At What Point in the Menstrual Cycle Are the Pelvic Floor Muscles at Their Weakest?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Instrumentation and Data Collection
2.3.1. Verification of Menstrual Cycle Phase
2.3.2. Sociodemographic Data and Questionnaires
2.3.3. First PFM Assessment: The Modified Oxford Scale (MOS)
2.3.4. Second PFM Assessment: Intravaginal Clinical Dynamometry
2.4. Data Analysis
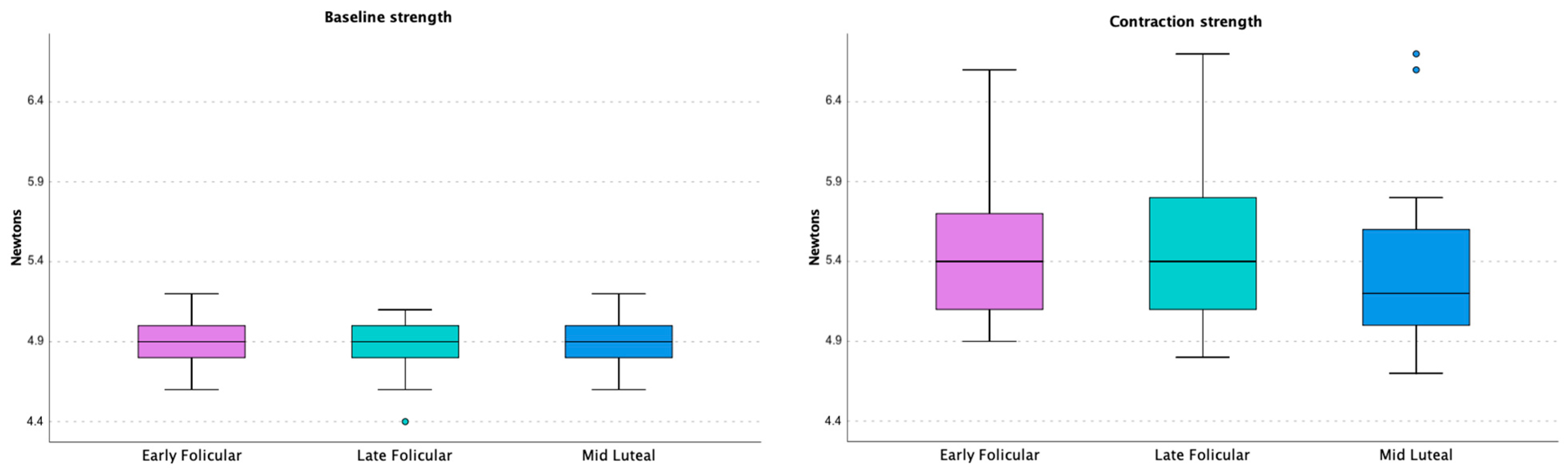
3. Results
4. Discussion
Strengths and Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros, R.P.A.; Gustafsson, J.Å. Estrogen Receptors and the Metabolic Network. Cell Metab. 2011, 14, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Blackburn, J.T.; Norcorss, M.F.; Ondrak, K.S.; Hudson, J.D.; Hackney, A.C.; Padua, D.A. Estrogen and Muscle Stiffness Have a Negative Relationship in Females. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Baltgalvis, K.A.; Greising, S.M. Mechanisms behind Estrogens’ Beneficial Effect on Muscle Strength in Females. Exerc. Sport. Sci. Rev. 2010, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.A.; Vitzthum, V.J. The Extent and Causes of Natural Variation in Menstrual Cycles: Integrating Empirically-Based Models of Ovarian Cycling into Research on Women’s Health. Drug Discov. Today Dis. Models 2020, 32, 41–49. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, R.C.; Bruinvels, G.; Pedlar, C.R. Variations in Strength-Related Measures during the Menstrual Cycle in Eumenorrheic Women: A Systematic Review and Meta-Analysis. J. Sci. Med. Sport 2020, 23, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Janse De Jonge, X.A.K. Effects of the Menstrual Cycle on Exercise Performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Myrick, M.P.; Troy Blackburn, J.; Shultz, S.J.; Guskiewicz, K.M.; Padua, D.A. The Effect of Menstrual-Cycle Phase on Hamstring Extensibility and Muscle Stiffness. J. Sport Rehabil. 2009, 18, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Janse De Jonge, X.A.K.; Boot, C.R.L.; Thom, J.M.; Ruell, P.A.; Thompson, M.W. The Influence of Menstrual Cycle Phase on Skeletal Muscle Contractile Characteristics in Humans. J. Physiol. 2001, 530, 161–166. [Google Scholar] [CrossRef]
- Miyazaki, M.; Maeda, S. Changes in Hamstring Flexibility and Muscle Strength during the Menstrual Cycle in Healthy Young Females. J. Phys. Ther. Sci. 2022, 34, 92–98. [Google Scholar] [CrossRef]
- Mannella, P.; Palla, G.; Bellini, M.; Simoncini, T. The Female Pelvic Floor through Midlife and Aging. Maturitas 2013, 76, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Rahn, D.D.; Ward, R.M.; Sanses, T.V.; Carberry, C.; Mamik, M.M.; Meriwether, K.V.; Olivera, C.K.; Abed, H.; Balk, E.M.; Murphy, M. Vaginal Estrogen Use in Postmenopausal Women with Pelvic Floor Disorders: Systematic Review and Practice Guidelines. Int. Urogynecol. J. 2015, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hextall, A.; Bidmead, J.; Cardozo, L.; Hooper, R. The Impact of the Menstrual Cycle on Urinary Symptoms and the Results of Urodynamic Investigation. Br. J. Obstet. Gynaecol. 2001, 108, 1193–1196. [Google Scholar] [CrossRef]
- Sánchez-García, O.; López-Juárez, R.; Corona-Quintanilla, D.L.; Ruiz, Á.C.; Martínez-Gómez, M.; Cuevas-Romero, E.; Castelán, F. Estrogens Influence Differentially on the Pelvic Floor Muscles Activation at Somatovisceral Reflexes Involved in Micturition of Rabbits. Menopause 2021, 28, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.C.; Arpke, R.W.; Larson, A.A.; Baumann, C.W.; Xie, N.; Cabelka, C.A.; Nash, N.L.; Juppi, H.K.; Laakkonen, E.K.; Sipilä, S.; et al. Estrogen Regulates the Satellite Cell Compartment in Females. Cell Rep. 2019, 28, 368–381.e6. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Functions of Estrogen and Estrogen Receptor Signaling on Skeletal Muscle. J. Steroid Biochem. Mol. Biol. 2019, 191, 105375. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.C.; Laakkonen, E.K.; Lowe, D.A. Aging of the Musculoskeletal System: How the Loss of Estrogen Impacts Muscle Strength. Bone 2019, 123, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lasak, A.M.; Jean-Michel, M.; Le, P.U.; Durgam, R.; Harroche, J. The Role of Pelvic Floor Muscle Training in the Conservative and Surgical Management of Female Stress Urinary Incontinence: Does the Strength of the Pelvic Floor Muscles Matter? PM&R 2018, 10, 1198–1210. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, X.; Yi, X.; Lai, P.; Lan, Y. Electromyographic Biofeedback for Stress Urinary Incontinence or Pelvic Floor Dysfunction in Women: A Systematic Review and Meta-Analysis. Adv. Ther. 2021, 38, 4163–4177. [Google Scholar] [CrossRef]
- Jorge, C.H.; Bø, K.; Chiazuto Catai, C.; Oliveira Brito, L.G.; Driusso, P.; Kolberg Tennfjord, M. Pelvic Floor Muscle Training as Treatment for Female Sexual Dysfunction: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2024, 231, 51–66.e1. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Pavone, P.; Vecchio, M. Diagnosis, Rehabilitation and Preventive Strategies for Pudendal Neuropathy in Cyclists, A Systematic Review. J. Funct. Morphol. Kinesiol. 2021, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.L.; Miller, L.D.; Mishra, K. Pelvic Floor Physical Therapy in the Treatment of Pelvic Floor Dysfunction in Women. Curr. Opin. Obstet. Gynecol. 2019, 31, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Almagro, J.; Hernández Martínez, A.; Martínez-Vázquez, S.; Peinado Molina, R.A.; Bermejo-Cantarero, A.; Martínez-Galiano, J.M. A Qualitative Exploration of the Perceptions of Women Living with Pelvic Floor Disorders and Factors Related to Quality of Life. J. Clin. Med. 2024, 13, 1896. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; De Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef]
- Nygaard, I.; Barber, M.D.; Burgio, K.L.; Kenton, K.; Meikle, S.; Schaffer, J.; Spino, C.; Whitehead, W.E.; Wu, J.; Brody, D.J. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA 2008, 300, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Frawley, H.; Shelly, B.; Morin, M.; Bernard, S.; Bø, K.; Digesu, G.A.; Dickinson, T.; Goonewardene, S.; McClurg, D.; Rahnama’i, M.S.; et al. An International Continence Society (ICS) Report on the Terminology for Pelvic Floor Muscle Assessment. Neurourol. Urodyn. 2021, 40, 1217–1260. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cullerés, G.; Peña-Pitarch, E.; Jané-Feixas, C.; Arnau, A.; Montesinos, J.; Abenoza-Guardiola, M. Intra-Rater Reliability and Diagnostic Accuracy of a New Vaginal Dynamometer to Measure Pelvic Floor Muscle Strength in Women With Urinary Incontinence. Neurourol. Urodyn. 2017, 36, 333–337. [Google Scholar] [CrossRef]
- Huang, W.-C.; Yang, J.-M.; Chen, H.-F. Four-Dimensional Introital Ultrasound in Assessing Perioperative Pelvic Floor Muscle Functions of Women with Cystoceles|4-Dimensionale Introitus-Sonografie Zur Beurteilung Der Perioperativen Funktion Der Beckenbodenmuskulatur Bei Frauen Mit Zystozelen. Ultraschall Der Med. 2021, 42, E31–E41. [Google Scholar] [CrossRef]
- Palmezoni, V.P.; Santos, M.D.; Pereira, J.M.; Bernardes, B.T.; Pereira-Baldon, V.S.; Resende, A.P.M. Pelvic Floor Muscle Strength in Primigravidae and Non-Pregnant Nulliparous Women: A Comparative Study. Int. Urogynecol. J. 2017, 28, 131–137. [Google Scholar] [CrossRef]
- Navarro Brazález, B.; Torres Lacomba, M.; de la Villa, P.; Sánchez Sánchez, B.; Prieto Gómez, V.; Asúnsolo Del Barco, Á.; McLean, L. The Evaluation of Pelvic Floor Muscle Strength in Women with Pelvic Floor Dysfunction: A Reliability and Correlation Study. Neurourol. Urodyn. 2018, 37, 269–277. [Google Scholar] [CrossRef]
- Romero-Cullerés, G.; Peña-Pitarch, E.; Jané-Feixas, C.; Vilaseca-Grané, A.; Montesinos, J.; Arnau, A. Reliability and Diagnostic Accuracy of a New Vaginal Dynamometer to Measure Pelvic Floor Muscle Strength. Female Pelvic Med. Reconstr. Surg. 2020, 26, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Martinho, N.M.; Marques, J.; Silva, V.R.; Silva, S.L.A.; Carvalho, L.C.; Botelho, S. Intra and Inter-Rater Reliability Study of Pelvic Floor Muscle Dynamometric Measurements. Braz. J. Phys. Ther. 2015, 19, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for the Conservative and Nonpharmacological Management of Female Pelvic Floor Dysfunction. Int. Urogynecol. J. 2017, 28, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Mittiku, Y.M.; Mekonen, H.; Wogie, G.; Tizazu, M.A.; Wake, G.E. Menstrual Irregularity and Its Associated Factors among College Students in Ethiopia, 2021. Front. Glob. Womens Health 2022, 3, 917643. [Google Scholar] [CrossRef] [PubMed]
- Al Wattar, B.H.; Fisher, M.; Bevington, L.; Talaulikar, V.; Davies, M.; Conway, G.; Yasmin, E. Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study. J. Clin. Endocrinol. Metab. 2021, 106, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ge, H.; Zhou, J.; Wang, J.; Wang, L. Polycystic Ovary Syndrome and Adverse Pregnancy Outcomes: Potential Role of Decidual Function. Drug Discov. Ther. 2023, 17, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, H.; Gull, B.; Stener-Victorin, E.; Hellström, M. Ovarian Volume and Antral Follicle Count Assessed by MRI and Transvaginal Ultrasonography: A Methodological Study. Acta Radiol. 2014, 55, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Zhang, M.M.; Duan, N.; Hu, X.Y.; Ren, S.; Cao, Y.Y.; Zhang, Y.P.; Wang, Z.Q. Using Transvaginal Ultrasonography and MRI to Evaluate Ovarian Volume and Follicle Count of Infertile Women: A Comparative Study. Clin. Radiol. 2022, 77, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Romero-Moraleda, B.; Del Coso, J.; Gutiérrez-Hellín, J.; Ruiz-Moreno, C.; Grgic, J.; Lara, B. Influence Of The Menstrual Cycle On Muscle Strength. J. Hum. Kinet. 2019, 21, 123–133. [Google Scholar] [CrossRef]
- Miller, P.B.; Soules, M.R. The Usefulness of a Urinary LH Kit for Ovulation Prediction during Menstrual Cycles of Normal Women. Obstet. Gynecol. 1996, 87, 13–17. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 1–11. [Google Scholar]
- Georgia, R.-C.; Cecilia, J.-F.; Montserrat, A.-G.; Anna, V.-G.; Anna, A.; Montesinos, J.; Abenoza-Guardiola, M. Inter-Rater Reliability of Th Digital Palpation of the Pelvic Floor Muscle by the Modified Oxford Grading Scale in Continent and Incontinent Women. Arch. Esp. Urol. 2019, 72, 602–607. [Google Scholar] [CrossRef]
- Ferreira, C.H.J.; Barbosa, P.B.; de Oliveira Souza, F.; Antônio, F.I.; Franco, M.M.; Bø, K. Inter-Rater Reliability Study of the Modified Oxford Grading Scale and the Peritron Manometer. Physiotherapy 2011, 97, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ben Ami, N.; Dar, G. What Is the Most Effective Verbal Instruction for Correctly Contracting the Pelvic Floor Muscles? Neurourol. Urodyn. 2018, 37, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis Nagano, R.C.; Biasotto-Gonzalez, D.A.; da Costa, G.L.; Amorim, K.M.; Fumagalli, M.A.; Amorim, C.F.; Politti, F. Test-Retest Reliability of the Different Dynamometric Variables Used to Evaluate Pelvic Floor Musculature during the Menstrual Cycle. Neurourol. Urodyn. 2018, 37, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Dumoulin, C.; Gravel, D.; Bourbonnais, D.; Lemieux, M.-C. Reliability of Speed of Contraction and Endurance Dynamometric Measurements of the Pelvic Floor Musculature in Stress Incontinent Parous Women. Neurourol. Urodyn. 2007, 26, 397–403; discussion 404. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Dumoulin, C.; Bourbonnais, D.; Gravel, D.; Lemieux, M.-C. Pelvic Floor Maximal Strength Using Vaginal Digital Assessment Compared to Dynamometric Measurements. Neurourol. Urodyn. 2004, 23, 336–341. [Google Scholar] [CrossRef] [PubMed]
- El-Sayegh, B.; Cacciari, L.P.; Primeau, F.L.; Sawan, M.; Dumoulin, C. The State of Pelvic Floor Muscle Dynamometry: A Scoping Review. Neurourol. Urodyn. 2023, 42, 478–499. [Google Scholar] [CrossRef] [PubMed]
- Thereza Micussi, M.; Pegado FreiTas, R.; Helouyse Angelo, P.; Maria Soares, E.; Maria leMos, T.; Maria Maranhão, T. Is there a difference in the electromyographic activity of the pelvic floor muscles across the phases of the menstrual cycle? J. Phys. Ther. Sci. 2015, 27, 2233–2237. [Google Scholar] [CrossRef][Green Version]
- Morin, M.; Gravel, D.; Bourbonnais, D.; Dumoulin, C.; Ouellet, S. Reliability of Dynamometric Passive Properties of the Pelvic Floor Muscles in Postmenopausal Women with Stress Urinary Incontinence. Neurourol. Urodyn. 2008, 27, 819–825. [Google Scholar] [CrossRef]
- Czyrnyj, C.S.; Bérubé, M.-È.; Varette, K.; McLean, L. The Impact of a Familiarization Session on the Magnitude and Stability of Active and Passive Pelvic Floor Muscle Forces Measured through Intravaginal Dynamometry. Neurourol. Urodyn. 2019, 38, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Quartly, E.; Hallam, T.; Kilbreath, S.; Refshauge, K. Strength and Endurance of the Pelvic Floor Muscles in Continent Women: An Observational Study. Physiotherapy 2010, 96, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Castro-Pardiñas, M.A.; Torres-Lacomba, M.; Navarro-Brazález, B. Muscle Function of the Pelvic Floor in Healthy, Puerperal Women with Pelvic Floor Dysfunction. Actas Urol. Esp. 2017, 41, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lamerton, T.J.; Torquati, L.; Brown, W.J. Overweight and Obesity as Major, Modifiable Risk Factors for Urinary Incontinence in Young to Mid-aged Women: A Systematic Review and Meta-analysis. Obes. Rev. 2018, 19, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.R.; Avery, N.C.; Tarlton, J.F.; Eckford, S.D.; Abrams, P.; Bailey, A.J. Changes in Metabolism of Collagen in Genitourinary Prolapse. Lancet 1996, 347, 1658–1661. [Google Scholar] [CrossRef]
- Weintraub, A.Y.; Glinter, H.; Marcus-Braun, N. Narrative Review of the Epidemiology, Diagnosis and Pathophysiology of Pelvic Organ Prolapse. Int. Braz. J. Urol. 2020, 46, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Vita, M.; Sedlackova, Z.; Herman, M.; Furst, T.; Smekal, D.; Cech, Z. Influence of Female Hormones on Fascia Elasticity: An Elastography Study. Clin. Anat. 2019, 32, 941–947. [Google Scholar] [CrossRef]
- Piasecki, J.; Guo, Y.; Jones, E.J.; Phillips, B.E.; Stashuk, D.W.; Atherton, P.J.; Piasecki, M. Menstrual Cycle Associated Alteration of Vastus Lateralis Motor Unit Function. Sports Med. Open 2023, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Aburto-Corona, J.A.; Gil González, I.J.; Natasha, V.; Aguilar, V.; Calleja Núñez, J.J. El Ciclo Menstrual No Afecta El Desempeño Físico de Jóvenes Eumenorreicas. Retos 2021, 39, 264–266. [Google Scholar]
- Vecchio, M.; Chiaramonte, R.; DI Benedetto, P. Management of Bladder Dysfunction in Multiple Sclerosis: A Systematic Review and Meta-Analysis of Studies Regarding Bladder Rehabilitation. Eur. J. Phys. Rehabil. Med. 2022, 58, 387. [Google Scholar] [CrossRef]
- Niering, M.; Wolf-Belala, N.; Seifert, J.; Tovar, O.; Coldewey, J.; Kuranda, J.; Muehlbauer, T. The Influence of Menstrual Cycle Phases on Maximal Strength Performance in Healthy Female Adults: A Systematic Review with Meta-Analysis. Sports 2024, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, X.J.; Thompson, B.; Ahreum, H.A.N. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
| EFP | LFP | MLP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | p Value a | Mean (SD) | 95% CI | p Value a | Mean (SD) | 95% CI | p Value a | Between Phases p Value b | |
| Strength (Modified Oxford Grading Scale) | 3.67 (0.49) | (3.43–3.91) | - | 3.67 (0.59) | (3.37–3.96) | - | 3.56 (0.62) | (3.25–3.86) | - | 0.449 |
| Baseline strength (N) 1st measurement * | 4.84 (0.10) | (4.79–4.89) | 0.333 | 4.91 (0.18) | (4.81–5.00) | 0.790 | 4.83 (0.12) | (4.77–4.89) | 0.248 | 0.513 |
| Baseline strength (N) 2nd measurement * | 4.91 (0.15) | (4.84–4.99) | 4.89 (0.18) | (4.80–4.98) | 4.87 (0.16) | (4.79–4.95) | 0.886 | |||
| Contraction strength (N) 1st measurement * | 5.25 (0.30) | (5.10–5.40) | 0.230 | 5.44 (0.68) | (5.11–5.78) | 0.381 | 5.42 (0.74) | (5.05–5.78) | 0.321 | 0.407 |
| Contraction strength (N) 2nd measurement * | 5.46 (0.47) | (5.22–5.69) | 5.52 (0.54) | (5.25–5.79) | 5.47 (0.66) | (5.14–5.80) | 0.756 | |||
| Strength (N) 1st measurement * | 0.41 (0.29) | (0.27–0.55) | 0.327 | 0.54 (0.69) | (0.20–0.89) | 0.414 | 0.58 (0.79) | (0.19–0.98) | 0.775 | 0.727 |
| Strength (N) 2nd measurement * | 0.54 (0.53) | (0.28–0.81) | 0.63 (0.59) | (0.33–0.92) | 0.60 (0.74) | (0.23–0.97) | 0.717 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojedo-Martín, C.; Rodríguez-López, E.S.; Acevedo-Gómez, M.B.; Úbeda-D’Ocasar, E.; de-Diego, M.V.; Lara, B. At What Point in the Menstrual Cycle Are the Pelvic Floor Muscles at Their Weakest? J. Funct. Morphol. Kinesiol. 2024, 9, 135. https://doi.org/10.3390/jfmk9030135
Ojedo-Martín C, Rodríguez-López ES, Acevedo-Gómez MB, Úbeda-D’Ocasar E, de-Diego MV, Lara B. At What Point in the Menstrual Cycle Are the Pelvic Floor Muscles at Their Weakest? Journal of Functional Morphology and Kinesiology. 2024; 9(3):135. https://doi.org/10.3390/jfmk9030135
Chicago/Turabian StyleOjedo-Martín, Cristina, Elena Sonsoles Rodríguez-López, María Barbaño Acevedo-Gómez, Edurne Úbeda-D’Ocasar, María Victoria de-Diego, and Beatriz Lara. 2024. "At What Point in the Menstrual Cycle Are the Pelvic Floor Muscles at Their Weakest?" Journal of Functional Morphology and Kinesiology 9, no. 3: 135. https://doi.org/10.3390/jfmk9030135
APA StyleOjedo-Martín, C., Rodríguez-López, E. S., Acevedo-Gómez, M. B., Úbeda-D’Ocasar, E., de-Diego, M. V., & Lara, B. (2024). At What Point in the Menstrual Cycle Are the Pelvic Floor Muscles at Their Weakest? Journal of Functional Morphology and Kinesiology, 9(3), 135. https://doi.org/10.3390/jfmk9030135






