholar] [CrossRef]
- Cicibas, H.; Yildirim, S.O. Adoption factors of health monitoring systems for smart healthcare: A systematic review. In Proceedings of the 12th IADIS International Conference Information Systems 2019, Utrecht, The Netherlands, 11–13 April 2019; pp. 43–50. [Google Scholar] [CrossRef]
- 60% of Americans Would be Uncomfortable with Provider Relying on AI in Their Own Health Care. Available online: https://www.pewresearch.org/science/2023/02/22/60-of-americans-would-be-uncomfortable-with-provider-relying-on-ai-in-their-own-health-care/ (accessed on 1 December 2024).
- Yang, H.; Lee, H.; Zo, H. User acceptance of smart home services: An extension of the theory of planned behavior. Ind. Manag. Data Syst. 2017, 117, 68–89. [Google Scholar] [CrossRef]
- Walker, R.C.; Tong, A.; Howard, K.; Palmer, S.C. Patient expectations and experiences of remote monitoring for chronic diseases: Systematic review and thematic synthesis of qualitative studies. Int. J. Med. Inform. 2019, 124, 78–85. [Google Scholar] [CrossRef]
- Health Canada Act. Available online: https://www.canada.ca/en/health-canada/services/health-care-system/canada-health-care-system-medicare/canada-health-act.html (accessed on 7 December 2024).
- International Survey Shows Canada Lags Behind Peer Countries in Access to Primary Health Care. Available online: https://www.cihi.ca/en/international-survey-shows-canada-lags-behind-peer-countries-in-access-to-primary-health-care (accessed on 7 December 2024).
- Duong, D.; Vogel, L. National survey highlights worsening primary care access. Can. Med. Assoc. J. 2023, 195, E592–E593. [Google Scholar] [CrossRef] [PubMed]
- Doty, M.M.; Shah, A.; Fields, K.; FitzGerald, M.; Williams, R.D., II. Comparing Nations on Timeliness and Coordination of Health Care; Commonwealth Fund: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Canadian Institute for Health Information. How Canada Compares: Results from the Commonwealth Fund’s 2020 International Health Policy Survey of the General Population in 11 Countries; CIHI: Ottawa, ON, Canada, 2021. [Google Scholar]
- Ross University School of Medicine. US vs. Canadian Healthcare: What Is the Difference? 2021. Available online: https://medical.rossu.edu/about/blog/us-vs-canadian-healthcare (accessed on 7 December 2024).
- Ghorayeb, A.; Comber, R.; Gooberman-Hill, R. Older adults’ perspectives of smart home technology: Are we developing the technology that older people want? Int. J. Hum.-Comput. Stud. 2020, 147, 102571. [Google Scholar] [CrossRef]
- Cimperman, M.; Brenčič, M.M.; Trkman, P.; De Leonni Stanonik, M. Older adults’ perceptions of home telehealth services. Telemed. J. E-Health 2013, 19, 786–790. [Google Scholar] [CrossRef]
- Kruger, J.; Dunning, D. Unskilled and unaware of it: How difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J. Pers. Soc. Psychol. 1999, 77, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, K.; Tsotsos, L.E. Technology for remote health monitoring in an older population: A role for mobile devices. Multimodal Technol. Interact. 2018, 2, 43. [Google Scholar] [CrossRef]
- Verloo, H.; Kampel, T.; Vidal, N.; Pereira, F. Perceptions about technologies that help community-dwelling older adults remain at home: Qualitative study. J. Med. Internet Res. 2020, 22, e17930. [Google Scholar] [CrossRef] [PubMed]
- Giger, J.T.; Pope, N.D.; Vogt, H.B.; Gutierrez, C.; Newland, L.A.; Lemke, J.; Lawler, M.J. Remote patient monitoring acceptance trends among older adults residing in a frontier state. Comput. Hum. Behav. 2014, 44, 174–182. [Google Scholar] [CrossRef]
- Cooper-Patrick, L. Race, gender, and partnership in the patient-physician relationship. J. Am. Med. Assoc. 1999, 282, 583. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.; Stoddard, J.J. Patient-physician pairing: Does racial and ethnic congruity influence selection of a regular physician? J. Community Health 1997, 22, 247–259. [Google Scholar] [CrossRef]
- Saha, S.; Taggart, S.H.; Komaromy, M.; Bindman, A.B. Do patients choose physicians of their own race? Health Aff. 2000, 19, 76–83. [Google Scholar] [CrossRef]
- Smith, G.H.; Hampton, C.; Brandon, W.P. Physicians, physician extenders and health outcomes: Race, gender and patient-health provider concordance. J. Health Care Poor Underserved 2018, 29, 530–555. [Google Scholar] [CrossRef] [PubMed]
- Meyer, O.L.; Zane, N. The influence of race and ethnicity in clients’ experiences of mental health treatment. J. Community Psychol. 2013, 41, 884–901. [Google Scholar] [CrossRef]
- Alizadeh, S.; Chavan, M. Cultural competence dimensions and outcomes: A systematic review of the literature. Health Soc. Care Community 2015, 24, e117–e130. [Google Scholar] [CrossRef]
- Alegría, M.; Roter, D.L.; Valentine, A.; Chen, C.; Li, X.; Lin, J.; Rosen, D.; Lapatin, S.; Normand, S.-L.; Larson, S.; et al. Patient–clinician ethnic concordance and communication in mental health intake visits. Patient Educ. Couns. 2013, 93, 188–196. [Google Scholar] [CrossRef]
- Nazione, S.; Perrault, E.K.; Keating, D.M. Finding common ground: Can provider-patient race concordance and self-disclosure bolster patient trust? J. Racial Ethn. Health Disparities 2019, 6, 962–972. [Google Scholar] [CrossRef]
- Moore, C.; Coates, E.; Watson, A.; de Heer, R.; McLeod, A.; Prudhomme, A. Correction to: “It’s important to work with people that look like me”: Black patients’ preferences for patient-provider race concordance. J. Racial Ethn. Health Disparities 2022, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- van Ryn, M. Research on the provider contribution to race/ethnicity disparities in medical care. Med. Care 2002, 40, I-140–I-151. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Devenney, E.; Hodges, J.R. The Mini-Mental State Examination: Pitfalls and limitations. Pract. Neurol. 2016, 17, 79–80. [Google Scholar] [CrossRef]
- Hsieh, S.; Schubert, S.; Hoon, C.; Mioshi, E.; Hodges, J.R. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementias and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 242–250. [Google Scholar] [CrossRef]
- Mathuranath, P.; Nestor, P.; Berrios, G.; Rakowicz, W.; Hodges, J. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology 2000, 55, 1613–1620. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Dautzenberg, G.; Lijmer, J.; Beekman, A. Diagnostic accuracy of the Montreal Cognitive Assessment (MoCA) for cognitive screening in old age psychiatry. Int. J. Geriatr. Psychiatry 2019, 35, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the MoCA test better suited than the MMSE in MCI detection among people aged over 60? Psychiatr. Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Lintner, T. A systematic review of AI literacy scales. npj Sci. Learn. 2024, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Bispo Júnior, J.P. Social desirability bias in qualitative health research. Rev. Saude Publica. 2022, 56, 101. [Google Scholar] [CrossRef] [PubMed]




| In-Person Assessments (n = 60) | Follow-Up Phone Interview (n = 51) | |
|---|---|---|
| Age (Years) | 75.0 ± 6.2 | 74.8 ± 6.4 |
| Gender (%) | ||
| Male | 30 | 28 |
| Female | 70 | 72 |
| Intersex | 0 | 0 |
| Prefer not to say | 0 | 0 |
| Ethnicity (%) | ||
| Arabic (Middle East, North Africa) | 2 | 1.9 |
| Black (e.g., African, American, Caribbean, etc.) | 1.7 | 1.9 |
| Latin American (e.g., Mexican, Chilean, Costa Rican, etc.) | 1.7 | 1.9 |
| South Asian (e.g., East Indian, Pakistani, Sri Lankan, Bangladeshi, etc.) | 3.3 | 3.7 |
| White | 86.7 | 85.2 |
| I would like to specify an identity not listed | 5 | 5.6 |
| Education (%) | ||
| High school | 6.7 | 7.4 |
| College diploma | 31.7 | 27.8 |
| University degree | 35.0 | 38.9 |
| Post graduate degree | 20.0 | 18.5 |
| Other | 6.7 | 7.4 |
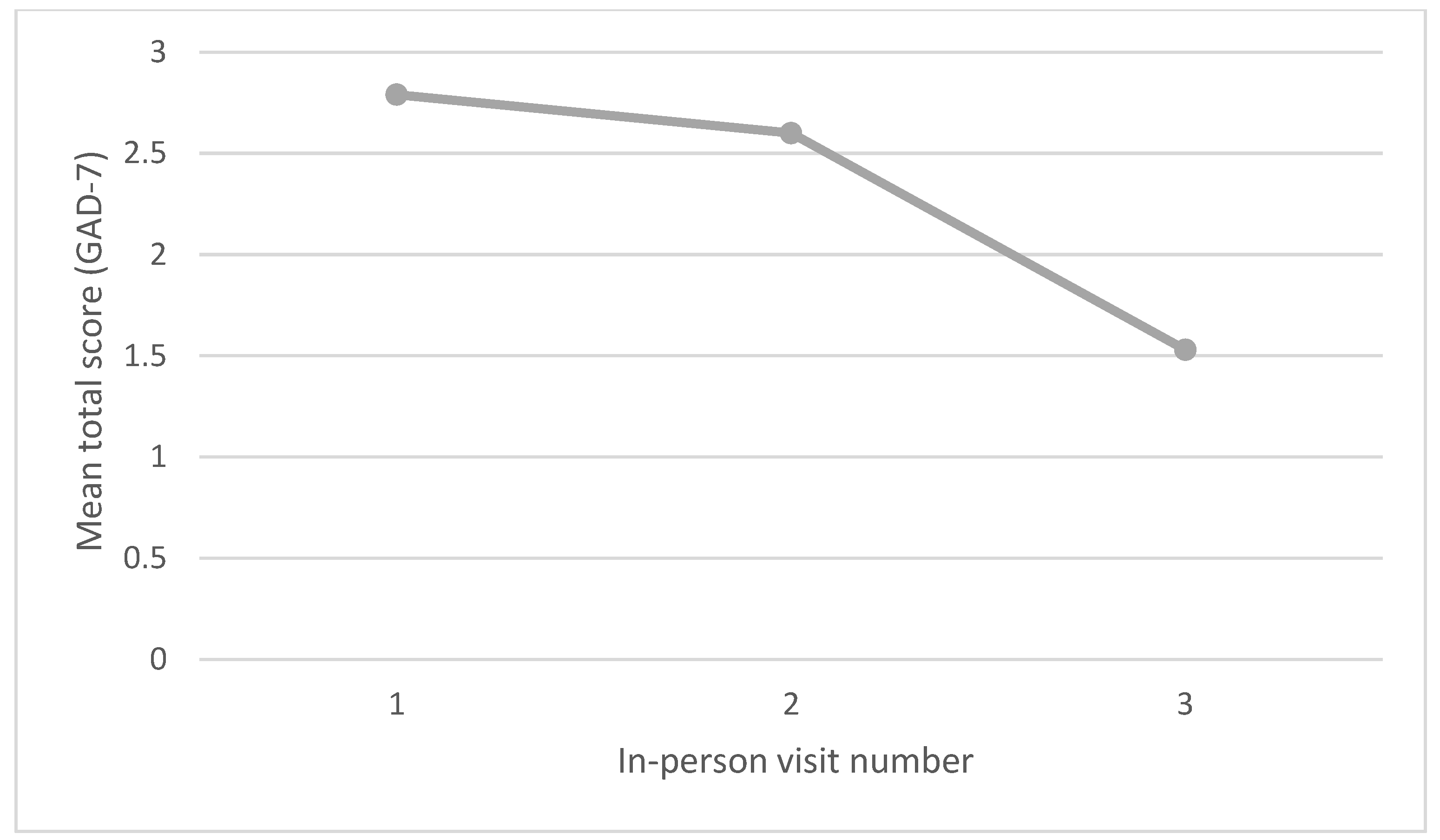
| Patient Health Questionnaire (PHQ-8) Total Score | Generalized Anxiety Disorder Questionnaire (GAD-7) Total Score | ||
|---|---|---|---|
| Visit 1 | Minimum | 0 | 0 |
| Maximum | 16 | 21 | |
| Mean | 3.25 | 2.79 | |
| Standard Deviation | 3.26 | 3.94 | |
| Visit 2 | Minimum | 0 | 0 |
| Maximum | 16 | 18 | |
| Mean | 3.11 | 2.60 | |
| Standard Deviation | 3.11 | 3.31 | |
| Visit 3 | Minimum | 0 | 0 |
| Maximum | 7 | 6 | |
| Mean | 2.67 | 1.53 | |
| Standard Deviation | 2.26 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paniak, I.; Cohen, E.; Studzinski, C.; Tsotsos, L. Testing a New Approach to Monitor Mild Cognitive Impairment and Cognition in Older Adults at the Community Level. Multimodal Technol. Interact. 2025, 9, 109. https://doi.org/10.3390/mti9100109
Paniak I, Cohen E, Studzinski C, Tsotsos L. Testing a New Approach to Monitor Mild Cognitive Impairment and Cognition in Older Adults at the Community Level. Multimodal Technologies and Interaction. 2025; 9(10):109. https://doi.org/10.3390/mti9100109
Chicago/Turabian StylePaniak, Isabel, Ethan Cohen, Christa Studzinski, and Lia Tsotsos. 2025. "Testing a New Approach to Monitor Mild Cognitive Impairment and Cognition in Older Adults at the Community Level" Multimodal Technologies and Interaction 9, no. 10: 109. https://doi.org/10.3390/mti9100109
APA StylePaniak, I., Cohen, E., Studzinski, C., & Tsotsos, L. (2025). Testing a New Approach to Monitor Mild Cognitive Impairment and Cognition in Older Adults at the Community Level. Multimodal Technologies and Interaction, 9(10), 109. https://doi.org/10.3390/mti9100109






