A Randomised Controlled Trial of YOGa and Strengthening Exercise for Knee OsteoArthritis: Protocol for a Comparative Effectiveness Trial (YOGA Trial)
Abstract
1. Introduction
2. Study Aims and Hypotheses
3. Method and Analysis
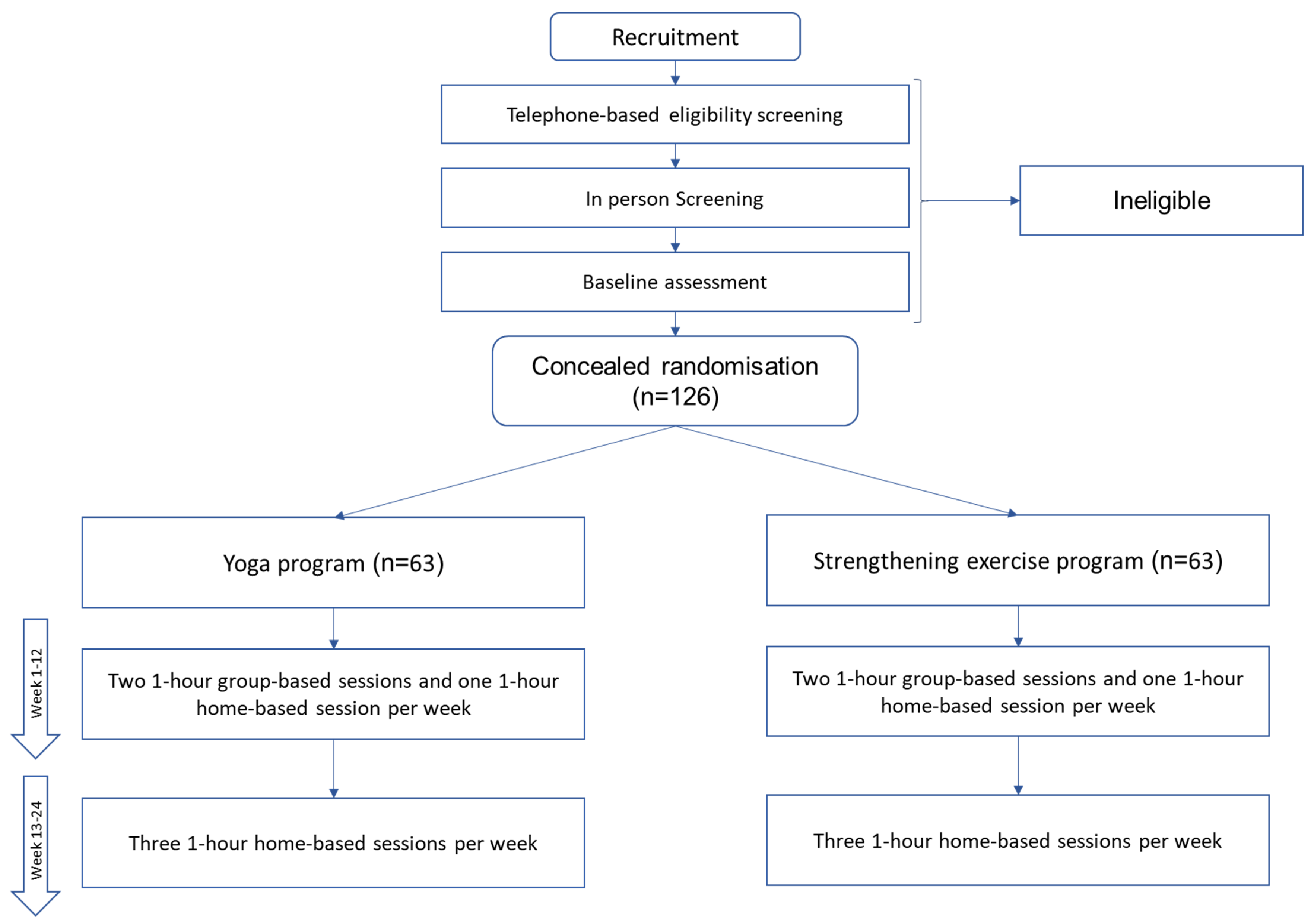
3.1. Trial Design
3.2. Study Participants
- Aged ≥40 years, both males and females;
- Knee pain on most days for at least six months;
- Average VAS knee pain intensity of ≥40 mm in the last month;
- Meet the American College of Rheumatology (ACR) clinical criteria for the diagnosis of knee OA;
- Be willing to participate in a group yoga program or group strengthening exercise program two times per week for the first 12 weeks and can attend on the days/times of the week that scheduled classes are running.
- Patients currently or in the past three months engaged in strengthening exercise or yoga programs for the treatment of any disease;
- Other forms of inflammatory arthritis (especially rheumatoid arthritis and gout);
- A significant knee injury that required treatment within the last six months;
- Arthroscopy or open surgery in the index knee in the last six months or planned in the next 6–8 months;
- Partial or total knee replacement;
- Injections of corticosteroids (last three months) or hyaluronic acid (last six months) in the index knee;
- Pregnancy or breastfeeding;
- Currently participating in any other drug/device/exercise clinical trial related to OA;
- Presence of any serious medical illness or condition that may preclude a 24-week follow up;
- Any condition that precludes safe participation in exercise (i.e., fails the safety for exercise clearance; see below for the procedure for this);
- Unable to walk without a gait aid;
- Inability to provide informed consent in English;
- Plan to start an exercise-based treatment program (e.g., GLA:D) or another new treatment for knee OA in the next six months;
- Planned absences (e.g., trips away) of >2 weeks maximum during the 12-week period.
3.3. Screening
3.4. Safety for Exercise Clearance
3.5. Randomisation and Blinding:
3.6. Treatments
3.7. Safety
3.8. Primary Outcome
3.9. Secondary Outcomes: The Overall Change from Baseline to Week 12 and Overall Change from Baseline to Week 24 Are Separate Outcomes
- Change in VAS knee pain over 12 weeks in patients with painDETECT > 12;
- Change in VAS knee pain over 24 weeks;
- Change in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) knee pain, WOMAC stiffness, and WOMAC knee function assessed using VAS over 12 weeks and over 24 weeks;
- Change in core physical function as assessed by 30 s chair stand test, 40 m fast walk test, and stair climb test over 12 and over 24 weeks.
- Change in biomarkers (urinary CTX-II, serum COMP, and serum hyaluronan) and systemic inflammatory markers (hs-CRP, IL-6, TNF-a) over 12 and over 24 weeks;
- Change in Patient Health Questionnaire (PHQ-9) scales over 12 and over 24 weeks.
- Change in patient global satisfaction score (assessed using a 100 mm VAS) over 12 and over 24 weeks [40]. Patients will be asked, “Considering all the ways in which illness and health conditions may affect you at this time, please indicate on the line below how you are doing ?”, along with a 0–100 VAS, where 0 is very well, and 100 is very poor;
- Change in neuropathic pain, as assessed by the painDETECT questionnaire, over 12 and over 24 weeks.
- Change in leg muscle strength will be assessed by leg muscle strength dynamometry at the lower limb (involving both legs simultaneously) over 12 and over 24 weeks.
- 2.
- Change in gait characteristics such as gait speed, step length, double support time, step width, and step time from baseline to week 12 and baseline to 24 weeks.
- 3.
- Change in physical activity will be assessed, and the participants will wear accelerometers for a week before the start of the intervention at 12 weeks and 24 weeks.
- 4.
- Self-reported adherence to the yoga or strengthening exercise program from baseline to 12 and baseline to 24 weeks will be assessed using an online logbook and defined as the percentage of prescribed sessions undertaken;
- 5.
- Change in body fat will be assessed using bioelectrical impedance analysis (BIA) (BIA analyser, Quantum II, RJL Systems, MI, USA) at baseline, week 12, and week 24. We will assess fat-free mass, percentage of fat-free mass, fat mass, and percentage of fat mass;
- 6.
- The OARSI-OMERACT responder criteria: This will be employed to generate a responder categorical variable (0 = non-responder, 1 = responder) based on improvement in WOMAC pain, function, and patient’s global assessment;
- 7.
- Pain medication use: There will be no constraints with regard to the use of analgesic medications. All participants will be allowed to continue taking the medications they are taking at their screening visit for the duration of the trial. Participants will be asked to keep medications as stable as possible, but if a participant requires an increase in analgesics, this will be permitted, and the reason for the dose increase and the dose used will be documented. Any medication changes will be documented with the reason, drug name, and dose. Medication change will be classified as commenced or increased, discontinued, or decreased, or stable use or non-use, and the change in total number of pain medications. A rescue medication, paracetamol, will be provided if the participant requests it. Medication use will be recorded at baseline and during each follow-up period.
4. Health Economics Outcomes (Secondary Outcomes):
- 8.
- Change in concomitant medications (assessed using self-reported medication history questionnaire) from baseline to 12 and baseline to 24 weeks and associated costs;
- 9.
- 10.
- Change in health resource utilisation (assessed using a self-reported questionnaire) at 12 weeks.
4.1. Data Integrity and Management
4.2. Sample Size Calculation
4.3. Statistical Analyses
4.4. Cost-Effectiveness Analysis
4.4.1. Measurement of Costs
4.4.2. Measurement of Benefit
4.4.3. Uncertainty and Sensitivity Analysis
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welfare AIoHa. Osteoarthritis Canberra: AIHW. 2019. Available online: https://www.aihw.gov.au/reports/chronic-musculoskeletal-conditions/osteoarthritis (accessed on 1 September 2021).
- Antony, B.; Singh, A. Imaging and Biochemical Markers for Osteoarthritis. Diagnostics 2021, 11, 1205. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.-L.; Persson, M.S.; Stocks, J.; Hou, Y.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Efficacy and potential determinants of exercise therapy in knee and hip osteoarthritis: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 356–365. [Google Scholar] [CrossRef]
- Wang, S.Y.; Olson-Kellogg, B.; Shamliyan, T.A.; Choi, J.Y.; Ramakrishnan, R.; Kane, R.L. Physical therapy interventions for knee pain secondary to osteoarthritis: A systematic review. Ann. Intern. Med. 2012, 157, 632–644. [Google Scholar] [CrossRef] [PubMed]
- DeRogatis, M.; Anis, H.K.; Sodhi, N.; Ehiorobo, J.O.; Chughtai, M.; Bhave, A.; Mont, M.A. Non-operative treatment options for knee osteoarthritis. Ann. Transl. Med. 2019, 7, S245. [Google Scholar] [CrossRef] [PubMed]
- Taibi, D.M.; Vitiello, M.V. A pilot study of gentle yoga for sleep disturbance in women with osteoarthritis. Sleep Med. 2011, 12, 512–517. [Google Scholar] [CrossRef]
- Cramer, H.; Ward, L.; Steel, A.; Lauche, R.; Dobos, G.; Zhang, Y. Prevalence, Patterns, and Predictors of Yoga Use: Results of a U.S. Nationally Representative Survey. Am. J. Prev. Med. 2016, 50, 230–235. [Google Scholar] [CrossRef]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl. Health Stat. Rep. 2015, 79, 1–16. [Google Scholar]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and Alternative Medicine Use Among Adults and Children: United States, 2007; National Health Statistics Reports; CDC: Atlanta, GA, USA, 2008; pp. 1–23. [Google Scholar] [CrossRef]
- Cheung, C.; Park, J.; Wyman, J.F. Effects of Yoga on Symptoms, Physical Function, and Psychosocial Outcomes in Adults with Osteoarthritis: A Focused Review. Am. J. Phys. Med. Rehabil. 2016, 95, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Juhl, C.; Christensen, R.; Roos, E.; Zhang, W.; Lund, H. Impact of Exercise Type and Dose on Pain and Disability in Knee Osteoarthritis: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Arthritis Rheumatol. 2014, 66, 622–636. [Google Scholar] [CrossRef]
- Patel, N.K.; Newstead, A.H.; Ferrer, R.L. The Effects of Yoga on Physical Functioning and Health Related Quality of Life in Older Adults: A Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 2012, 18, 902–917. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef]
- Bernstein, S. Yoga Benefits for Arthritis: Arthritis Foundation. 2020. Available online: https://www.arthritis.org/health-wellness/healthy-living/physical-activity/yoga/yoga-benefits-for-arthritis (accessed on 1 September 2021).
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Lauche, R.; Hunter, D.J.; Adams, J.; Cramer, H. Yoga for Osteoarthritis: A Systematic Review and Meta-analysis. Curr. Rheumatol. Rep. 2019, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Young, L.; Li, F. Network Meta-Analysis of Various Nonpharmacological Interventions on Pain Relief in Older Adults With Osteoarthritis. Am. J. Phys. Med. Rehabil. 2019, 98, 469–478. [Google Scholar] [CrossRef]
- Royal Australian College of General Practitioners. Guideline for the Management of Knee and Hip Osteoarthritis: The Royal Australian College of General Practitioners Ltd. 2018. Available online: https://www.racgp.org.au/download/Documents/Guidelines/Musculoskeletal/guideline-for-the-management-of-knee-and-hip-oa-2nd-edition.pdf (accessed on 1 September 2021).
- Park, J.; McCaffrey, R.; Dunn, D.; Goodman, R. Managing osteoarthritis: Comparisons of chair yoga, Reiki, and education (pilot study). Holist Nurs. Pract. 2011, 25, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Deveza, L.A.; Melo, L.; Yamato, T.P.; Mills, K.; Ravi, V.; Hunter, D.J. Knee osteoarthritis phenotypes and their relevance for outcomes: A systematic review. Osteoarthr. Cartil. 2017, 25, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Kittelson, A.J.; Stevens-Lapsley, J.E.; Schmiege, S.J. Determination of Pain Phenotypes in Knee Osteoarthritis: A Latent Class Analysis Using Data From the Osteoarthritis Initiative. Arthritis Care Res. 2016, 68, 612–620. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Steultjens, M. Classification of patients with knee osteoarthritis in clinical phenotypes: Data from the osteoarthritis initiative. PLoS ONE 2018, 13, e0191045. [Google Scholar] [CrossRef]
- Thakur, M.; Dickenson, A.H.; Baron, R. Osteoarthritis pain: Nociceptive or neuropathic? Nat. Rev. Rheumatol. 2014, 10, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Scopaz, K.A.; Piva, S.R.; Wisniewski, S.; Fitzgerald, G.K. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2009, 90, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Somers, T.J.; Keefe, F.J.; Pells, J.J.; Dixon, K.E.; Waters, S.J.; Riordan, P.A.; Blumenthal, J.A.; McKee, D.C.; LaCaille, L.; Tucker, J.M.; et al. Pain Catastrophizing and Pain-Related Fear in Osteoarthritis Patients: Relationships to Pain and Disability. J. Pain Symptom Manag. 2009, 37, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Heuts, P.H.; Vlaeyen, J.; Roelofs, J.; De Bie, R.A.; Aretz, K.; van Weel, C.; Van Schayck, O.C. Pain-related fear and daily functioning in patients with osteoarthritis. Pain 2004, 110, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Nie, H.; Laursen, M.B.; Laursen, B.S.; Madeleine, P.; Simonsen, O.H.; Graven-Nielsen, T. Sensitization in patients with painful knee osteoarthritis. Pain 2010, 149, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Egsgaard, L.; Petersen, K.; Eskehave, T.; Graven-Nielsen, T.; Hoeck, H.; Simonsen, O. A mechanism-based pain sensitivity index to characterize knee osteoarthritis patients with different disease stages and pain levels. Eur. J. Pain 2014, 19, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Buenaver, L.F.; Bounds, S.C.; Hussain, S.; Park, R.J.; Haque, U.J.; Campbell, C.M.; Haythornthwaite, J.A.; Edwards, R.R.; Smith, M.T. Discordance between pain and radiographic severity in knee osteoarthritis: Findings from quantitative sensory testing of central sensitization. Arthritis Care Res. 2012, 65, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Wylde, V.; Hewlett, S.; Learmonth, I.D.; Dieppe, P. Persistent pain after joint replacement: Prevalence, sensory qualities, and postoperative determinants. Pain 2011, 152, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulas, T.; Duarte, R.V.; Behura, A.; Kitas, G.D.; Raphael, J.H. Neuropathic pain in osteoarthritis: A review of pathophysiological mechanisms and implications for treatment. Semin. Arthritis Rheum. 2014, 44, 145–154. [Google Scholar] [CrossRef]
- Lluch, E.; Torres, R.; Nijs, J.; Van Oosterwijck, J. Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. Eur. J. Pain 2014, 18, 1367–1375. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Moonaz, S.H.; Bingham, C.O., 3rd; Wissow, L.; Bartlett, S.J. Yoga in Sedentary Adults with Arthritis: Effects of a Randomized Controlled Pragmatic Trial. J. Rheumatol. 2015, 42, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Dobson, F.; Bennell, K.L.; Hinman, R.S.; Abbott, J.H.; Roos, E.M. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- OARSI. Physical Performance Measures: Osteoarthritis Research Society International. 2022. Available online: https://oarsi.org/research/physical-performance-measures (accessed on 4 January 2022).
- Löwe, B.; Kroenke, K.; Herzog, W.; Gräfe, K. Measuring depression outcome with a brief self-report instrument: Sensitivity to change of the Patient Health Questionnaire (PHQ-9). J. Affect. Disord. 2004, 81, 61–66. [Google Scholar] [CrossRef]
- Pham, T.; van der Heijde, D.; Altman, R.; Anderson, J.; Bellamy, N.; Hochberg, M.; Simon, L.; Strand, V.; Woodworth, T.; Dougados, M. OMERACT-OARSI Initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr. Cartil. 2004, 12, 389–399. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. Pain DETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Jones, G.; Glisson, M.; Hynes, K.; Cicuttini, F. Sex and site differences in cartilage development: A possible explanation for variations in knee osteoarthritis in later life. Arthritis Care Res. 2000, 43, 2543–2549. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Manca, A.; Hawkins, N.; Sculpher, M.J. Estimating mean QALYs in trial-based cost-effectiveness analysis: The importance of controlling for baseline utility. Health Econ. 2005, 14, 487–496. [Google Scholar] [CrossRef]
- Horsman, J.; Furlong, W.; Feeny, D.; Torrance, G. The Health Utilities Index (HUI): Concepts, measurement properties and applications. Health Qual Life Outcomes 2003, 1, 54. [Google Scholar] [CrossRef]
- Maxwell, A.; Özmen, M.; Iezzi, A.; Richardson, J. Deriving population norms for the AQoL-6D and AQoL-8D multi-attribute utility instruments from web-based data. Qual. Life Res. 2016, 25, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Centre for Health Economics. AQoL-8D 2014. Available online: https://www.aqol.com.au/index.php/aqolinstruments?id=58 (accessed on 1 September 2021).
- EuroQol Research Foundation EQ-5D-5L about 2022. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed on 1 September 2021).
- Online, M. Indexation of Medicare Benefits Schedule (MBS) Items from 1 July 2018: MBS. Available online: http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/MBSIndexation-July2018 (accessed on 1 September 2021).
- Singh, A.; Wang, Z.; Dissanayaka, T.; Das, S.; Antony, B. Efficacy and safety of hydroxychloroquine in osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Korean J. Intern. Med. 2021, 37, 210. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.; Dickson, K.; Hallett, R.; Grant, R.; Hauari, H.; Walsh, N.; Stansfield, C.; Oliver, S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: A mixed methods review. Cochrane Database Syst. Rev. 2018, 4, CD010842. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin. Arthritis Rheum. 2014, 43, 701–712. [Google Scholar] [CrossRef]
- Maddux, R.E.; Daukantaité, D.; Tellhed, U. The effects of yoga on stress and psychological health among employees: An 8- and 16-week intervention study. Anxiety Stress Coping 2018, 31, 121–134. [Google Scholar] [CrossRef]
- De Manincor, M.; Bensoussan, A.; Smith, C.; Fahey, P.; Bourchier, S. Establishing key components of yoga interventions for reducing depression and anxiety, and improving well-being: A Delphi method study. BMC Complement Altern Med. 2015, 15, 85. [Google Scholar] [CrossRef][Green Version]
- Dobson, J.L.; McMillan, J.; Li, L. Benefits of exercise intervention in reducing neuropathic pain. Front. Cell. Neurosci. 2014, 8, 102. [Google Scholar] [CrossRef]
- Telles, S.; Sayal, N.; Nacht, C.; Chopra, A.; Patel, K.; Wnuk, A.; Dalvi, P.; Bhatia, K.; Miranpuri, G.; Anand, A. Yoga: Can it be integrated with treatment of neuropathic pain? Ann. Neurosci. 2019, 26, 82–91. [Google Scholar] [CrossRef]
- Vallath, N. Perspectives on yoga inputs in the management of chronic pain. Indian J. Palliat. Care 2010, 16, 1–7. [Google Scholar] [CrossRef]
- Kan, L.; Zhang, J.; Yang, Y.; Wang, P. The Effects of Yoga on Pain, Mobility, and Quality of Life in Patients with Knee Osteoarthritis: A Systematic Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 6016532. [Google Scholar] [CrossRef]
- Antony, B.; Singh, A. A Randomised Comparative Effectiveness Trial of YOGa and Strengthening Exercise for Knee Osteo Arthritis (YOGA Trial): ANZCTR. 2021. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=380369&isReview=true (accessed on 4 January 2022).
| Items/Variables | Screening | Baseline (week 0) | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 |
|---|---|---|---|---|---|---|---|---|
| Informed consent | x | |||||||
| Randomisation | x | |||||||
| Safety for exercise clearance | x | |||||||
| ACR clinical criteria for knee OA | x | |||||||
| Medicare number | x | |||||||
| Clinical measures | ||||||||
| Blood (stored for cartilage/synovium/ inflammatory markers) | x | x | x | |||||
| Core physical function tests | x | x | x | |||||
| Leg muscle strength test | x | x | x | |||||
| Height and weight | x | x | x | |||||
| Gait characteristics | x | x | x | |||||
| Body composition (using BIA) | x | x | x | |||||
| Physical activity (using accelerometers) | x | x | ||||||
| Questionnaires | ||||||||
| Knee pain VAS | x | x | x | x | x | x | x | x |
| Knee WOMAC | x | x | x | x | x | x | x | |
| PainDETECT | x | x | x | |||||
| PHQ-9 | x | x | x | |||||
| Patient global evaluation | x | x | x | x | x | x | x | |
| Pain medication use/change in use | x | x | x | x | x | x | x | |
| Health Economics Outcomes: Medication cost diary Health service utilisation (visit to GP, practice nurses, and any other health professionals (e.g., physiotherapists)) Employment/days off work Concession/health care card Private health insurance Transport and specialised equipment costs | x | x | ||||||
| Safety (AEs) | x | x | x | x | x | x | ||
| EQ-5D and AQoL-8D | x | x | x | |||||
| Consent to contact for future studies | x | |||||||
| Early withdrawal information | As required | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Aitken, D.; Moonaz, S.; Palmer, A.J.; Blizzard, L.; Ding, C.; Drummen, S.; Jones, G.; Bennell, K.; Antony, B. A Randomised Controlled Trial of YOGa and Strengthening Exercise for Knee OsteoArthritis: Protocol for a Comparative Effectiveness Trial (YOGA Trial). J. Funct. Morphol. Kinesiol. 2022, 7, 84. https://doi.org/10.3390/jfmk7040084
Singh A, Aitken D, Moonaz S, Palmer AJ, Blizzard L, Ding C, Drummen S, Jones G, Bennell K, Antony B. A Randomised Controlled Trial of YOGa and Strengthening Exercise for Knee OsteoArthritis: Protocol for a Comparative Effectiveness Trial (YOGA Trial). Journal of Functional Morphology and Kinesiology. 2022; 7(4):84. https://doi.org/10.3390/jfmk7040084
Chicago/Turabian StyleSingh, Ambrish, Dawn Aitken, Steffany Moonaz, Andrew J. Palmer, Leigh Blizzard, Changhai Ding, Stan Drummen, Graeme Jones, Kim Bennell, and Benny Antony. 2022. "A Randomised Controlled Trial of YOGa and Strengthening Exercise for Knee OsteoArthritis: Protocol for a Comparative Effectiveness Trial (YOGA Trial)" Journal of Functional Morphology and Kinesiology 7, no. 4: 84. https://doi.org/10.3390/jfmk7040084
APA StyleSingh, A., Aitken, D., Moonaz, S., Palmer, A. J., Blizzard, L., Ding, C., Drummen, S., Jones, G., Bennell, K., & Antony, B. (2022). A Randomised Controlled Trial of YOGa and Strengthening Exercise for Knee OsteoArthritis: Protocol for a Comparative Effectiveness Trial (YOGA Trial). Journal of Functional Morphology and Kinesiology, 7(4), 84. https://doi.org/10.3390/jfmk7040084









