The Usefulness of Synovial Fluid Proteome Analysis in Orthopaedics: Focus on Osteoarthritis and Periprosthetic Joint Infections
Abstract
1. Introduction
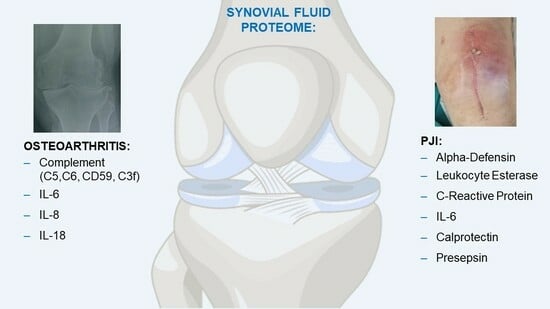
2. Synovial Fluid Proteome in Osteoarthritis
Synovial Fluid Proteome and Periprosthetic Joint Infections
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balakrishnan, L.; Nirujogi, R.; Ahmad, S.; Bhattacharjee, M.; Manda, S.S.; Renuse, S.; Kelkar, D.S.; Subbannayya, Y.; Raju, R.; Goel, R.; et al. Proteomic Analysis of Human Osteoarthritis Synovial Fluid. Clin. Proteom. 2014, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, L.; Bhattacharjee, M.; Ahmad, S.; Nirujogi, R.; Renuse, S.; Subbannayya, Y.; Marimuthu, A.; Srikanth, S.M.; Raju, R.; Dhillon, M.; et al. Differential Proteomic Analysis of Synovial Fluid from Rheumatoid Arthritis and Osteoarthritis Patients. Clin. Proteom. 2014, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Bizzoca, D.; Rocchetti, M.T.; Scacco, S.; Taurino, F.; Vicenti, G.; Spadaccino, F.; Moretti, L.; Ranieri, E.; Gesualdo, L.; Moretti, F.; et al. Beyond Pre-Analytical and Analytical Concerns in the Study of Synovial Fluid Proteome: Description of an Optimized Gel-Based Protocol. J. Biol. Regul. Homeost. Agents 2021, 35, 827–832. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Nappi, V.S.; Moretti, F.; Carrozzo, M.; Belviso, V.; Moretti, B. Biophysical Stimulation of the Knee with PEMFs: From Bench to Bedside. J. Biol. Regul. Homeost. Agents 2018, 32, 23–28. [Google Scholar] [PubMed]
- Hui, A.Y.; McCarty, W.J.; Masuda, K.; Firestein, G.S.; Sah, R.L. A Systems Biology Approach to Synovial Joint Lubrication in Health, Injury, and Disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 15–37. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and Synovial Joint: Function, Distribution and Healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M.; et al. Identification of a Central Role for Complement in Osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef]
- Peng, B.; Li, H.; Peng, X.-X. Functional Metabolomics: From Biomarker Discovery to Metabolome Reprogramming. Protein Cell 2015, 6, 628–637. [Google Scholar] [CrossRef]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.K.; Spector, T.D. Interleukin-6 Is a Significant Predictor of Radiographic Knee Osteoarthritis: The Chingford Study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef]
- Zheng, K.; Shen, N.; Chen, H.; Ni, S.; Zhang, T.; Hu, M.; Wang, J.; Sun, L.; Yang, X. Global and Targeted Metabolomics of Synovial Fluid Discovers Special Osteoarthritis Metabolites. J. Orthop. Res. 2017, 35, 1973–1981. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Carrozzo, M.; Solarino, G.; Moretti, B. Multi-Omics Analysis of Synovial Fluid: A Promising Approach in the Study of Osteoarthritis. J. Biol. Regul. Homeost. Agents 2018, 32, 9–13. [Google Scholar]
- Mobasheri, A.; Bay-Jensen, A.-C.; van Spil, W.E.; Larkin, J.; Levesque, M.C. Osteoarthritis Year in Review 2016: Biomarkers (Biochemical Markers). Osteoarthr. Cartil. 2017, 25, 199–208. [Google Scholar] [CrossRef]
- Hunter, D.J.; Nevitt, M.; Losina, E.; Kraus, V. Biomarkers for Osteoarthritis: Current Position and Steps towards Further Validation. Best Pract. Res. Clin. Rheumatol. 2014, 28, 61–71. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Bizzoca, D.; Vicenti, G.; Solarino, G.; Moretti, F.; Gnoni, A.; Maccagnano, G.; Noia, G.; Moretti, B. Gut Microbiota and Osteoarthritis: A Deep Insight into a New Vision of the Disease. J. Biol. Regul. Homeost. Agents 2020, 34, 51–55. [Google Scholar]
- Bizzoca, D.; Solarino, G.; Vicenti, G.; Moretti, L.; Nappi, V.S.; Belluati, A.; Moretti, B. Novel Directions in the Study of Osteoporosis: Focus on Gut Microbiota as a Potential Therapeutic Target. J. Biol. Regul. Homeost. Agents 2020, 34, 29–35. [Google Scholar]
- Vicenti, G.; Bortone, I.; Bizzoca, D.; Sardone, R.; Belluati, A.; Solarino, G.; Moretti, B. Bridging the Gap between Serum Biomarkers and Biomechanical Tests in Musculoskeletal Ageing. J. Biol. Regul. Homeost. Agents 2020, 34, 263–274. [Google Scholar]
- Blanco, F.J.; Ruiz-Romero, C. Metabolomic Characterization of Metabolic Phenotypes in OA. Nat. Rev. Rheumatol. 2012, 8, 130–132. [Google Scholar] [CrossRef]
- Moretti, L.; Bizzoca, D.; Giancaspro, G.A.; Cassano, G.D.; Moretti, F.; Setti, S.; Moretti, B. Biophysical Stimulation in Athletes’ Joint Degeneration: A Narrative Review. Medicina 2021, 57, 1206. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Solarino, G.; Moretti, F.; Ottaviani, G.; Simone, F.; Zavattini, G.; Maccagnano, G.; Noia, G.; Moretti, B. The Role of Biophysical Stimulation with Pemfs in Fracture Healing: From Bench to Bedside. J. Biol. Regul. Homeost. Agents 2020, 34, 131–135. [Google Scholar]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The Global Burden of Hip and Knee Osteoarthritis: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Hepinstall, M.S.; Rutledge, J.R.; Bornstein, L.J.; Mazumdar, M.; Westrich, G.H. Factors That Impact Expectations before Total Knee Arthroplasty. J. Arthroplast. 2011, 26, 870–876. [Google Scholar] [CrossRef]
- Mahendran, S.M.; Oikonomopoulou, K.; Diamandis, E.P.; Chandran, V. Synovial Fluid Proteomics in the Pursuit of Arthritis Mediators: An Evolving Field of Novel Biomarker Discovery. Crit. Rev. Clin. Lab. Sci. 2017, 54, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Corigliano, A.; Preianò, M.; Terracciano, R.; Savino, R.; De Gori, M.; Galasso, O.; Gasparini, G. C3f Is a Potential Tool for the Staging of Osteoarthritis. J. Biol. Regul. Homeost. Agents 2017, 31, 29–35. [Google Scholar] [PubMed]
- Hillmen, P.; Young, N.S.; Schubert, J.; Brodsky, R.A.; Socié, G.; Muus, P.; Röth, A.; Szer, J.; Elebute, M.O.; Nakamura, R.; et al. The Complement Inhibitor Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2006, 355, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, R.A.; Young, N.S.; Antonioli, E.; Risitano, A.M.; Schrezenmeier, H.; Schubert, J.; Gaya, A.; Coyle, L.; de Castro, C.; Fu, C.-L.; et al. Multicenter Phase 3 Study of the Complement Inhibitor Eculizumab for the Treatment of Patients with Paroxysmal Nocturnal Hemoglobinuria. Blood 2008, 111, 1840–1847. [Google Scholar] [CrossRef]
- Wanner, J.P.; Subbaiah, R.; Skomorovska-Prokvolit, Y.; Shishani, Y.; Boilard, E.; Mohan, S.; Gillespie, R.; Miyagi, M.; Gobezie, R. Proteomic Profiling and Functional Characterization of Early and Late Shoulder Osteoarthritis. Arthritis Res. Ther. 2013, 15, R180. [Google Scholar] [CrossRef]
- Sánchez Romero, E.A.; Fernández-Carnero, J.; Calvo-Lobo, C.; Ochoa Sáez, V.; Burgos Caballero, V.; Pecos-Martín, D. Is a Combination of Exercise and Dry Needling Effective for Knee OA? Pain Med. 2020, 21, 349–363. [Google Scholar] [CrossRef]
- Sánchez-Romero, E.A.; González-Zamorano, Y.; Arribas-Romano, A.; Martínez-Pozas, O.; Fernández Espinar, E.; Pedersini, P.; Villafañe, J.H.; Alonso Pérez, J.L.; Fernández-Carnero, J. Efficacy of Manual Therapy on Facilitatory Nociception and Endogenous Pain Modulation in Older Adults with Knee Osteoarthritis: A Case Series. Appl. Sci. 2021, 11, 1895. [Google Scholar] [CrossRef]
- Sinatti, P.; Sánchez Romero, E.A.; Martínez-Pozas, O.; Villafañe, J.H. Effects of Patient Education on Pain and Function and Its Impact on Conservative Treatment in Elderly Patients with Pain Related to Hip and Knee Osteoarthritis: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 6194. [Google Scholar] [CrossRef]
- Turcotte, J.J.; Kelly, M.E.; Aja, J.M.; King, P.J.; MacDonald, J.H. Risk factors for progression to total knee arthroplasty within two years of presentation for knee osteoarthritis. J. Clin. Orthop. Trauma 2021, 16, 257–263. [Google Scholar] [CrossRef]
- Tsuchida, A.I.; Beekhuizen, M.; Rutgers, M.; van Osch, G.J.; Bekkers, J.E.; Bot, A.G.; Geurts, B.; Dhert, W.J.; Saris, D.B.; Creemers, L.B. Interleukin-6 Is Elevated in Synovial Fluid of Patients with Focal Cartilage Defects and Stimulates Cartilage Matrix Production in an in Vitro Regeneration Model. Arthritis Res. Ther. 2012, 14, R262. [Google Scholar] [CrossRef]
- Kokebie, R.; Aggarwal, R.; Lidder, S.; Hakimiyan, A.A.; Rueger, D.C.; Block, J.A.; Chubinskaya, S. The Role of Synovial Fluid Markers of Catabolism and Anabolism in Osteoarthritis, Rheumatoid Arthritis and Asymptomatic Organ Donors. Arthritis Res. Ther. 2011, 13, R50. [Google Scholar] [CrossRef]
- Kaneko, S.; Satoh, T.; Chiba, J.; Ju, C.; Inoue, K.; Kagawa, J. Interleukin-6 and Interleukin-8 Levels in Serum and Synovial Fluid of Patients with Osteoarthritis. Cytokines Cell. Mol. Ther. 2000, 6, 71–79. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D.; Long, L.; Deng, X.; Tao, R.; Huang, G. Correlation between Plasma, Synovial Fluid and Articular Cartilage Interleukin-18 with Radiographic Severity in 33 Patients with Osteoarthritis of the Knee. Clin. Exp. Med. 2014, 14, 297–304. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Nappi, V.; Pesce, V.; Solarino, G.; Carrozzo, M.; Moretti, F.; Dicuonzo, F.; Moretti, B. Serum Biomarkers in the Diagnosis of Periprosthetic Joint Infection: Consolidated Evidence and Recent Developments. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 43–50. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Cotugno, D.; Carrozzo, M.; Riefoli, F.; Rifino, F.; Belviso, V.; Elia, R.; Solarino, G.; Moretti, B. The Use of a Gentamicin-Coated Titanium Nail, Combined with RIA System, in the Management of Non-Unions of Open Tibial Fractures: A Single Centre Prospective Study. Injury 2020, 51, S86–S91. [Google Scholar] [CrossRef]
- Demitri, S.; Vicenti, G.; Carrozzo, M.; Bizzoca, D.; De Franceschi, D.; Moretti, B. The Masquelet Technique in the Treatment of a Non-Infected Open Complex Fracture of the Distal Tibia with Severe Bone and Soft Tissue Loss: A Case Report. Injury 2018, 49, S58–S62. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Spinarelli, A.; Bizzoca, D.; Moretti, L.; Vicenti, G.; Garofalo, R.; Moretti, B. The Autoclaving and Re-Implantation of an Infected Prosthesis as a Spacer during Resection Knee Arthroplasty: A Systematic Review. Musculoskelet. Surg. 2022, 106, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins. Natural Peptide Antibiotics of Human Neutrophils. J. Clin. Investig. 1985, 76, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Chisari, E.; Yacovelli, S.; Goswami, K.; Shohat, N.; Woloszyn, P.; Parvizi, J. Leukocyte Esterase Versus ICM 2018 Criteria in the Diagnosis of Periprosthetic Joint Infection. J. Arthroplast. 2021, 36, 2942–2945.e1. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Hirschmann, M.T.; Becker, R.; Shaker, A.; Ateschrang, A.; Keel, M.J.B.; Albers, C.E.; Buetikofer, L.; Maqungo, S.; Stöckle, U.; et al. A Meta-Analysis of Synovial Biomarkers in Periprosthetic Joint Infection: SynovasureTM Is Less Effective than the ELISA-Based Alpha-Defensin Test. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Kwapisz, L.; Gregor, J.; Chande, N.; Yan, B.; Ponich, T.; Mosli, M. The Utility of Fecal Calprotectin in Predicting the Need for Escalation of Therapy in Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2017, 52, 846–850. [Google Scholar] [CrossRef]
- Vicenti, G.; Pesce, V.; Bizzoca, D.; Nappl, V.; Palmiotto, F.; Carrozzo, M.; Moretti, B. Perioperative Plasmatic Presepsin Levels in Patients Undergoing Total Hip or Knee Replacement: A Preliminary Study. J. Biol. Regul. Homeost. Agents 2017, 31, 1081–1085. [Google Scholar]
- Vicenti, G.; Solarino, G.; Bizzoca, D.; Caringella, N.; Nappi, V.S.; Moretti, L.; Belluati, A.; Moretti, B. The Limits and Potentials of Presepsin in Orthopaedic Surgery: State of the Art and Future Directions. J. Biol. Regul. Homeost. Agents 2020, 34, 259–262. [Google Scholar]
- Marazzi, M.G.; Randelli, F.; Brioschi, M.; Drago, L.; Romanò, C.L.; Banfi, G.; Massaccesi, L.; Crapanzano, C.; Morelli, F.; Corsi Romanelli, M.M.; et al. Presepsin: A Potential Biomarker of PJI? A Comparative Analysis with Known and New Infection Biomarkers. Int. J. Immunopathol. Pharmacol. 2018, 31, 039463201774935. [Google Scholar] [CrossRef]
- Alonso Pérez, J.L.; Martín Pérez, S.; Turroni, S.; Marchese, L.; Villafañe, J.H. Relationship between the Gut Microbiome and Osteoarthritis Pain: Review of the Literature. Nutrients 2021, 13, 716. [Google Scholar] [CrossRef]
- Sánchez-Romero, E.; Battaglino, A.; Campanella, W.; Turroni, S.; Bishop, M.; Villafañe, J.H. Impact on Blood Tests of Lower Limb Joint Replacement for the Treatment of Osteoarthritis: Hip and Knee. In Topics in Geriatric Rehabilitation; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2021; Volume 37, pp. 227–229. [Google Scholar] [CrossRef]
- Yaegashi, Y.; Shirakawa, K.; Sato, N.; Suzuki, Y.; Kojika, M.; Imai, S.; Takahashi, G.; Miyata, M.; Furusako, S.; Endo, S. Evaluation of a Newly Identified Soluble CD14 Subtype as a Marker for Sepsis. J. Infect. Chemother. 2005, 11, 234–238. [Google Scholar] [CrossRef]
- Yu, B.Z.; Li, R.; Li, X.; Chai, W.; Zhou, Y.G.; Chen, J.Y. The Relationship of C-Reactive Protein/Interleukin-6 Concentrations between Serum and Synovial Fluid in the Diagnosis of Periprosthetic Joint Infection. J. Orthop. Surg. Res. 2021, 16, 733. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Ploegmakers, J.J.W.; Kampinga, G.A.; Wagenmakers-Huizenga, L.; Jutte, P.C.; Muller Kobold, A.C. Synovial Calprotectin: A Potential Biomarker to Exclude a Prosthetic Joint Infection. Bone Jt. J. 2017, 99-B, 660–665. [Google Scholar] [CrossRef]
- Imagama, T.; Tokushige, A.; Seki, K.; Seki, T.; Nakashima, D.; Ogasa, H.; Sakai, T.; Taguchi, T. Early Diagnosis of Septic Arthritis Using Synovial Fluid Presepsin: A Preliminary Study. J. Infect. Chemother. 2019, 25, 170–174. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzoca, D.; Moretti, L.; Gnoni, A.; Moretti, F.L.; Scacco, S.; Banfi, G.; Piazzolla, A.; Solarino, G.; Moretti, B. The Usefulness of Synovial Fluid Proteome Analysis in Orthopaedics: Focus on Osteoarthritis and Periprosthetic Joint Infections. J. Funct. Morphol. Kinesiol. 2022, 7, 97. https://doi.org/10.3390/jfmk7040097
Bizzoca D, Moretti L, Gnoni A, Moretti FL, Scacco S, Banfi G, Piazzolla A, Solarino G, Moretti B. The Usefulness of Synovial Fluid Proteome Analysis in Orthopaedics: Focus on Osteoarthritis and Periprosthetic Joint Infections. Journal of Functional Morphology and Kinesiology. 2022; 7(4):97. https://doi.org/10.3390/jfmk7040097
Chicago/Turabian StyleBizzoca, Davide, Lorenzo Moretti, Antonio Gnoni, Francesco Luca Moretti, Salvatore Scacco, Giuseppe Banfi, Andrea Piazzolla, Giuseppe Solarino, and Biagio Moretti. 2022. "The Usefulness of Synovial Fluid Proteome Analysis in Orthopaedics: Focus on Osteoarthritis and Periprosthetic Joint Infections" Journal of Functional Morphology and Kinesiology 7, no. 4: 97. https://doi.org/10.3390/jfmk7040097
APA StyleBizzoca, D., Moretti, L., Gnoni, A., Moretti, F. L., Scacco, S., Banfi, G., Piazzolla, A., Solarino, G., & Moretti, B. (2022). The Usefulness of Synovial Fluid Proteome Analysis in Orthopaedics: Focus on Osteoarthritis and Periprosthetic Joint Infections. Journal of Functional Morphology and Kinesiology, 7(4), 97. https://doi.org/10.3390/jfmk7040097