Significantly Delayed Medium-Latency Response of the Stretch Reflex in Delayed-Onset Muscle Soreness of the Quadriceps Femoris Muscles Is Indicative of Sensory Neuronal Microdamage
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Procedures
2.3. Exercise Protocol
2.4. Electromyography
2.5. Statistics
2.6. Muscle Soreness Questionnaire
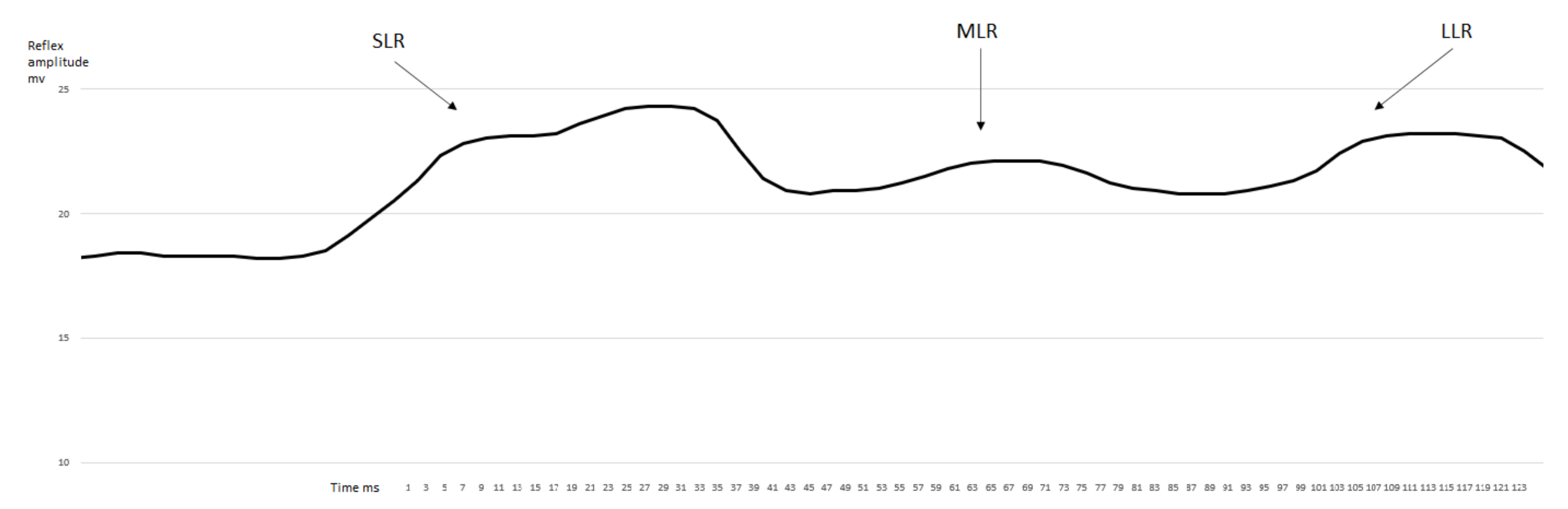
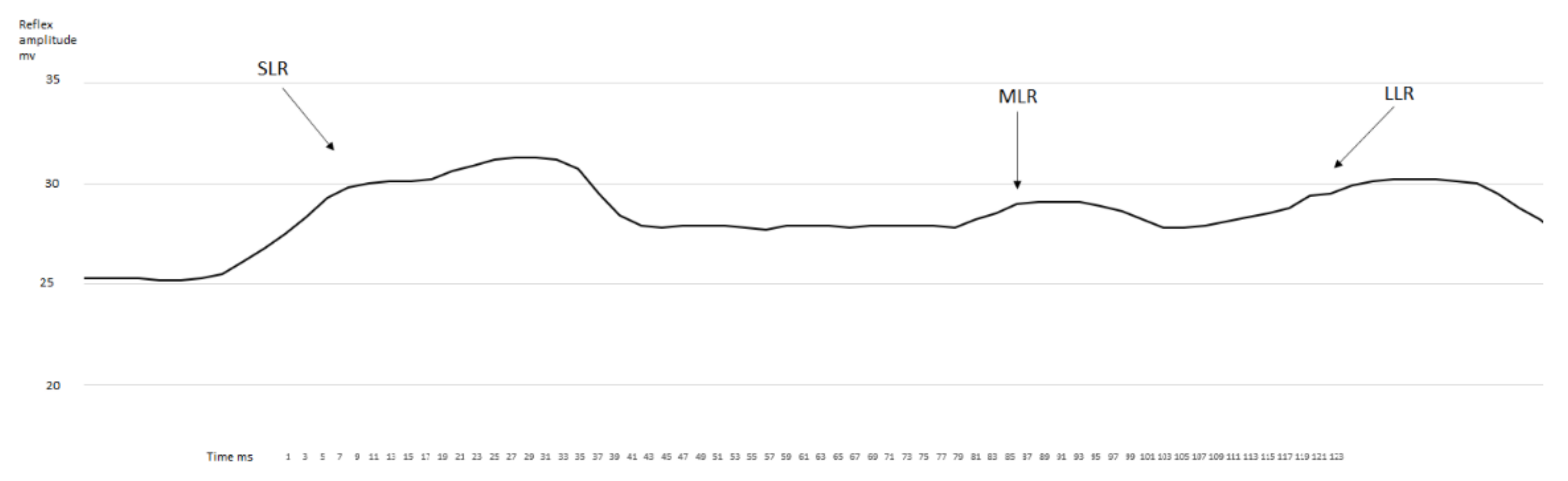
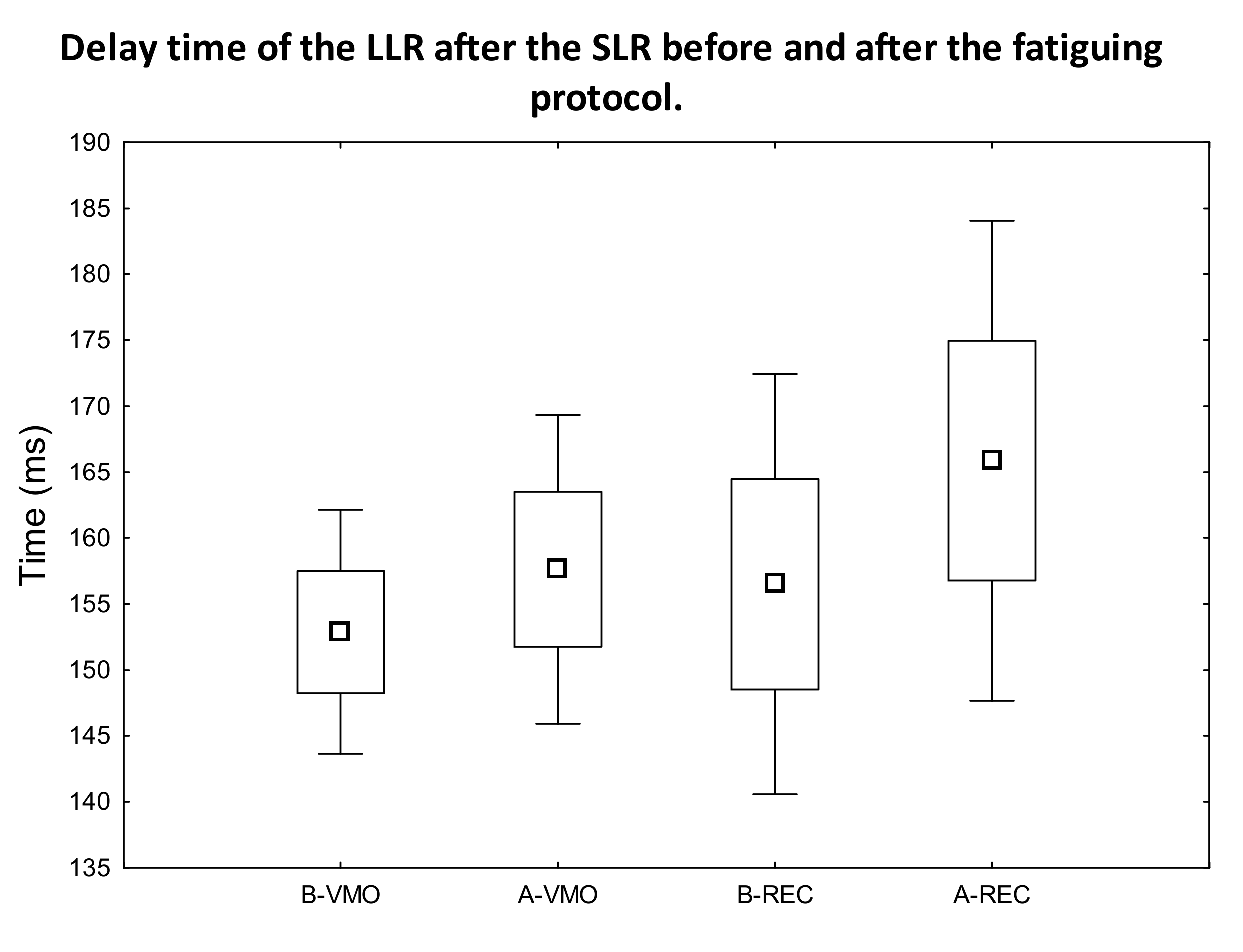
3. Results
3.1. EMG Activity of the Observed Muscles
3.2. Muscle Soreness Questionnaire
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarkson, P.M.; Nosaka, K.; Braun, B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med. Sci. Sports Exerc. 1992, 24, 512–520. [Google Scholar] [CrossRef]
- Newham, D.J. The consequences of eccentric contractions and their relationship to delayed onset muscle pain. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 57, 353–359. [Google Scholar] [CrossRef]
- Cheung, K.; Hume, P.; Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Sonkodi, B.; Berkes, I.; Koltai, E. Have We Looked in the Wrong Direction for More Than 100 Years? Delayed Onset Muscle Soreness Is, in Fact, Neural Microdamage Rather Than Muscle Damage. Antioxidants 2020, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Sonkodi, B.; Kopa, Z.; Nyirady, P. Post Orgasmic Illness Syndrome (POIS) and Delayed Onset Muscle Soreness (DOMS): Do They Have Anything in Common? Cells 2021, 10, 1867. [Google Scholar] [CrossRef]
- Miles, M.P.; Clarkson, P.M. Exercise-induced muscle pain, soreness, and cramps. J. Sports Med. Phys. Fit. 1994, 34, 203–216. [Google Scholar]
- Hayashi, K.; Abe, M.; Yamanaka, A.; Mizumura, K.; Taguchi, T. Degenerative histological alteration is not required for the induction of muscular mechanical hyperalgesia after lengthening contraction in rats. J. Physiol. Sci. 2015, 65, S277. [Google Scholar]
- Weerakkody, N.S.; Percival, P.; Hickey, M.W.; Morgan, D.L.; Gregory, J.E.; Canny, B.J.; Proske, U. Effects of local pressure and vibration on muscle pain from eccentric exercise and hypertonic saline. Pain 2003, 105, 425–435. [Google Scholar] [CrossRef]
- Semark, A.; Noakes, T.D.; St Clair Gibson, A.; Lambert, M.I. The effect of a prophylactic dose of flurbiprofen on muscle soreness and sprinting performance in trained subjects. J. Sports Sci. 1999, 17, 197–203. [Google Scholar] [CrossRef]
- Mizumura, K.; Taguchi, T. Delayed onset muscle soreness: Involvement of neurotrophic factors. J. Physiol. Sci. 2016, 66, 43–52. [Google Scholar] [CrossRef]
- Sonkodi, B.; Bardoni, R.; Hangody, L.; Radák, Z.; Berkes, I. Does Compression Sensory Axonopathy in the Proximal Tibia Contribute to Noncontact Anterior Cruciate Ligament Injury in a Causative Way?—A New Theory for the Injury Mechanism. Life 2021, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Lukacs, V.; de Nooij, J.C.; Zaytseva, D.; Criddle, C.R.; Francisco, A.; Jessell, T.M.; Wilkinson, K.A.; Patapoutian, A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015, 18, 1756–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesler, A.T.; Szczot, M.; Bharucha-Goebel, D.; Ceko, M.; Donkervoort, S.; Laubacher, C.; Hayes, L.H.; Alter, K.; Zampieri, C.; Stanley, C.; et al. The Role of PIEZO2 in Human Mechanosensation. N. Engl. J. Med. 2016, 375, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Woo, S.H.; Dubin, A.E.; Moshourab, R.A.; Wetzel, C.; Petrus, M.; Mathur, J.; Begay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hody, S.; Croisier, J.L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric Muscle Contractions: Risks and Benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.L.; Allen, D.G. Early events in stretch-induced muscle damage. J. Appl. Physiol. 1999, 87, 2007–2015. [Google Scholar] [CrossRef] [Green Version]
- Torres, R.; Vasques, J.; Duarte, J.A.; Cabri, J.M. Knee proprioception after exercise-induced muscle damage. Int. J. Sports Med. 2010, 31, 410–415. [Google Scholar] [CrossRef]
- Bennett, G.J.; Liu, G.K.; Xiao, W.H.; Jin, H.W.; Siau, C. Terminal arbor degeneration--a novel lesion produced by the antineoplastic agent paclitaxel. Eur. J. Neurosci. 2011, 33, 1667–1676. [Google Scholar] [CrossRef] [Green Version]
- Kouzaki, K.; Nosaka, K.; Ochi, E.; Nakazato, K. Increases in M-wave latency of biceps brachii after elbow flexor eccentric contractions in women. Eur. J. Appl. Physiol. 2016, 116, 939–946. [Google Scholar] [CrossRef]
- Vincent, J.A.; Nardelli, P.; Gabriel, H.M.; Deardorff, A.S.; Cope, T.C. Complex impairment of IA muscle proprioceptors following traumatic or neurotoxic injury. J. Anat. 2015, 227, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.A.; Wieczerzak, K.B.; Gabriel, H.M.; Nardelli, P.; Rich, M.M.; Cope, T.C. A novel path to chronic proprioceptive disability with oxaliplatin: Distortion of sensory encoding. Neurobiol. Dis. 2016, 95, 54–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullinger, K.L.; Nardelli, P.; Pinter, M.J.; Alvarez, F.J.; Cope, T.C. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J. Neurophysiol. 2011, 106, 2471–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, F.J.; Titus-Mitchell, H.E.; Bullinger, K.L.; Kraszpulski, M.; Nardelli, P.; Cope, T.C. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J. Neurophysiol. 2011, 106, 2450–2470. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Delayed Onset Muscle Soreness (DOMS): The Repeated Bout Effect and Chemotherapy-Induced Axonopathy May Help Explain the Dying-Back Mechanism in Amyotrophic Lateral Sclerosis and Other Neurodegenerative Diseases. Brain Sci 2021, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.K.; Mrachacz-Kersting, N.; Sinkjaer, T.; Andersen, J.B. Modulation of soleus stretch reflexes during walking in people with chronic incomplete spinal cord injury. Exp. Brain Res. 2019, 237, 2461–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corna, S.; Grasso, M.; Nardone, A.; Schieppati, M. Selective depression of medium-latency leg and foot muscle responses to stretch by an alpha 2-agonist in humans. J. Physiol. 1995, 484, 803–809. [Google Scholar] [CrossRef]
- Sinkjaer, T.; Andersen, J.B.; Nielsen, J.F. Impaired stretch reflex and joint torque modulation during spastic gait in multiple sclerosis patients. J. Neurol. 1996, 243, 566–574. [Google Scholar] [CrossRef]
- Schieppati, M.; Nardone, A. Medium-latency stretch reflexes of foot and leg muscles analysed by cooling the lower limb in standing humans. J. Physiol. 1997, 503, 691–698. [Google Scholar] [CrossRef]
- Nardone, A.; Schieppati, M. Medium-latency response to muscle stretch in human lower limb: Estimation of conduction velocity of group II fibres and central delay. Neurosci. Lett. 1998, 249, 29–32. [Google Scholar] [CrossRef]
- Sinkjaer, T.; Andersen, J.B.; Nielsen, J.F.; Hansen, H.J. Soleus long-latency stretch reflexes during walking in healthy and spastic humans. Clin. Neurophysiol. 1999, 110, 951–959. [Google Scholar] [CrossRef]
- Grey, M.J.; Ladouceur, M.; Andersen, J.B.; Nielsen, J.B.; Sinkjaer, T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J. Physiol. 2001, 534, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Uysal, H.; Larsson, L.E.; Efendi, H.; Burke, D.; Ertekin, C. Medium-latency reflex response of soleus elicited by peroneal nerve stimulation. Exp. Brain Res. 2009, 193, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Af Klint, R.; Mazzaro, N.; Nielsen, J.B.; Sinkjaer, T.; Grey, M.J. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J. Neurophysiol. 2010, 103, 2747–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, H.; Boyraz, I.; Yagcioglu, S.; Oktay, F.; Kafali, P.; Tonuk, E. Ankle clonus and its relationship with the medium-latency reflex response of the soleus by peroneal nerve stimulation. J. Electromyogr. Kinesiol. 2011, 21, 438–444. [Google Scholar] [CrossRef]
- Hjortskov, N.; Essendrop, M.; Skotte, J.; Fallentin, N. The effect of delayed-onset muscle soreness on stretch reflexes in human low back muscles. Scand. J. Med. Sci. Sports 2005, 15, 409–415. [Google Scholar] [CrossRef]
- Bencke, J.; Naesborg, H.; Simonsen, E.B.; Klausen, K. Motor pattern of the knee joint muscles during side-step cutting in European team handball. Influence on muscular co-ordination after an intervention study. Scand. J. Med. Sci. Sports 2000, 10, 68–77. [Google Scholar] [CrossRef]
- Horvath, M.; Fazekas, G. Assessment of motor impairment with electromyography--the kinesiological EMG. Ideggyogy. Szle. 2003, 56, 360–369. [Google Scholar]
- Nosaka, K. Muscle Soreness and Damage and the Repeated-Bout Effect. In Skeletal Muscle Damage and Repair; Tiidus, P.M., Ed.; Human Kinetics: Champaign, IL, USA, 2008; pp. 59–76. [Google Scholar]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Sonkodi, B.; Hortobágyi, T. Amyotrophic lateral sclerosis and delayed onset muscle soreness in light of the impaired blink and stretch reflexes – watch out for Piezo2. Open Med. 2022, 17, 397–402. [Google Scholar] [CrossRef]
- Balestra, C.; Duchateau, J.; Hainaut, K. Effects of fatigue on the stretch reflex in a human muscle. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 46–52. [Google Scholar] [CrossRef]
- Duchateau, J.; Hainaut, K. Behaviour of short and long latency reflexes in fatigued human muscles. J. Physiol. 1993, 471, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Suchyna, T.M. Piezo channels and GsMTx4: Two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog. Biophys. Mol. Biol. 2017, 130, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Bewick, G.S.; Banks, R.W. Spindles are doin’ it for themselves: Glutamatergic autoexcitation in muscle spindles. J. Physiol. 2021, 599, 2781–2783. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B.; Resch, M.D.; Hortobágyi, T. Is the Sex Difference a Clue to the Pathomechanism of Dry Eye Disease? Watch out for the NGF-TrkA-Piezo2 Signaling Axis and the Piezo2 Channelopathy. J. Mol. Neurosci. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Kukley, M. Glutamate receptors and glutamatergic signalling in the peripheral nerves. Neural Regen Res. 2020, 15, 438–447. [Google Scholar] [CrossRef]
- Spitzer, S.; Volbracht, K.; Lundgaard, I.; Karadottir, R.T. Glutamate signalling: A multifaceted modulator of oligodendrocyte lineage cells in health and disease. Neuropharmacology 2016, 110, 574–585. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Koyama, M.; Tamura, R.; Takagishi, Y.; Murase, S.; Mizumura, K. Absence of mechanical hyperalgesia after exercise (delayed onset muscle soreness) in neonatally capsaicin-treated rats. Neurosci. Res. 2012, 73, 56–60. [Google Scholar] [CrossRef]
- Sufka, K.J.; Price, D.D. Gate Control Theory Reconsidered. Brain Mind 2002, 3, 277–290. [Google Scholar] [CrossRef]
- Borghi, S.M.; Bussulo, S.K.D.; Pinho-Ribeiro, F.A.; Fattori, V.; Carvalho, T.T.; Rasquel-Oliveira, F.S.; Zaninelli, T.H.; Ferraz, C.R.; Casella, A.M.B.; Cunha, F.Q.; et al. Intense Acute Swimming Induces Delayed-Onset Muscle Soreness Dependent on Spinal Cord Neuroinflammation. Front. Pharmacol. 2021, 12, 734091. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonkodi, B.; Hegedűs, Á.; Kopper, B.; Berkes, I. Significantly Delayed Medium-Latency Response of the Stretch Reflex in Delayed-Onset Muscle Soreness of the Quadriceps Femoris Muscles Is Indicative of Sensory Neuronal Microdamage. J. Funct. Morphol. Kinesiol. 2022, 7, 43. https://doi.org/10.3390/jfmk7020043
Sonkodi B, Hegedűs Á, Kopper B, Berkes I. Significantly Delayed Medium-Latency Response of the Stretch Reflex in Delayed-Onset Muscle Soreness of the Quadriceps Femoris Muscles Is Indicative of Sensory Neuronal Microdamage. Journal of Functional Morphology and Kinesiology. 2022; 7(2):43. https://doi.org/10.3390/jfmk7020043
Chicago/Turabian StyleSonkodi, Balázs, Ádám Hegedűs, Bence Kopper, and István Berkes. 2022. "Significantly Delayed Medium-Latency Response of the Stretch Reflex in Delayed-Onset Muscle Soreness of the Quadriceps Femoris Muscles Is Indicative of Sensory Neuronal Microdamage" Journal of Functional Morphology and Kinesiology 7, no. 2: 43. https://doi.org/10.3390/jfmk7020043
APA StyleSonkodi, B., Hegedűs, Á., Kopper, B., & Berkes, I. (2022). Significantly Delayed Medium-Latency Response of the Stretch Reflex in Delayed-Onset Muscle Soreness of the Quadriceps Femoris Muscles Is Indicative of Sensory Neuronal Microdamage. Journal of Functional Morphology and Kinesiology, 7(2), 43. https://doi.org/10.3390/jfmk7020043





