Functional Recovery and Regenerative Effects of Direct Transcutaneous Electrical Nerve Stimulation in Treatment of Post-COVID-19 Guillain–Barré and Acute Transverse Myelitis Overlap Syndrome: A Clinical Case
Abstract
1. Introduction
2. Case Presentation
2.1. Patient History
2.2. Before Direct TENS Treatment
2.2.1. Physical Examination
- (1)
- Higher nervous activity: Normal waking consciousness was established. No cognitive impairments were registered, but a focus on reducing self-criticism was found.
- (2)
- Motor functions: In the lower extremities, paraparesis was noted in proximal and distal muscles with an average of 3/5 points on the right side and 4/5 points on the left. More pronounced paraparesis was recorded in ankle dorsiflexion with a decrease of up to 2/5 points on the right side and up to 3/5 points on the left. No motor deficit was noted in the hands and face. No muscle atrophy was detected. A mixed type of gait disorder was disclosed (ataxic, spastic and paretic). Carporadial, flexion elbow and extensor elbow reflexes were symmetrically reduced. Patellar reflexes were hyperactive, more so on the right side, but Achilles reflexes diminished, especially on the left side. Babinski reflex and variations of the Babinski reflex, such as the Schaeffer, Oppenheim, Gordon and Chaddock reflexes, were evoked on the right side, and only the Babinski reflex was evoked on the left side.
- (3)
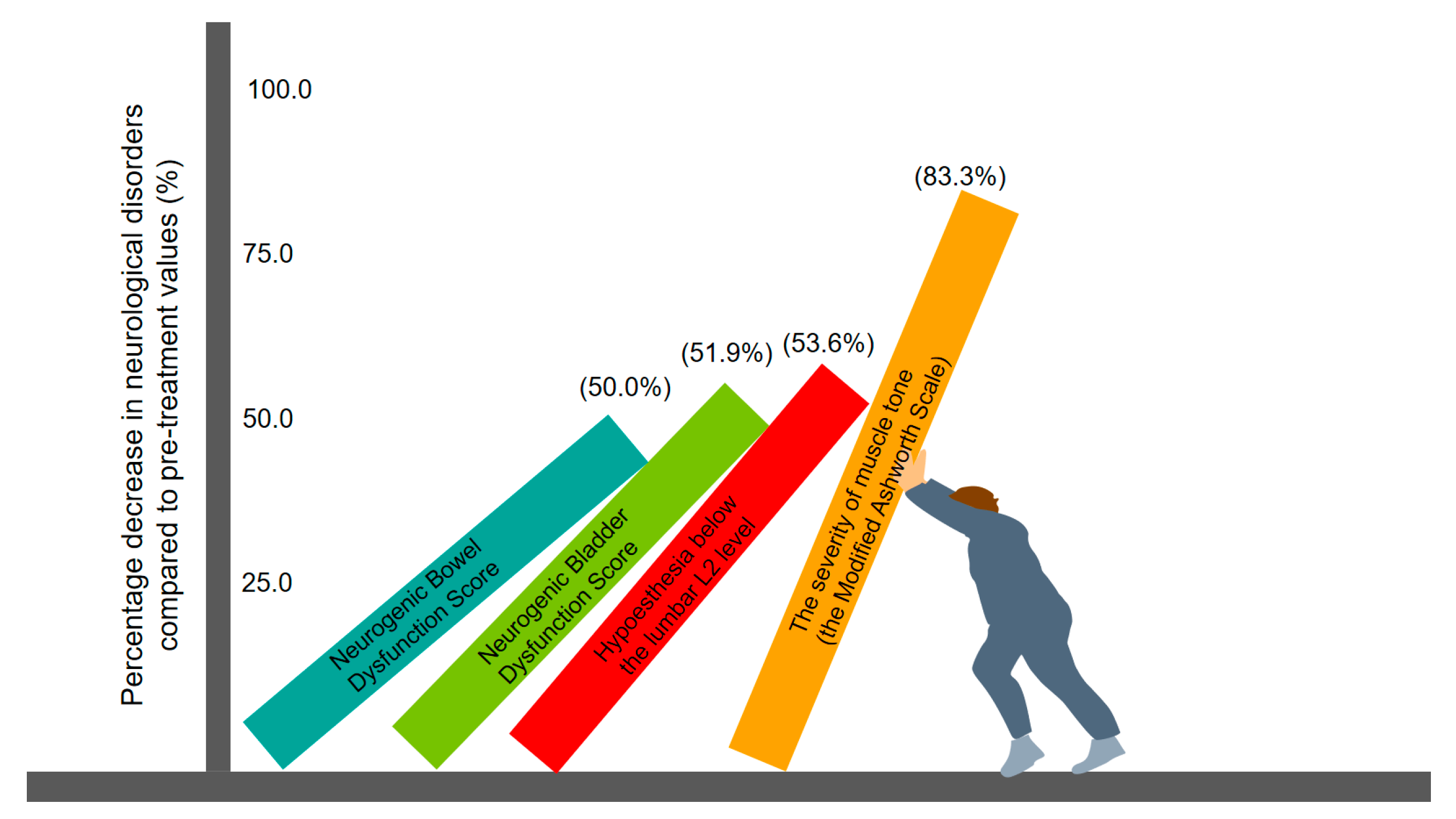
- Muscle tone was slightly decreased in the upper extremities, symmetrically, but asymmetrically increased in the lower extremities, with greater spasticity in the right leg. The severity of muscle tone according to the Modified Ashworth Scale was 2 in the left leg and 3 in the right leg. Nocturnal painful spasticity was assessed on a 10-point scale: 7 points in the right leg and 5 points in the left leg.
- (4)
- Sensory functions: No sensation abnormality was found on the face, torso or arms. Temperature, touch and pinprick perception were reduced in the upper extremities in a glove-type fashion. The level of temperature hypoesthesia in the upper extremities was detected in the middle third of the forearm and below (4/5), and tactile hypoesthesia was detected in the lower third of the forearm and below (3/5) in both hands. All sensations, including vibration and position perception, were reduced below the L2 lumbar level and were more pronounced on the right half of the body. The decrease in sensation was assessed by the patient on a 10-point scale compared to sensation on the face and was 10 points on the right side and 7 points on the left side.
- (5)
- Pain syndrome on the Visual Analog Scale (VAS) was 3 points in the hands and 6 points in the legs. Paresthesia (symptoms of numbness, burning and tingling) was assessed on a 10-point scale: 5 points in the hands and 7 points in the legs.
- (6)
- Bladder and bowel dysfunction: Control over voluntary urination was not complete. The patient had difficulty urinating in small portions. Intermittent catheterization was performed every 8 h. Neurogenic bladder dysfunction was assessed by the specific health-related Russian-translated Short Form of Qualiveen (SF-Qualiveen) questionnaire and averaged 2.6/4.0 [34]. Despite the use of polyethylene glycol-based suppositories, bowel care time increased to 1–1:20 h. However, the patient did not require digital stimulation for bowel care sessions, but a mini-enema was administered on average once a week. The frequency of bowel movements was 1–3 times a week. No fecal incontinence was observed. The severity of bowel dysfunction assessed by the Neurogenic Bowel Dysfunction Score was 8/47 [35].
2.2.2. Laboratory Results
2.2.3. Neuroimaging
- (1)
- Brain MRI: There are no abnormal focal areas of altered signal intensity in the cerebral hemispheres, brainstem or cerebellum.
- (2)
- Cervical MRI: T1- and T2-weighted MRI images of the cervical spine showed signs of spondylosis, spondyloarthrosis and multiple extrusions of intervertebral discs at cervical level C5-D1 without compression of the dural sac and spinal cord. No spinal cord pathology was detected.
- (3)
- Lumbar MRI: T1- and T2-weighted MRI scans of the thoracic spine showed signs of spondylosis, spondyloarthrosis and multiple protrusion of intervertebral discs at lumbar level Th12-S1 without compression of the dural sac and spinal cord. No spinal cord pathology was detected.
- (4)
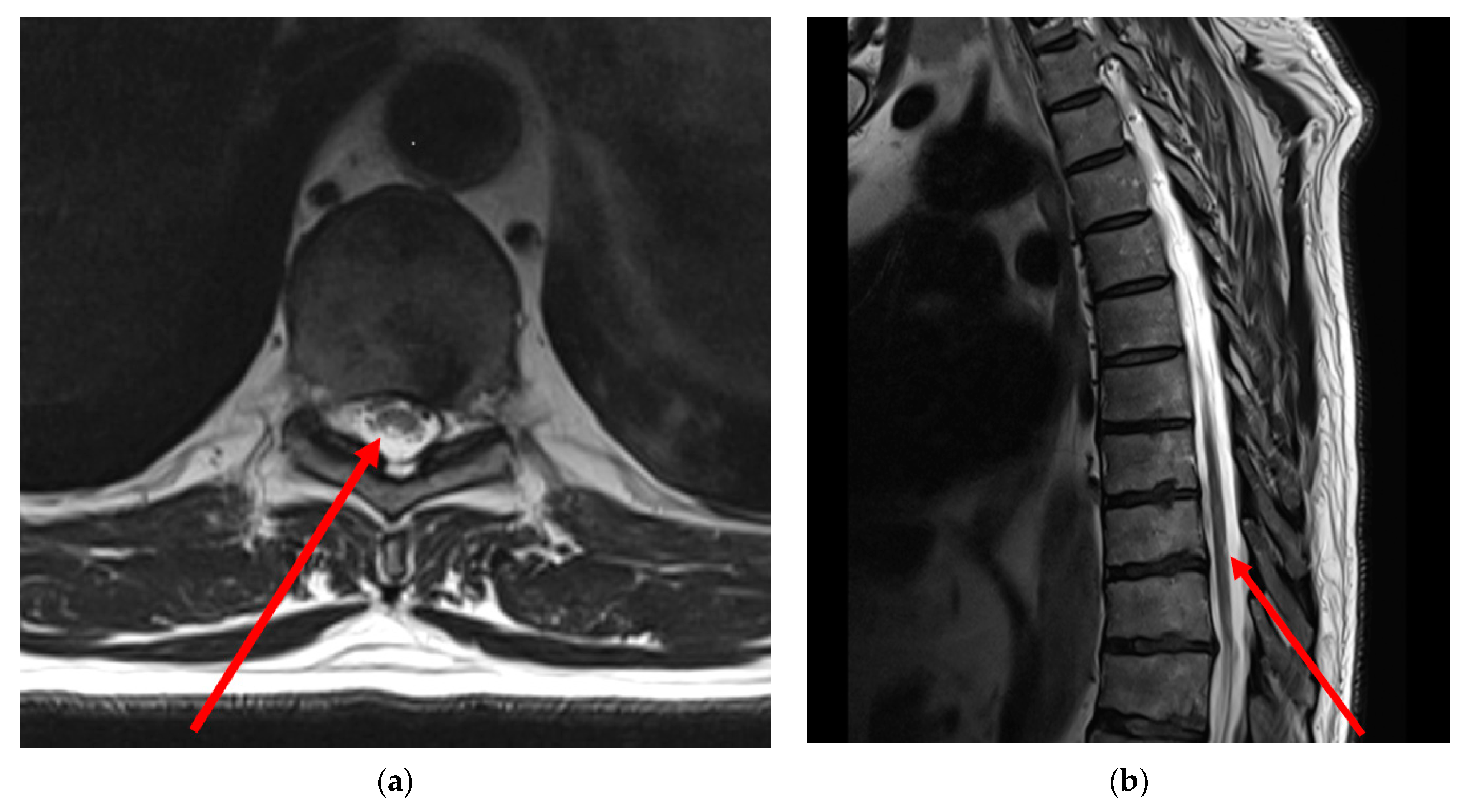
- Thoracic MRI: T2-weighted MRI images of the thoracic spine showed asymmetrical mild patchy high-signal changes in the spinal cord between levels D9 and D10, affecting the posterolateral sides of the spinal cord bilaterally, but predominantly on the right side with minimal swelling (Figure 1).
2.2.4. Electrophysiology
Evoked Electroneurography
- (1)
- In the upper extremities, electroneurography of median and ulnar nerves showed a slight decrease in motor conduction velocity, an increase in terminal latency of the compound muscle action potential (CMAP) and normal CMAP amplitudes and duration. Additional temporal dispersion in the elbow–wrist nerve segment was registered in an examination of ulnar nerves. A pronounced decrease in sensory nerve conduction velocity and sensory nerve action potential (SNAP) amplitude was registered in the median and ulnar nerves bilaterally. No decremental motor responses to repetitive nerve stimulation of the right axillary and median nerves were registered.
- (2)
- In the lower extremities, a significant decrease in motor conduction velocity of the common peroneal and tibial nerves was recorded with a marked decrease in CMAP amplitude and an increase in terminal latency and duration of CMAP bilaterally. Temporal dispersion was recorded in the ankle–knee segment of the tibial nerves and in the ankle–fibular head segment of the common peroneal nerves. SNAP of the bilateral superficial peroneal nerves, medial plantar nerves and sural nerves could not be recorded. No decremental motor responses to repetitive nerve stimulation of the right tibial nerve were registered.
Quantitative Needle Electromyography
Brain Evoked Potentials Tests
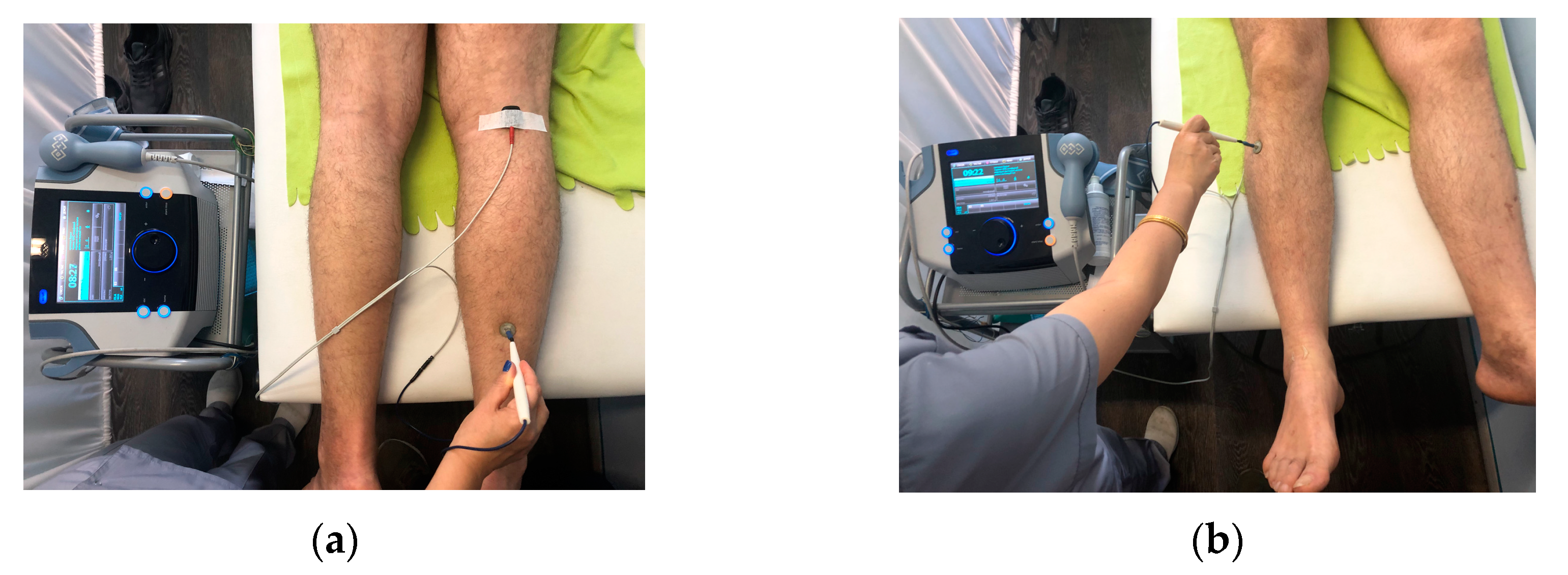
2.3. Direct TENS Treatment
2.3.1. Equipment
2.3.2. Characteristics of Current
- (1)
- HF TENS: monopolar square-wave pulse was used in the stimulation with a 100 Hz frequency and 50 μs duration. The amplitude of stimulation was increased until a painless sensation of vibration was achieved.
- (2)
- LF TENS: monopolar square-wave pulse was used in the stimulation with a 1 Hz frequency and 200 μs duration. The amplitude of stimulation was increased until painless muscle contraction was achieved.
2.3.3. Electrode Fixation
2.4. After Direct TENS Treatment
2.4.1. Physical Examination
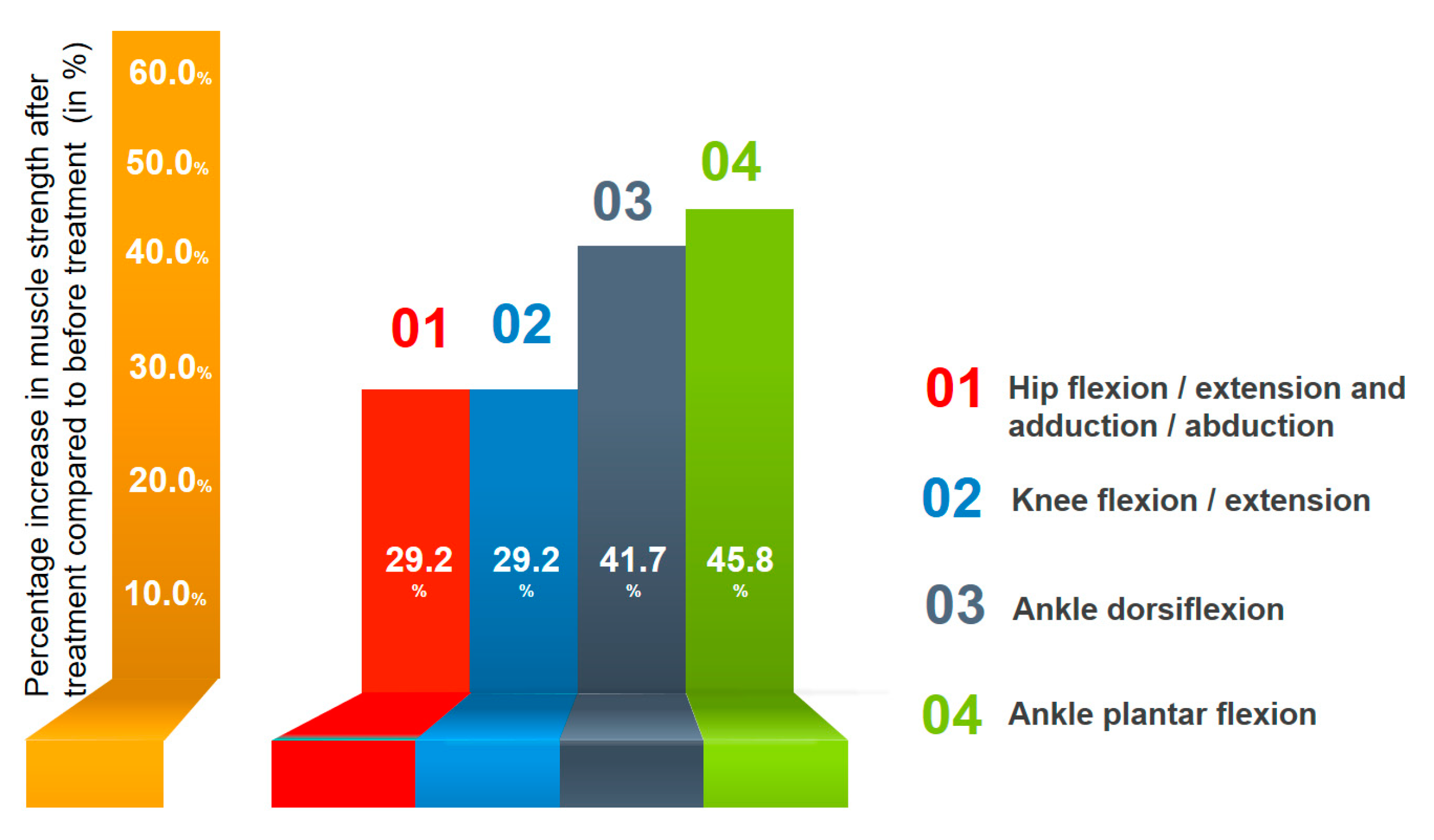
- (1)
- Motor functions: Motor deficit completely regressed in the left leg with the exception of ankle dorsiflexion (strength = 4.5/5). In the right leg, slight residual motor deficits were registered proximally and distally (strength = 4/5). However, ankle dorsiflexion strength improved to a 3/5. A marked improvement in walking was noted with the preservation of elements of spasticity and steppage gait. Gait velocity and functional mobility increased in good lighting, but in the dark, some difficulties were noted. Asymmetrical dysmetria was detected during the heel-to-shin test, mainly in the right leg, but not during the finger-to-nose test. Compared to the previous examination before treatment, no significant changes in reflexes were detected. The Babinski reflex was evoked only on the right, with a total reduction in the Schaeffer, Oppenheim, Gordon and Chaddock reflexes and the absence of spontaneous activity. The muscle tone of the upper extremities was normalized. Moderate spasticity was detected in the right leg but not in the left. The severity of muscle tone according to the Modified Ashworth Scale was 0 in the left leg and 1 in the right leg. Nocturnal painful spasticity regressed to 3 points in the right leg and 1 point in the left.
- (2)
- Sensory functions: Temperature and tactile hypoesthesia in the upper extremities decreased significantly and was observed only on the fingers. Comparing the current status with the previous one, the patient began to distinguish tactile and painful stimuli on the right half of the body below the lumbar level of L2. No improvement was found in vibration and position perception. According to the patient’s assessment on a 10-point scale, hypoesthesia was 5 points in the right half and 3 points in the left. Pain syndrome in the hands completely decreased, but in the legs, it remained at 3 points. Paresthesia averaged 2 points in the hands and 3 points in the legs.
- (3)
- Neurogenic bladder dysfunction improved significantly and scored 1.25 on the SF-Qualiveen scale. With the use of polyethylene glycol-based suppositories, bowel care time decreased to 30–40 min. The severity of bowel dysfunction assessed by the Neurogenic Bowel Dysfunction Score decreased to 4 points.
2.4.2. Electrophysiology
Evoked Electromyography
- (1)
- In the upper extremities, the CMAP of the median and ulnar nerves remained normal. A slight increase in conduction velocity and a moderate decrease in distal latency were registered in motor nerves. In sensory nerves, an increase in the SNAP amplitudes of the median nerves was registered, with an average increase of 20.1%, and the ulnar nerves increased by 84.9%. At the same time, changes in conduction velocity were not significant.
- (2)
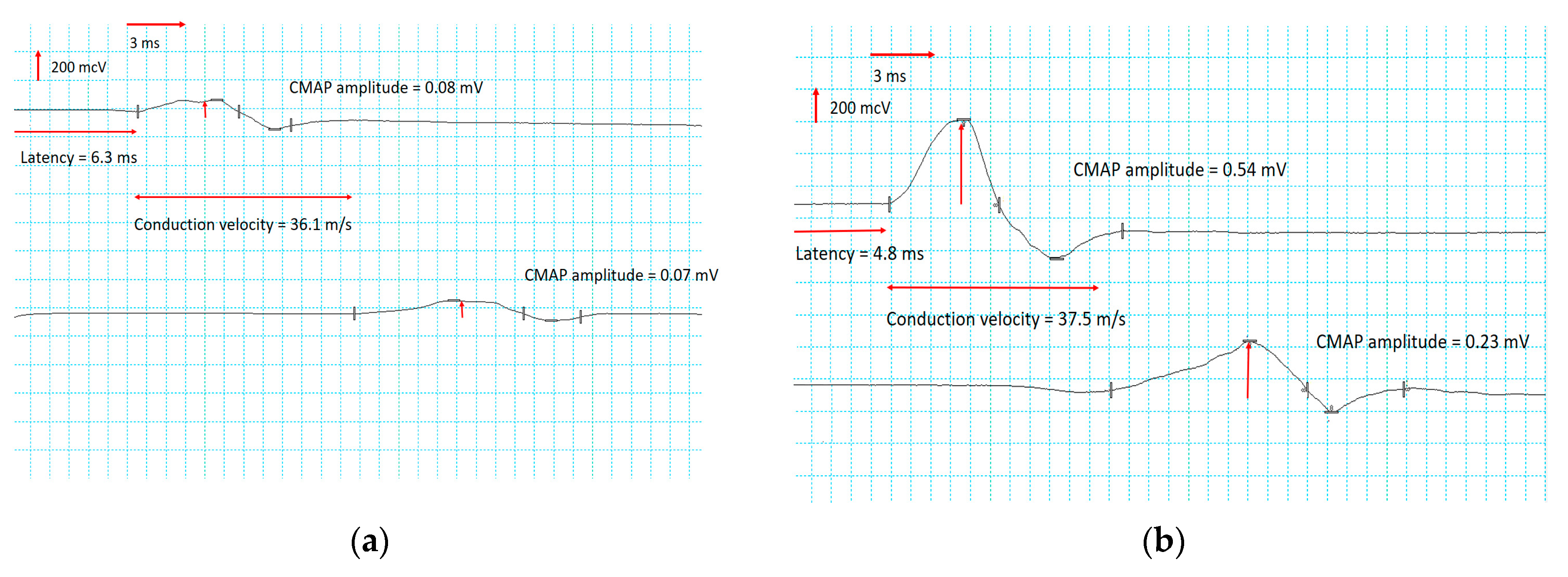
- In the lower extremities, significant increases in the CMAP of the peroneal and tibial nerves were recorded, especially in the right peroneal (Figure 3) and the left tibial nerves (Figure 4). Motor conduction velocity did not change in peroneal nerves. Electroneurography of the tibial nerves showed an increase in conduction velocity of 18% in the left nerve and 21% in the right. Terminal latency decreased to 19% in the left peroneal nerve and to 24% in the right. No improvement was noted in SNAP amplitude and sensory conduction velocity of peroneal, tibial and sural nerves.
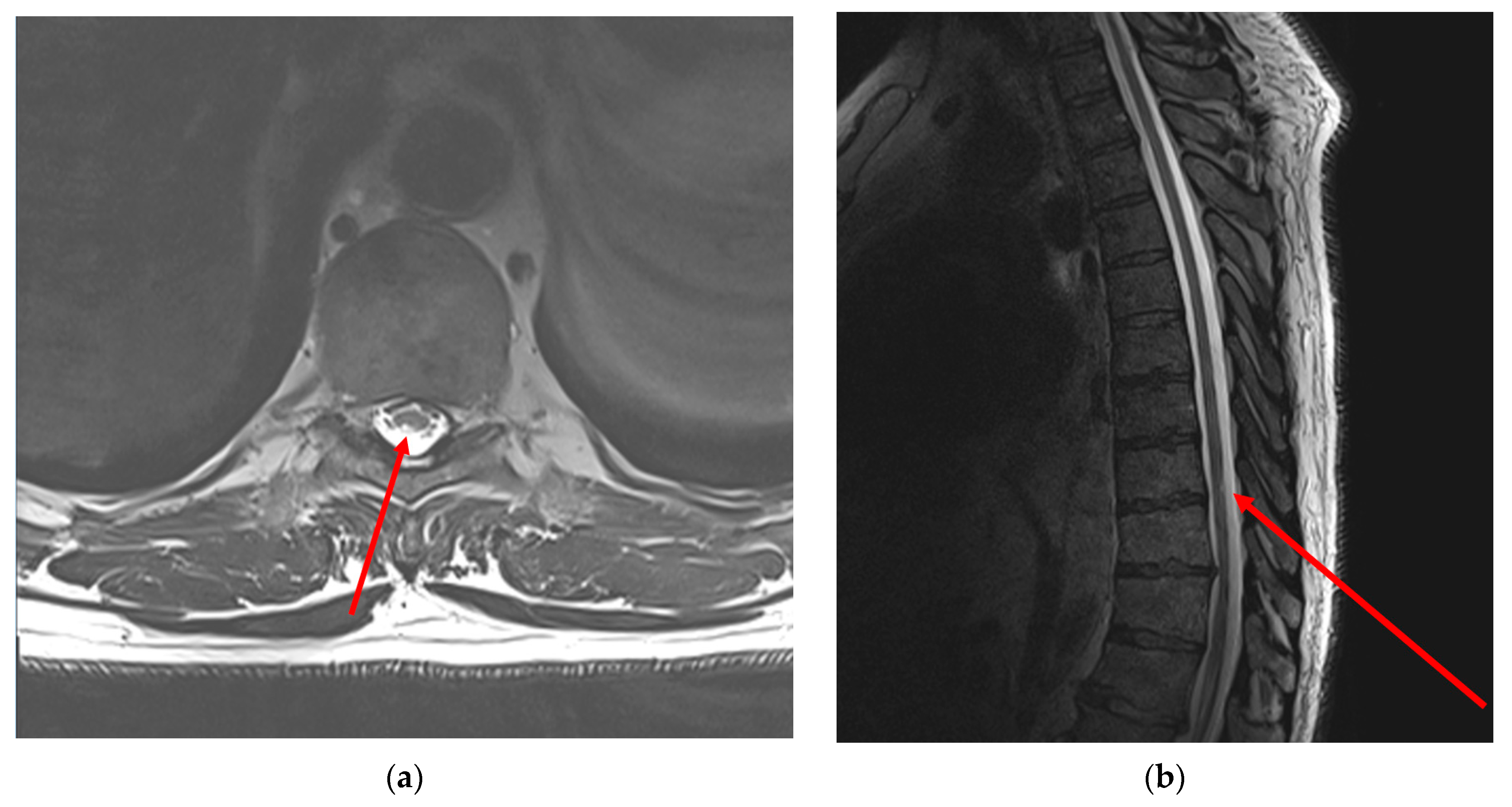
2.4.3. Neuroimaging
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, H.; Joa, K.L.; Kim, H.S.; Kim, C.H.; Jung, H.Y.; Kim, M.O. Concomitant acute transverse myelitis and sensory motor axonal polyneuropathy in two children: Two case reports. Ann. Rehabil. Med. 2015, 39, 142–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Román, G.C.; Gracia, F.; Torres, A.; Palacios, A.; Gracia, K.; Harris, D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients with COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events with the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front. Immunol. 2021, 26, 653786. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.G.; Emmady, P.D. Transverse Myelitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Farrokhi, M.R.; Iravanpour, F.; Nejabat, N. Development of Acute Transverse Myelitis following COVID-19 Infection: A Review on the Potential Pathways. Eur. Neurol. 2023, 86, 209–216. [Google Scholar] [CrossRef]
- Gulati, N.; Kapila, S.; Bhalla Sehgal, L.; Sehgal, V.; Lnu, P. Myelitis Following COVID-19 Illness. Cureus 2022, 18, e28134. [Google Scholar] [CrossRef] [PubMed]
- Prete, S.; McShannic, J.D.; Fertel, B.S.; Simon, E.L. Acute transverse myelitis progressing to permanent quadriplegia following COVID-19 infection. Am. J. Emerg. Med. 2022, 56, 391.e1–391.e3. [Google Scholar] [CrossRef]
- Alrubaye, R.; Bondugula, V.; Baleguli, V.; Chofor, R. A possible Guillain-Barré syndrome/transverse myelitis overlap syndrome after recent COVID-19. BMJ Case Rep. 2022, 9, e246967. [Google Scholar] [CrossRef] [PubMed]
- Canavero, I.; Ravaglia, S.; Valentino, F.; Micieli, G. Guillain Barrè syndrome and myelitis associated with SARS-CoV-2 infection. Neurosci. Lett. 2021, 10, 136040. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jain, E.; Sharma, V.; Sinha, A.; Khaliq, W. Guillain-Barré Syndrome Presenting as Painful Weakness and Edema of the Legs: A Case Report. Cureus 2023, 19, e40641. [Google Scholar] [CrossRef]
- Pidcock, F.S.; Krishnan, C.; Crawford, T.O.; Salorio, C.F.; Trovato, M.; Kerr, D.A. Acute transverse myelitis in childhood: Center-based analysis of 47 cases. Neurology 2007, 1, 1474–1480. [Google Scholar] [CrossRef]
- Beh, S.C.; Greenberg, B.M.; Frohman, T.; Frohman, E.M. Transverse myelitis. Neurol. Clin. 2013, 31, 79–138. [Google Scholar] [CrossRef]
- Martinez Villegas, H.X.; Hallo, A.; Cruz-Loor, S.; Jacome-Calderon, K. Spinal cord stimulator for neuropathic pain in a patient with severe disability due to transverse myelitis. BMJ Case Rep. 2021, 19, e242522. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Prasad, R.; Rejai, S.; Halter, K.; Chen, J. Relief of Neuropathic Pain After Spinal Cord Stimulator Implantation in a Patient With Idiopathic Thoracic Transverse Myelitis: A Case Report. A&A Pract. 2019, 13, 409–412. [Google Scholar] [CrossRef]
- Voitenkov, V.B.; Klimkin, A.V.; Skripchenko, N.V.; Pulman, N.F.; Ivanova, M.V. Diagnostic transcranial magnetic stimulation as a prognostic tool in children with acute transverse myelitis. Spinal Cord. 2016, 54, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Shrestha, R.L. Effect of Electroacupuncture Rehabilitation in Transverse Myelitis: A Case Report. J. Acupunct. Meridian Stud. 2017, 10, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Somoza Argibay, I.; Casal-Beloy, I.; Seoane Rodríguez, S. Transcutaneous electrical nerve stimulation (TENS) in neuropathic bladder due to transverse myelitis. Neurologia 2021, 36, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I.; Paley, C.A.; Jones, G.; Mulvey, M.R.; Wittkopf, P.G. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: A systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open 2022, 10, e051073. [Google Scholar] [CrossRef]
- Alarcón, J.B.; Chuhuaicura, P.B.; Sluka, K.A.; Vance, C.G.T.; Fazan, V.P.S.; Godoy, K.A.; Fuentes, R.E.; Dias, F.J. Transcutaneous Electrical Nerve Stimulation in Nerve Regeneration: A Systematic Review of In Vivo Animal Model Studies. Neuromodulation 2022, 25, 1248–1258. [Google Scholar] [CrossRef]
- Al-Zamil, M.; Minenko, I.A.; Kulikova, N.G.; Alade, M.; Petrova, M.M.; Pronina, E.A.; Romanova, I.V.; Narodova, E.A.; Nasyrova, R.F.; Shnayder, N.A. Clinical Experience of High Frequency and Low Frequency TENS in Treatment of Diabetic Neuropathic Pain in Russia. Healthcare 2022, 10, 250. [Google Scholar] [CrossRef]
- Al-Zamil, M.; Minenko, I.A.; Kulikova, N.G.; Mansur, N.; Nuvakhova, M.B.; Khripunova, O.V.; Shurygina, I.P.; Topolyanskaya, S.V.; Trefilova, V.V.; Petrova, M.M.; et al. Efficiency of Direct Transcutaneous Electroneurostimulation of the Median Nerve in the Regression of Residual Neurological Symptoms after Carpal Tunnel Decompression Surgery. Biomedicines 2023, 11, 2396. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Z.-P.; Zhang, N.-N.; Ni, J.; Wang, Z.-Y. Investigation into the Effectiveness of Combining Transcranial Direct Current Stimulation and Transcutaneous Electrical Nerve Stimulation as Treatment Options for Poststroke Shoulder Pain by Utilizing Functional Near-Infrared Spectroscopy. Ther. Clin. Risk Manag. 2023, 19, 875–887. [Google Scholar] [CrossRef]
- Plotas, P.; Papadopoulos, A.; Tsiamaki, E.; Apostolou, M.-D.; Chaniotaki, M.-A.; Ganiatsou, E.; Goutzeri, E.-M.; Kalogeraki, T.; Karra, E.; Malliou, M.; et al. Effects of Transcutaneous Electrical Nervous Stimulation (TENS) on Dysphonic Patients: A Systematic Review Study. Medicina 2023, 59, 1737. [Google Scholar] [CrossRef]
- Do Carmo Almeida, T.C.; Dos Santos Figueiredo, F.W.; Barbosa Filho, V.C.; de Abreu, L.C.; Fonseca, F.L.A.; Adami, F. Effects of Transcutaneous Electrical Nerve Stimulation on Proinflammatory Cytokines: Systematic Review and Meta-Analysis. Mediators Inflamm. 2018, 2, 1094352. [Google Scholar] [CrossRef]
- Gürgen, S.G.; Sayın, O.; Cetin, F.; Tuç Yücel, A. Transcutaneous electrical nerve stimulation (TENS) accelerates cutaneous wound healing and inhibits pro-inflammatory cytokines. Inflammation 2014, 37, 775–784. [Google Scholar] [CrossRef]
- Su, H.L.; Chiang, C.Y.; Lu, Z.H.; Cheng, F.C.; Chen, C.J.; Sheu, M.L.; Sheehan, J.; Pan, H.C. Late administration of high-frequency electrical stimulation increases nerve regeneration without aggravating neuropathic pain in a nerve crush injury. BMC Neurosci. 2018, 25, 37. [Google Scholar] [CrossRef]
- Chu, X.L.; Song, X.Z.; Lim, Y.R.; Wu, Z.R.; Li, Q.; Li, Q.W.; Gu, X.S.; Ming, D. An ultrasound-guided percutaneous electrical nerve stimulation regimen devised using finite element modeling promotes functional recovery after median nerve transection. Neural Regen. Res. 2023, 18, 683–688. [Google Scholar] [CrossRef]
- Mills, P.B.; Dossa, F. Transcutaneous Electrical Nerve Stimulation for Management of Limb Spasticity: A Systematic Review. Am. J. Phys. Med. Rehabil. 2016, 95, 309–318. [Google Scholar] [CrossRef]
- Logosu, D.; Tagoe, T.A.; Adjei, P. Transcutaneous electrical nerve stimulation in the management of calf muscle spasticity in cerebral palsy: A pilot study. IBRO Neurosci. Rep. 2021, 2, 194–199. [Google Scholar] [CrossRef] [PubMed]
- In, T.S.; Jung, J.H.; Jung, K.S.; Cho, H.Y. Effectiveness of Transcutaneous Electrical Nerve Stimulation with Taping for Stroke Rehabilitation. Biomed. Res. Int. 2021, 25, 9912094, Erratum in Biomed. Res. Int. 2022, 2022, 9763093. [Google Scholar] [CrossRef] [PubMed]
- Kwong, P.W.H.; Ng, G.Y.F.; Chung, R.C.K.; Ng, S.S.M. Bilateral Transcutaneous Electrical Nerve Stimulation Improves Lower-Limb Motor Function in Subjects With Chronic Stroke: A Randomized Controlled Trial. J. Am. Heart Assoc. 2018, 8, e007341. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lu, J.; Wang, M.; Zhang, P.; Fang, H.; Yang, K.; Wang, L.; Zhuang, J.; Tian, Z.; Yang, J.; et al. Ultrasound-Guided Median Nerve Electrical Stimulation to Promote Upper Limb Function Recovery after Stroke. Evid. Based Complement. Alternat. Med. 2022, 2022, 3590057. [Google Scholar] [CrossRef]
- Eginyan, G.; Zhou, X.; Williams, A.M.M.; Lam, T. Effects of motor stimulation of the tibial nerve on corticospinal excitability of abductor hallucis and pelvic floor muscles. Front. Rehabil. Sci. 2023, 16, 1089223. [Google Scholar] [CrossRef]
- Lai, M.I.; Pan, L.L.; Tsai, M.W.; Shih, Y.F.; Wei, S.H.; Chou, L.W. Investigating the Effects of Peripheral Electrical Stimulation on Corticomuscular Functional Connectivity Stroke Survivors. Top. Stroke Rehabil. 2016, 23, 154–162. [Google Scholar] [CrossRef]
- Philippova, E.S.; Bazhenov, I.V.; Ziryanov, A.V.; Moskvina, E.Y. Evaluation of Lower Urinary Tract Dysfunction Impact on Quality of Life in Multiple Sclerosis Patients: Russian Translation and Validation of SF-Qualiveen. Mult. Scler. Int. 2020, 25, 4652439. [Google Scholar] [CrossRef]
- van Doorn, T.; Groenendijk, I.M.; Scheepe, J.R.; Blok, B.F.M. Neurogenic bowel dysfunction score in spinal cord-injured patients: Translation and validation of the Dutch-language NBD score. Spinal Cord. 2022, 60, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M. General characteristics of electroneuromyography values of median, ulnar, peroneal and tibial nerves in healthy population. Bull. Med. Dent. Inst. 2013, 2, 39–52. [Google Scholar]
- Kućma, K. Principles of electric stimulation in flaccid paralysis. Neurol. Neurochir Pol. 1984, 18, 319–325. [Google Scholar]
- Jung, K.S.; In, T.S.; Cho, H.Y. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke: A randomized controlled study. Gait Posture 2017, 54, 183–187. [Google Scholar] [CrossRef]
- Krarup, C.; Boeckstyns, M.; Ibsen, A.; Moldovan, M.; Archibald, S. Remodeling of motor units after nerve regeneration studied by quantitative electromyography. Clin. Neurophysiol. 2016, 127, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.J.; Woo, D.H.; Lee, P.R.; Pajevic, S.; Bukalo, O.; Huffman, W.C.; Wake, H.; Basser, P.J.; SheikhBahaei, S.; Lazarevic, V.; et al. Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl. Acad. Sci. USA 2018, 13, 11832–11837, Erratum in Proc. Natl. Acad. Sci. USA 2019, 116, 12574. [Google Scholar] [CrossRef]
- Chevret, S.; Hughes, R.A.; Annane, D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst. Rev. 2017, 27, CD001798. [Google Scholar] [CrossRef] [PubMed]
- Osterman, P.O.; Fagius, J.; Lundemo, G.; Pihlstedt, P.; Pirskanen, R.; Sidén, A.; Säfwenberg, J. Beneficial effects of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet 1984, 8, 1296–1299. [Google Scholar] [CrossRef]
- McCarthy, J.A.; Zigenfus, R.W. Transcutaneous electrical nerve stimulation: An adjunct in the pain management of Guillain-Barré syndrome. Phys. Ther. 1978, 58, 23–24. [Google Scholar] [CrossRef]
- Harbo, T.; Markvardsen, L.K.; Hellfritzsch, M.B.; Severinsen, K.; Nielsen, J.F.; Andersen, H. Neuromuscular electrical stimulation in early rehabilitation of Guillain-Barré syndrome: A pilot study. Muscle Nerve 2019, 59, 481–484. [Google Scholar] [CrossRef]
- Jain, N.; Cortez-Garcia, E.; Cartwright, M.S. Cross-sectional area reference values of the median nerve at the palm using ultrasound. Muscle Nerve 2020, 62, 389–392. [Google Scholar] [CrossRef]
- Zeb, A.; Arsh, A.; Bahadur, S.; Ilyas, S.M. Effectiveness of transcutaneous electrical nerve stimulation in management of neuropathic pain in patients with post traumatic incomplete spinal cord injuries. Pak. J. Med. Sci. 2018, 34, 1177–1180. [Google Scholar] [CrossRef]
- Stampas, A.; Gustafson, K.; Korupolu, R.; Smith, C.; Zhu, L.; Li, S. Bladder Neuromodulation in Acute Spinal Cord Injury via Transcutaneous Tibial Nerve Stimulation: Cystometrogram and Autonomic Nervous System Evidence From a Randomized Control Pilot Trial. Front. Neurosci. 2019, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Birkhäuser, V.; Liechti, M.D.; Anderson, C.E.; Bachmann, L.M.; Baumann, S.; Baumberger, M.; Birder, L.A.; Botter, S.M.; Büeler, S.; Cruz, C.D.; et al. TASCI-transcutaneous tibial nerve stimulation in patients with acute spinal cord injury to prevent neurogenic detrusor overactivity: Protocol for a nationwide, randomised, sham-controlled, double-blind clinical trial. BMJ Open 2020, 13, e039164. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, R.M.; Berryman, C.F.; Lauto, A.; Leonard, A.V. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front. Cell. Neurosci. 2023, 3, 1095259. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Tillakaratne, N.J.; Bigbee, A.J.; de Leon, R.D.; Roy, R.R. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004, 27, 145–167. [Google Scholar] [CrossRef] [PubMed]

| Date | Anamnestic Data before TENS Treatment |
|---|---|
| 21 May 2022 | Contact with people with confirmed coronavirus infection (COVID-19). |
| 28 May 2022 | Fever up to 38.5 degrees Celsius with runny nose and cough. |
| 1 June 2022 | Positive PCR test for COVID-19. |
| 8 June 2022 | No substantive changes in lungs were detected in chest CT scan. |
| 10 June 2022 | Burning sensation on the inner surface of the right thigh. |
| 13 June 2022 | Spread of burning sensation to right foot. |
| 14 June 2022 | Numbness and hypoesthesia in the right leg. Fast progression of weakness in the right leg. |
| 15 June 2022 | Numbness and hypoesthesia in the left leg. Fast progression of mild weakness in the left leg. |
| 17 June 2022 | Hospitalization in the neurological department with a diagnosis of post-COVID-19 Guillain–Barré and acute transverse myelitis overlap syndrome. |
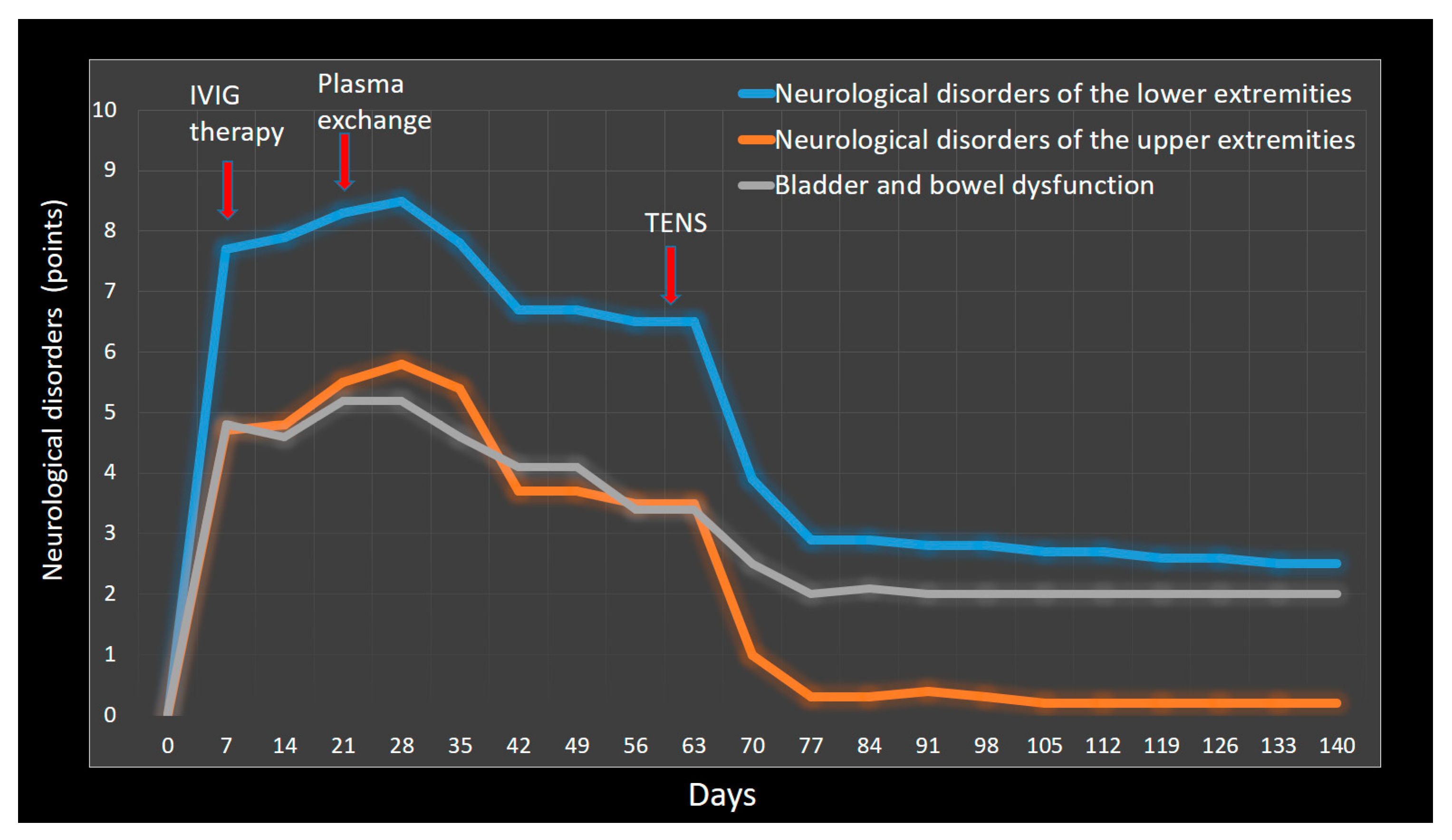
| 17 June 2022 | Neurological disorders: incomplete bladder evacuation, indwelling bladder catheterization, loss of the sensation of rectal fullness and ability to evacuate bowels. Administering of cleansing enema. SF-Qualiveen score 3/4, Neurogenic Bowel Dysfunction Score 10/47. Lower extremities: motor strength 2/5 points, hypoesthesia 9/10 points, neuropathic pain 8/10 points. Upper extremities: motor strength 5/5 points, hypoesthesia 4/5 points, neuropathic pain 6/10 points. Patient could not stand up unaided. |
| 17 June 2022 | Treatment was started with intravenous immunoglobulin 0.4 g/kg daily for 5 days. Immunoglobulin therapy was commenced without benefit and progressive clinical deterioration. Then, 10 days after completion of immunoglobulin treatment, plasma exchange 200 mL/kg was started for 5 sessions within 10 days. |
| 10 July 2023 | Incomplete course of electromagnetic field therapy (5 sessions). Physiotherapy was excluded due to increased pain, burning and tingling in the legs during procedures. |
| 31 July 2022 | Control over voluntary urination was not complete. Intermittent catheterization was performed every 8 h. Bowel care time increased to 1–1:20 h. Mini-enema was used on average once a week. SF-Qualiveen score 2.6/4.0, Neurogenic Bowel Dysfunction Score 8/47. Lower extremities: motor strength 3/5 points, hypoesthesia 9/10 points, neuropathic pain 7/10 points. Upper extremities: motor strength 5/5 points, hypoesthesia 3/5 points, neuropathic pain 5/10 points. Patient could stand up unaided and could walk with aid but not run. |
| 1 August 2022 | Hospital discharge. |
| 1 August 2022 | Home medication. |
| 17 August 2022 | Outpatient transcutaneous electrical neurostimulation therapy. |
| Before Treatment | After Treatment | Lower Limit of Normal [36] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nerve | CMAP | CV | TL | CMAP | CV | TL | CMAP | CV | TL | |
| Median nerve | Left | 10.2 | 43.0 | 5.8 | 7.65 | 47.2 | 4.7 | >5 mV | >49 m/s | <4.6 ms |
| Right | 7.76 | 43.4 | 5.7 | 6.34 | 46.3 | 4.8 | >5 mV | >49 m/s | <4.6 ms | |
| Ulnar nerve | Left | 9.90 | 44.3 | 5.02 | 9.10 | 49.6 | 4.6 | >5 mV | >55 m/s | <4.6 ms |
| Right | 6.40 | 40.1 | 5.20 | 6.20 | 45.2 | 4.9 | >5 mV | >55 m/s | <4.6 ms | |
| Common peroneal nerve | Left | 0.02 | 36.8 | 10.9 | 0.05 | 38.0 | 8.8 | >3 mV | >40 m/s | <4.9 ms |
| Right | 0.08 | 36.1 | 6.3 | 0.54 | 37.5 | 4.8 | >3 mV | >40 m/s | <4.9 ms | |
| Tibial nerve | Left | 0.26 | 32.3 | 4.8 | 1.6 | 38.1 | 4.6 | >3.5 mV | >40 m/s | <4.9 ms |
| Right | 0.21 | 34.3 | 6.5 | 1.3 | 41.6 | 5.8 | >3.5 mV | >40 m/s | <4.9 ms | |
| Before Treatment | After Treatment | Lower Limit of Normal [36] | |||||
|---|---|---|---|---|---|---|---|
| Nerve | SNAP | CV | SNAP | CV | SNAP | CV | |
| Median nerve | Left | 0.68 | 37.6 | 0.77 | 35.0 | >5 mcV | >50 m/s |
| Right | 0.52 | 37.2 | 0.66 | 38.9 | >5 mcV | >50 m/s | |
| Ulnar nerve | Left | 0.19 | 50.4 | 0.45 | 52.7 | >5 mcV | >50 m/s |
| Right | 0.97 | 50.1 | 1.29 | 53.1 | >5 mcV | >50 m/s | |
| Superficial peroneal nerve | Left | No response | No response | >5 mcV | >50 m/s | ||
| Right | No response | No response | >5 mcV | >50 m/s | |||
| Medial plantar nerve | Left | No response | No response | >5 mcV | >50 m/s | ||
| Right | No response | No response | >5 mcV | >50 m/s | |||
| Sural nerve | Left | No response | No response | >5 mcV | >50 m/s | ||
| Right | No response | No response | >5 mcV | >50 m/s | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zamil, M.; Kulikova, N.G.; Minenko, I.A.; Mansur, N.; Zalozhnev, D.M.; Uzdenov, M.B.; Dzhanibekova, A.A.; Gochiyayev, A.A.; Shnayder, N.A. Functional Recovery and Regenerative Effects of Direct Transcutaneous Electrical Nerve Stimulation in Treatment of Post-COVID-19 Guillain–Barré and Acute Transverse Myelitis Overlap Syndrome: A Clinical Case. J. Funct. Morphol. Kinesiol. 2024, 9, 40. https://doi.org/10.3390/jfmk9010040
Al-Zamil M, Kulikova NG, Minenko IA, Mansur N, Zalozhnev DM, Uzdenov MB, Dzhanibekova AA, Gochiyayev AA, Shnayder NA. Functional Recovery and Regenerative Effects of Direct Transcutaneous Electrical Nerve Stimulation in Treatment of Post-COVID-19 Guillain–Barré and Acute Transverse Myelitis Overlap Syndrome: A Clinical Case. Journal of Functional Morphology and Kinesiology. 2024; 9(1):40. https://doi.org/10.3390/jfmk9010040
Chicago/Turabian StyleAl-Zamil, Mustafa, Natalia G. Kulikova, Inessa A. Minenko, Numman Mansur, Denis M. Zalozhnev, Marat B. Uzdenov, Alina A. Dzhanibekova, Alikhan A. Gochiyayev, and Natalia A. Shnayder. 2024. "Functional Recovery and Regenerative Effects of Direct Transcutaneous Electrical Nerve Stimulation in Treatment of Post-COVID-19 Guillain–Barré and Acute Transverse Myelitis Overlap Syndrome: A Clinical Case" Journal of Functional Morphology and Kinesiology 9, no. 1: 40. https://doi.org/10.3390/jfmk9010040
APA StyleAl-Zamil, M., Kulikova, N. G., Minenko, I. A., Mansur, N., Zalozhnev, D. M., Uzdenov, M. B., Dzhanibekova, A. A., Gochiyayev, A. A., & Shnayder, N. A. (2024). Functional Recovery and Regenerative Effects of Direct Transcutaneous Electrical Nerve Stimulation in Treatment of Post-COVID-19 Guillain–Barré and Acute Transverse Myelitis Overlap Syndrome: A Clinical Case. Journal of Functional Morphology and Kinesiology, 9(1), 40. https://doi.org/10.3390/jfmk9010040








