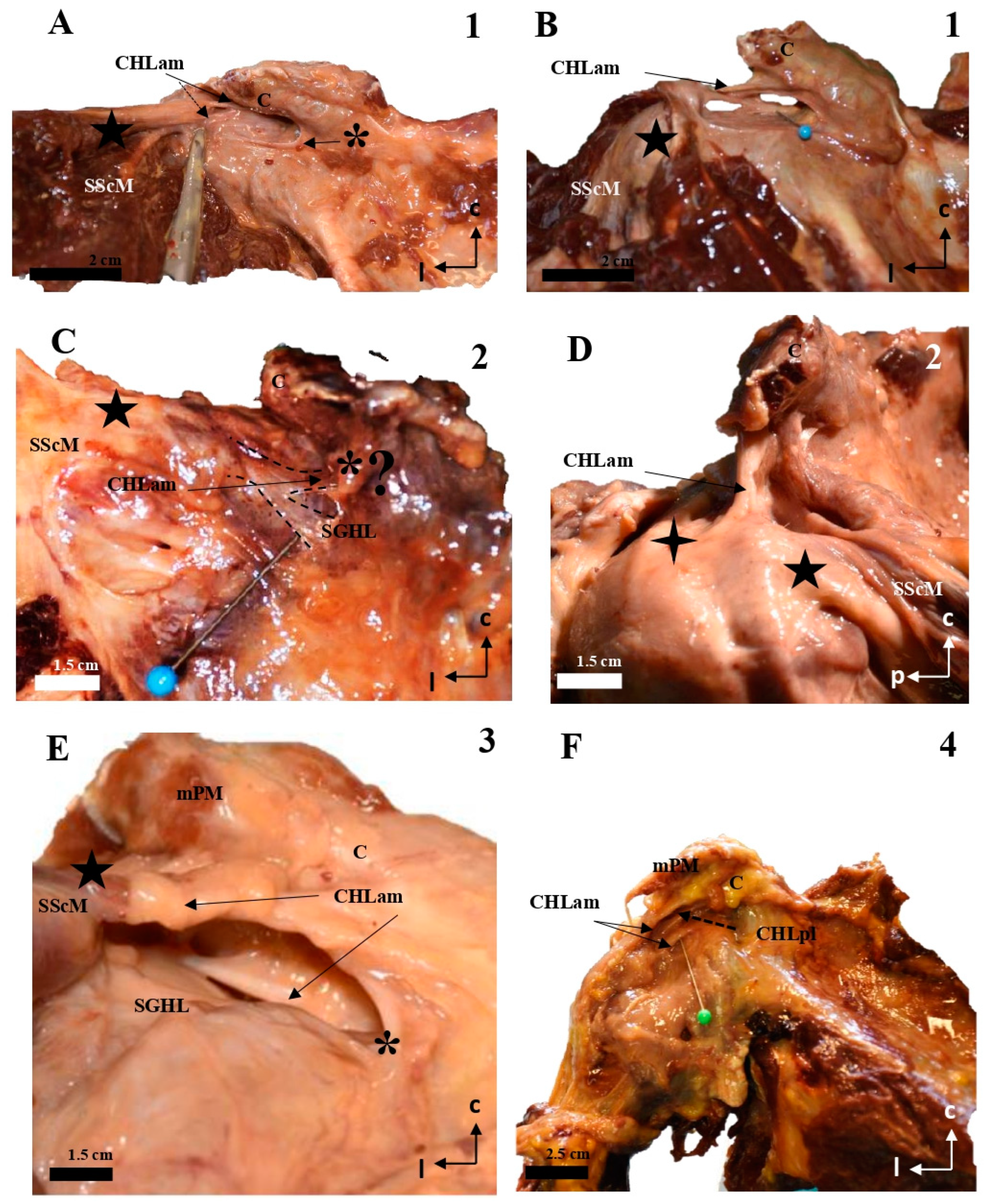
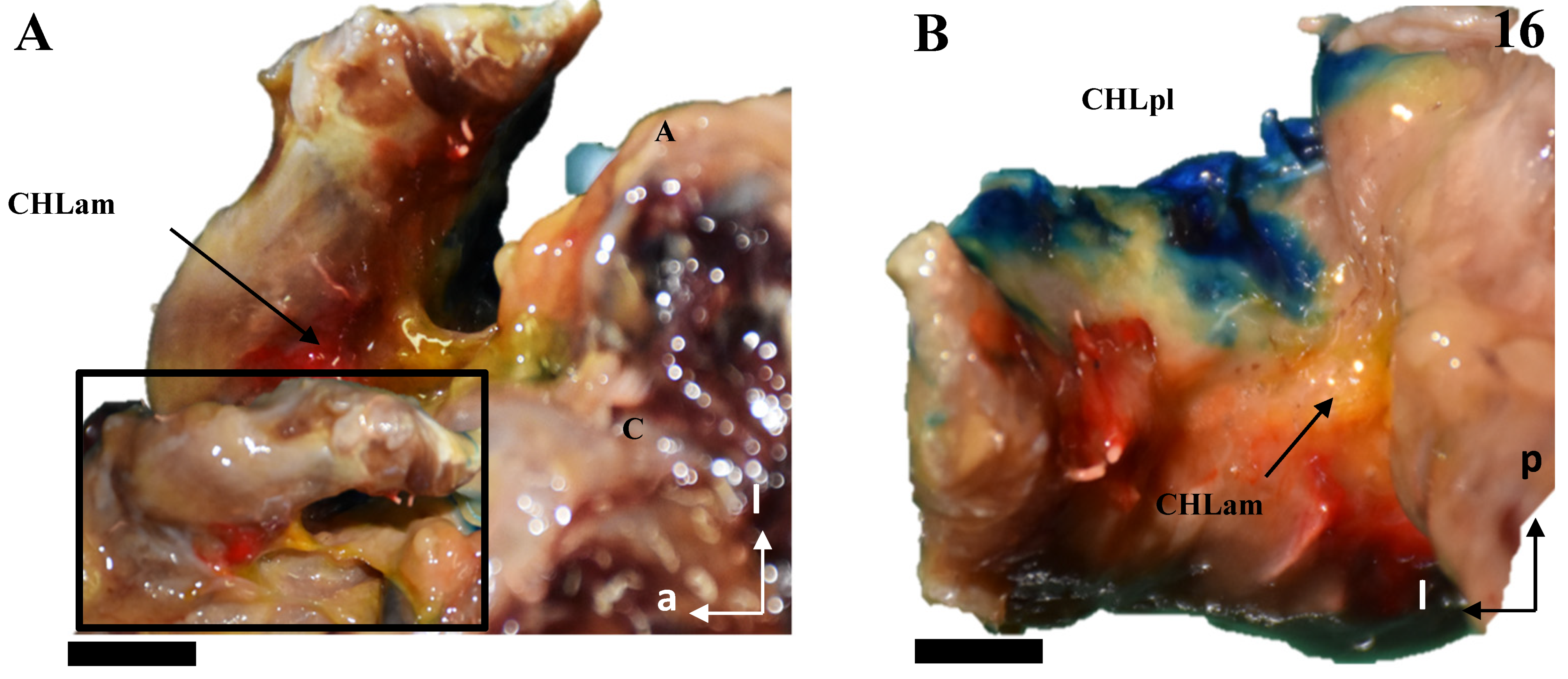
Figure 1.
Cadaveric dissections of the anterior column of the CHL. (A,B) (case 1): after disinsertion of the SScM, it was laterally retracted, revealing the anterior and medial column of the CHL (CHLam) divided into two fascicles: a superficial one that attached to the caudal surface of the coracoid process (C) (arrow in A,B) and the capsule-ligamentous tissue of the humeral head, positioned above the tendon of the SScM; and a smaller deep fascicle attached to the anterolateral aspect of the base of the coracoid process (dotted arrow in A and pin in B) and the capsuloligamentous tissue that covered the humeral head, located beneath (deep into) the SScM tendon (five-pointed star). In (B), the shoulder is in forced external rotation. (C,D) (case 2): In this shoulder, the CHLam exhibits a single superficial fascicle fully attached to the caudal surface of the coracoid process (D), while the deep fascicle is replaced by a loose band of tissue that fuses with the SGHL (C) and may correspond to an atypical coracoglenoid ligament based on its insertions (asterisk and dotted lines). The superficial fascicle (D) laterally fuses with the shoulder capsule at the rotator interval (located between the SScM tendon, marked with a five-pointed star, and the SSpM tendon, marked with a four-pointed star). In cases 3 (E) and 4 (F), the superficial fascicle attaches to the caudal surface of the coracoid and fuses with the shoulder capsule above the SScM tendon, while the caudal fascicle merges with the capsule beneath the SScM tendon, originating from the lateral aspect of the coracoid base (dotted arrow in F). The asterisk in (E) highlights a distinct coracoglenoid ligament.
Figure 1.
Cadaveric dissections of the anterior column of the CHL. (A,B) (case 1): after disinsertion of the SScM, it was laterally retracted, revealing the anterior and medial column of the CHL (CHLam) divided into two fascicles: a superficial one that attached to the caudal surface of the coracoid process (C) (arrow in A,B) and the capsule-ligamentous tissue of the humeral head, positioned above the tendon of the SScM; and a smaller deep fascicle attached to the anterolateral aspect of the base of the coracoid process (dotted arrow in A and pin in B) and the capsuloligamentous tissue that covered the humeral head, located beneath (deep into) the SScM tendon (five-pointed star). In (B), the shoulder is in forced external rotation. (C,D) (case 2): In this shoulder, the CHLam exhibits a single superficial fascicle fully attached to the caudal surface of the coracoid process (D), while the deep fascicle is replaced by a loose band of tissue that fuses with the SGHL (C) and may correspond to an atypical coracoglenoid ligament based on its insertions (asterisk and dotted lines). The superficial fascicle (D) laterally fuses with the shoulder capsule at the rotator interval (located between the SScM tendon, marked with a five-pointed star, and the SSpM tendon, marked with a four-pointed star). In cases 3 (E) and 4 (F), the superficial fascicle attaches to the caudal surface of the coracoid and fuses with the shoulder capsule above the SScM tendon, while the caudal fascicle merges with the capsule beneath the SScM tendon, originating from the lateral aspect of the coracoid base (dotted arrow in F). The asterisk in (E) highlights a distinct coracoglenoid ligament.
![Jfmk 10 00149 g001]()
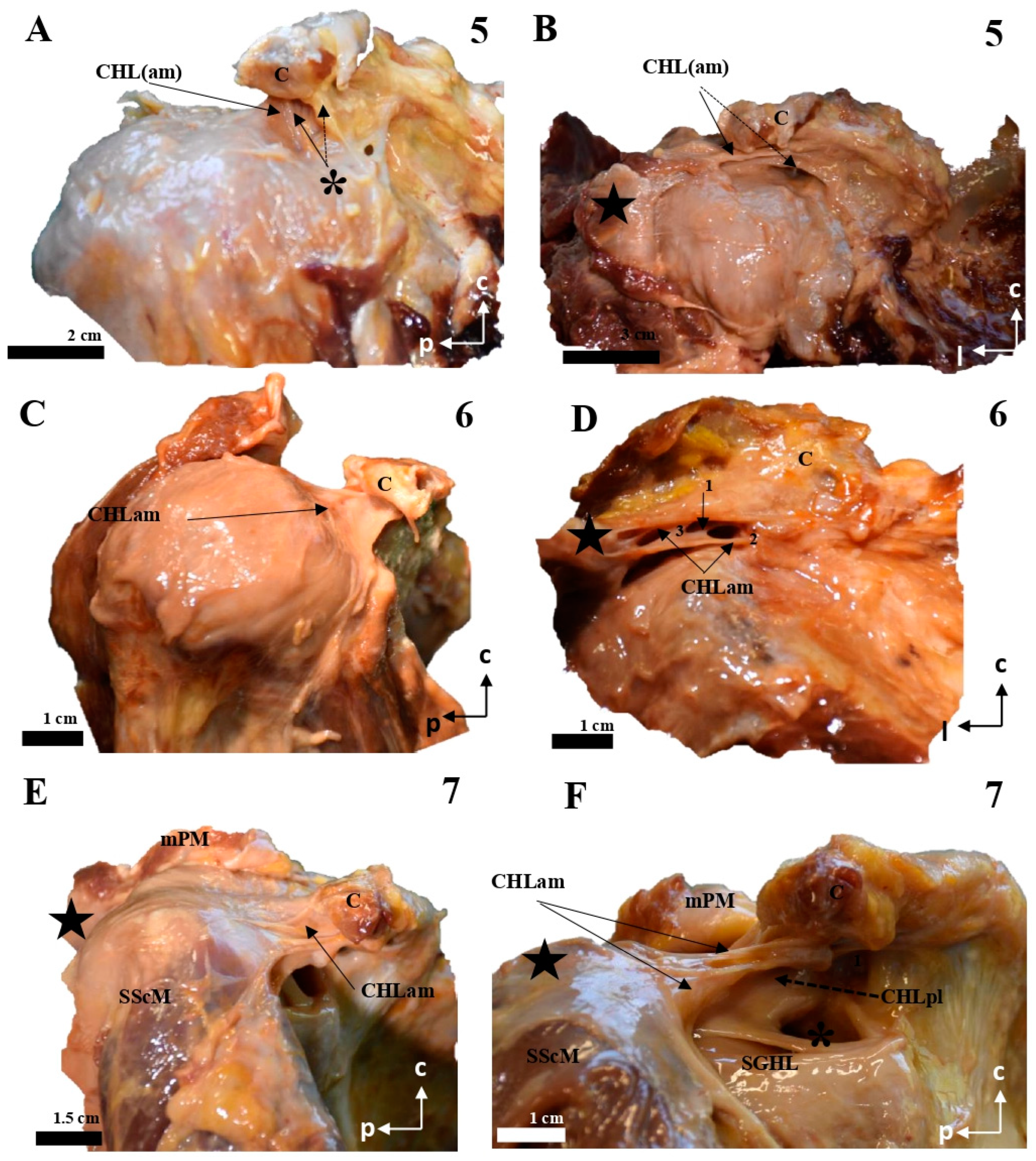
Figure 2.
Cadaveric dissections of the anterior column of the CHL. (A,B) (case 5): the anterior and medial column of the CHL (CHLam) is organized into two fascicles: a superficial one that is inserted in the caudal surface of the coracoid process (C) (arrow in A) immediately laterally to a lateral fascicle of a two-bundled coracoglenoid ligament (asterisk, the medial bundle marked with a dotted arrow), and broadly reaches the rotator interval; and a deep fascicle (dotted arrow in B) that is inserted in the caudal surface of the coracoid and the lateral aspect of its base, and adjoins the humeral capsuloligamentous tissue beneath the insertion of the SScM tendon (star in B). (C,D): Case 6 shows a similar pattern, although the deep fascicle (D) is organized into three bundles in its medial insertion: one directed to the posterior edge (1) of the coracoid base, a second one directed to the anterior edge of the coracoid base (2) and a third one merging with the inferior surface of the superficial fascicle (3). Case 7 shows a superficial fascicle ranging from the caudal surface of the coracoid process (E) to the rotator interval and the capsuloligamentous tissue of the humeral head superficial to the insertion of SScM, partially reaching the humeral tubercle. The deep fascicle is a strong band of connective tissue that arises from the most posterior and medial aspect of the caudal coracoid surface (F), whose lateral insertion is superimposed on the lateral fascicle of an accessory band of the SGHL (four-pointed star).
Figure 2.
Cadaveric dissections of the anterior column of the CHL. (A,B) (case 5): the anterior and medial column of the CHL (CHLam) is organized into two fascicles: a superficial one that is inserted in the caudal surface of the coracoid process (C) (arrow in A) immediately laterally to a lateral fascicle of a two-bundled coracoglenoid ligament (asterisk, the medial bundle marked with a dotted arrow), and broadly reaches the rotator interval; and a deep fascicle (dotted arrow in B) that is inserted in the caudal surface of the coracoid and the lateral aspect of its base, and adjoins the humeral capsuloligamentous tissue beneath the insertion of the SScM tendon (star in B). (C,D): Case 6 shows a similar pattern, although the deep fascicle (D) is organized into three bundles in its medial insertion: one directed to the posterior edge (1) of the coracoid base, a second one directed to the anterior edge of the coracoid base (2) and a third one merging with the inferior surface of the superficial fascicle (3). Case 7 shows a superficial fascicle ranging from the caudal surface of the coracoid process (E) to the rotator interval and the capsuloligamentous tissue of the humeral head superficial to the insertion of SScM, partially reaching the humeral tubercle. The deep fascicle is a strong band of connective tissue that arises from the most posterior and medial aspect of the caudal coracoid surface (F), whose lateral insertion is superimposed on the lateral fascicle of an accessory band of the SGHL (four-pointed star).
![Jfmk 10 00149 g002]()
Figure 3.
Cadaveric dissections of the anterior column of the CHL. (A,B) (case 8): the anterior and medial column of the CHL (CHLam) is organized into two fascicles: a superficial one that is inserted in the caudal surface of the coracoid process (C) (arrow in A), immediately laterally to a lateral fascicle of the coracoglenoid ligament (asterisk)—this superficial fascicle profusely fuses with the fascial tissue overlying the SScM tendon (star), before reaching the capsuloligamentous tissue of the shoulder; and a deep fascicle that is inserted in the caudal surface of the coracoid and the lateral aspect of its base, only revealed after careful removal of the superficial fascicle, and adjoins the humeral capsuloligamentous tissue beneath the insertion of the SScM tendon (star in B). The coracoglenoid ligament is present (asterisk in B), and is laterally prolonged towards the capsuloligamentous complex. Case 9 (C,D) shows two fascicles: the superficial one is broadly comparable to the one previously described (C); the deep fascicle (C,D) is a Y-shaped bundle that ranges from the lateral aspect of the coracoid base to the rotator interval (D) and the deep fascia of the SScM. Case 10 (E) is a good example of the canonical organization of the CHLam, with a superficial bundle that extends from the caudal surface of the coracoid to the rotator interval (cephalic and superficial to the SScM tendinous insertion) and broadly reaches the rotator interval, and a deep fascicle broadly extending from the lateral surface of the coracoid base (1), before fusing with the shoulder joint capsule underneath the SScM tendon. In case 11 (F,G) the deep fascicle of the CHLam is inserted almost in the posterior edge of the caudal surface of the coracoid limb, as well as in the anterior surface of the CHLpl (see dotted arrow in G), before reaching the shoulder joint capsule, as an accessory fascicle of the superior glenohumeral ligament (SGHL in G).
Figure 3.
Cadaveric dissections of the anterior column of the CHL. (A,B) (case 8): the anterior and medial column of the CHL (CHLam) is organized into two fascicles: a superficial one that is inserted in the caudal surface of the coracoid process (C) (arrow in A), immediately laterally to a lateral fascicle of the coracoglenoid ligament (asterisk)—this superficial fascicle profusely fuses with the fascial tissue overlying the SScM tendon (star), before reaching the capsuloligamentous tissue of the shoulder; and a deep fascicle that is inserted in the caudal surface of the coracoid and the lateral aspect of its base, only revealed after careful removal of the superficial fascicle, and adjoins the humeral capsuloligamentous tissue beneath the insertion of the SScM tendon (star in B). The coracoglenoid ligament is present (asterisk in B), and is laterally prolonged towards the capsuloligamentous complex. Case 9 (C,D) shows two fascicles: the superficial one is broadly comparable to the one previously described (C); the deep fascicle (C,D) is a Y-shaped bundle that ranges from the lateral aspect of the coracoid base to the rotator interval (D) and the deep fascia of the SScM. Case 10 (E) is a good example of the canonical organization of the CHLam, with a superficial bundle that extends from the caudal surface of the coracoid to the rotator interval (cephalic and superficial to the SScM tendinous insertion) and broadly reaches the rotator interval, and a deep fascicle broadly extending from the lateral surface of the coracoid base (1), before fusing with the shoulder joint capsule underneath the SScM tendon. In case 11 (F,G) the deep fascicle of the CHLam is inserted almost in the posterior edge of the caudal surface of the coracoid limb, as well as in the anterior surface of the CHLpl (see dotted arrow in G), before reaching the shoulder joint capsule, as an accessory fascicle of the superior glenohumeral ligament (SGHL in G).
![Jfmk 10 00149 g003]()
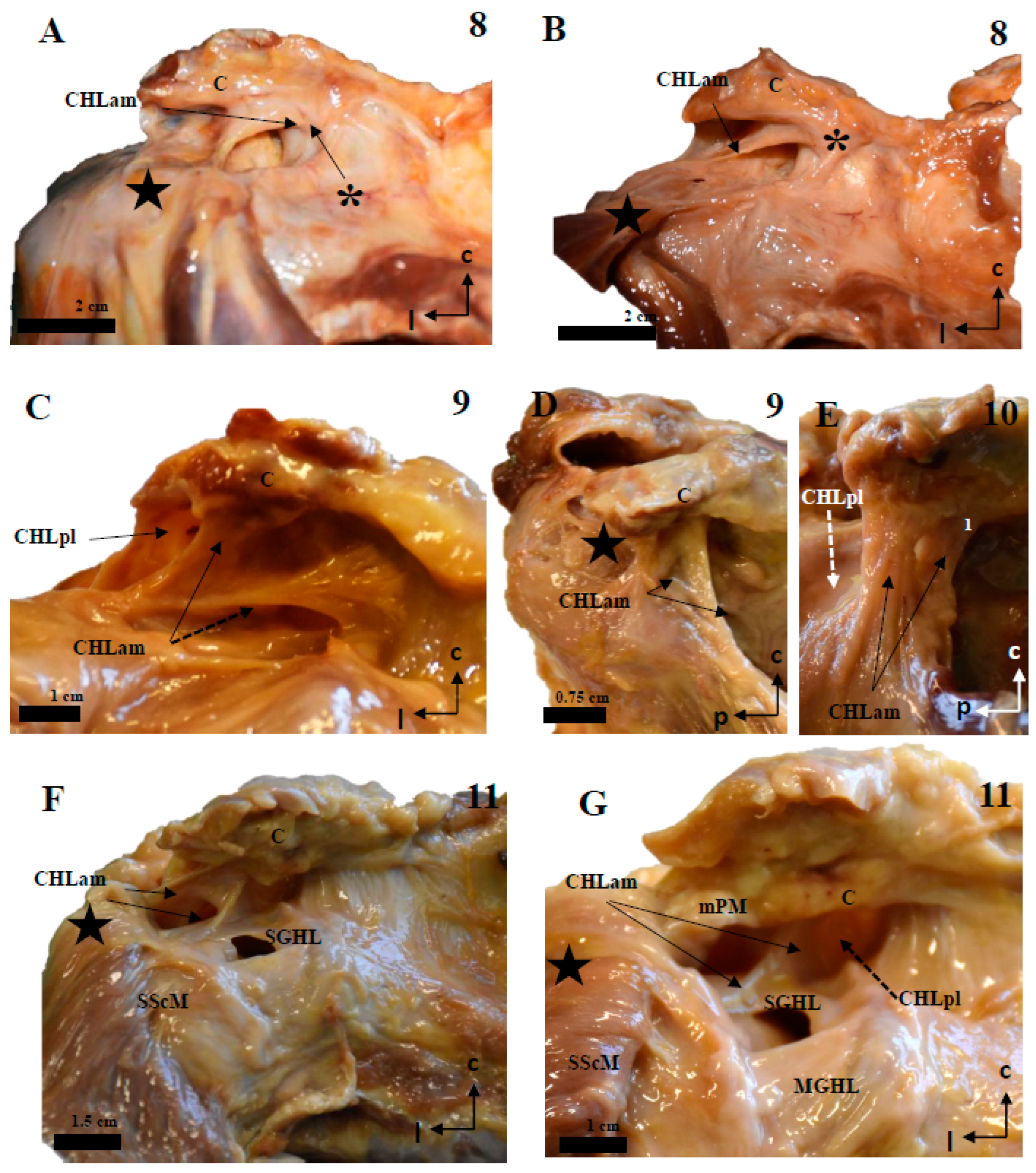
Figure 4.
Cadaveric dissections of the anterior column of the CHL in shoulders, as seen from the left side. (A) (case 12): The anterior and medial column of the CHL (CHLam) is inserted in the caudal surface of the coracoid process and reinforced both laterally (dotted arrow) and medially (arrow), being the medial reinforcement at the anterolateral aspect of the coracoid base, where it fuses with the coracoid insertion of a deep fascicle (black star). Laterally, the major superficial fascicle and the deep fascicle surround the insertion of the SScM tendon. (B): Case 13 shows a superficial fascicle inserted medially at the caudal surface of the coracoid process (and partially in the lateral aspect of the base); this fascicle is laterally inserted in the superficial fascia of the SScM. The deep fascicle (yellow in C) is inserted in the inferior space of the coracoid limb and the anterior surface of the CHLpl. Note the differences in the insertions of both the superficial (red) and deep (yellow) bundles. (D) shows a particular distribution of the anterior column of the CHL; the superficial fascicle blends with the superficial fascia of the SScM near its lateral insertion. In this lateral view of the coracoid process, it appears (arrow) as an intermediate bundle situated next to a particularly strong reinforcement of the edge (triangle), between the CHLam and the CHLpl and the deep fascicle of the anterior column, which is inserted in the anterolateral aspect of the coracoid base and blends laterally with the deep fascia of the SScM insertion (dotted arrow).
Figure 4.
Cadaveric dissections of the anterior column of the CHL in shoulders, as seen from the left side. (A) (case 12): The anterior and medial column of the CHL (CHLam) is inserted in the caudal surface of the coracoid process and reinforced both laterally (dotted arrow) and medially (arrow), being the medial reinforcement at the anterolateral aspect of the coracoid base, where it fuses with the coracoid insertion of a deep fascicle (black star). Laterally, the major superficial fascicle and the deep fascicle surround the insertion of the SScM tendon. (B): Case 13 shows a superficial fascicle inserted medially at the caudal surface of the coracoid process (and partially in the lateral aspect of the base); this fascicle is laterally inserted in the superficial fascia of the SScM. The deep fascicle (yellow in C) is inserted in the inferior space of the coracoid limb and the anterior surface of the CHLpl. Note the differences in the insertions of both the superficial (red) and deep (yellow) bundles. (D) shows a particular distribution of the anterior column of the CHL; the superficial fascicle blends with the superficial fascia of the SScM near its lateral insertion. In this lateral view of the coracoid process, it appears (arrow) as an intermediate bundle situated next to a particularly strong reinforcement of the edge (triangle), between the CHLam and the CHLpl and the deep fascicle of the anterior column, which is inserted in the anterolateral aspect of the coracoid base and blends laterally with the deep fascia of the SScM insertion (dotted arrow).
![Jfmk 10 00149 g004]()
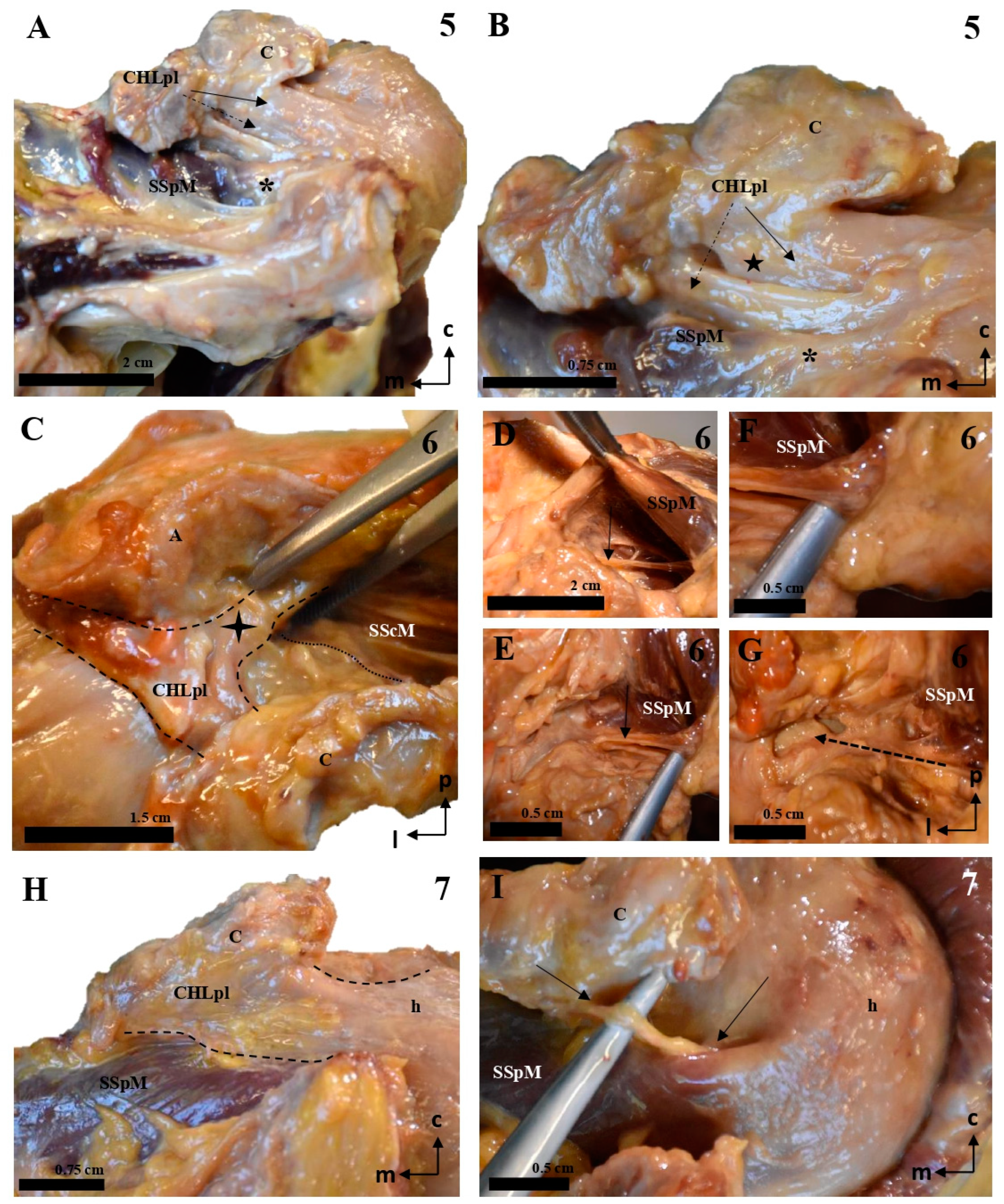
Figure 5.
Cadaveric dissections of the posterior column of the CHL. (A,B) (case 1): The posterior and lateral column of the CHL (CHLpl) is organized into two fascicles: a superficial one that is inserted in the caudal surface of the coracoid process (C) (arrow in A) and fuses with the fascial tissue overlying the SSpM tendon (star), before reaching the capsuloligamentous tissue of the shoulder; and a deep fascicle, bifurcated in this particular case, which is simply the posteromedial continuation of the superficial fascicle; its coracoid insertions allow it to blend into the capsuloligamentous tissue of the shoulder joint beneath the SSpM (dotted arrow in B). This organization is repeated in cases 2 (C), 3 (D–F, dotted lines representing the outline of the structure), and 4 (G–I). In all of them, the most lateral bundles of the CHLpl blend with the capsule underlying the SSpM (highlighted in (C) with a dotted line), and the most medial and posterior bundles of the CHL (E,F,I) reach the capsule under the same muscle. Additionally, case 4 shows an auxiliary deep fascicle (dotted arrow in I) that arises in the posterior aspect of the scapular notch (H). Note that in images (G,I), the SSpM (star) has been sectioned and displaced posterolaterally, while in images (E,F), its insertions have been respected.
Figure 5.
Cadaveric dissections of the posterior column of the CHL. (A,B) (case 1): The posterior and lateral column of the CHL (CHLpl) is organized into two fascicles: a superficial one that is inserted in the caudal surface of the coracoid process (C) (arrow in A) and fuses with the fascial tissue overlying the SSpM tendon (star), before reaching the capsuloligamentous tissue of the shoulder; and a deep fascicle, bifurcated in this particular case, which is simply the posteromedial continuation of the superficial fascicle; its coracoid insertions allow it to blend into the capsuloligamentous tissue of the shoulder joint beneath the SSpM (dotted arrow in B). This organization is repeated in cases 2 (C), 3 (D–F, dotted lines representing the outline of the structure), and 4 (G–I). In all of them, the most lateral bundles of the CHLpl blend with the capsule underlying the SSpM (highlighted in (C) with a dotted line), and the most medial and posterior bundles of the CHL (E,F,I) reach the capsule under the same muscle. Additionally, case 4 shows an auxiliary deep fascicle (dotted arrow in I) that arises in the posterior aspect of the scapular notch (H). Note that in images (G,I), the SSpM (star) has been sectioned and displaced posterolaterally, while in images (E,F), its insertions have been respected.
![Jfmk 10 00149 g005]()
Figure 6.
Cadaveric dissections of the posterior column of the CHL. (A,B) (case 5): The posterolateral column of the CHL (CHLpl) is inserted proximally in the posterior edge of the caudal surface of the coracoid process, and laterally, it fuses with the capsuloligamentous tissue of the shoulder joint capsule: its most lateral fibers are directed towards the rotator interval (solid arrow in A), where they superficially cover the lateral area of the SSpM tendon, while its most posterior fibers are directed deeply towards the tendon (dotted arrow in A). An auxiliary fascicle (dotted arrow in B) is inserted proximally in the posterior edge of the angle between the coracoid process and the coracoid base, and fuses with the capsuloligamentous tissue of the capsule (asterisk) underneath the SSpM tendon, albeit in a slightly more superficial plane than the posterior fibers of the main CHLpl (five-point star). (C–G) (case 6): almost all the fibers of the CHLpl are directed from the posterior edge of the inferior surface of the coracoid process (c) to the most superficial layer of the shoulder capsuloligamentous tissue (four-point star in C). Running parallel to the anterior/deep surface of the SSpM (D,E) appears an auxiliary fascicle that is proximally inserted in the posterior aspect of the scapular notch (F) and fuses with the deepest layer of the shoulder joint (G). Case 7 (H,I) follows a canonical pattern, with the most lateral fibers directed from the posterior edge of the inferior coracoid surface (c) to the rotator interval covering the insertion of the SSpM tendon (dotted line in H). A bundle of posteriorly inserted fibers envelops the SSpM tendon deeply (arrows in I).
Figure 6.
Cadaveric dissections of the posterior column of the CHL. (A,B) (case 5): The posterolateral column of the CHL (CHLpl) is inserted proximally in the posterior edge of the caudal surface of the coracoid process, and laterally, it fuses with the capsuloligamentous tissue of the shoulder joint capsule: its most lateral fibers are directed towards the rotator interval (solid arrow in A), where they superficially cover the lateral area of the SSpM tendon, while its most posterior fibers are directed deeply towards the tendon (dotted arrow in A). An auxiliary fascicle (dotted arrow in B) is inserted proximally in the posterior edge of the angle between the coracoid process and the coracoid base, and fuses with the capsuloligamentous tissue of the capsule (asterisk) underneath the SSpM tendon, albeit in a slightly more superficial plane than the posterior fibers of the main CHLpl (five-point star). (C–G) (case 6): almost all the fibers of the CHLpl are directed from the posterior edge of the inferior surface of the coracoid process (c) to the most superficial layer of the shoulder capsuloligamentous tissue (four-point star in C). Running parallel to the anterior/deep surface of the SSpM (D,E) appears an auxiliary fascicle that is proximally inserted in the posterior aspect of the scapular notch (F) and fuses with the deepest layer of the shoulder joint (G). Case 7 (H,I) follows a canonical pattern, with the most lateral fibers directed from the posterior edge of the inferior coracoid surface (c) to the rotator interval covering the insertion of the SSpM tendon (dotted line in H). A bundle of posteriorly inserted fibers envelops the SSpM tendon deeply (arrows in I).
![Jfmk 10 00149 g006]()
Figure 7.
The multi-layered structure of the humeral insertion of the CHL. (A): The most superficial layer corresponds to the CHLpl and its insertion in the rotator interval (arrow), which fuses itself with the most superficial tissue of the capsuloligamentous wall of the shoulder joint capsule and covers the LHBBT (discontinuous arrow). Its most posterior and medial fascicle follows a coracoacromial route and is, indeed, attached to the inferior surface of the coracoacromial ligament (sectioned) (five-point star in A,B). (B–D): The superficial layer is partially intermingled with a second layer extending between the superficial aspects of the rotator interval muscles, connecting their tendons; this layer originates (or at least attached to) both from the anteromedial and posterolateral columns of the CHL, and effectively represents most of what is classically understood as the CHL. (E,F): The deepest macroscopic layer is a similar bundle that extends between the deep fascicles (arrows) of both columns, therefore reinforcing the deep attachment of the muscular tendons at the boundaries of the rotator interval. The scale bars represent 1 cm.
Figure 7.
The multi-layered structure of the humeral insertion of the CHL. (A): The most superficial layer corresponds to the CHLpl and its insertion in the rotator interval (arrow), which fuses itself with the most superficial tissue of the capsuloligamentous wall of the shoulder joint capsule and covers the LHBBT (discontinuous arrow). Its most posterior and medial fascicle follows a coracoacromial route and is, indeed, attached to the inferior surface of the coracoacromial ligament (sectioned) (five-point star in A,B). (B–D): The superficial layer is partially intermingled with a second layer extending between the superficial aspects of the rotator interval muscles, connecting their tendons; this layer originates (or at least attached to) both from the anteromedial and posterolateral columns of the CHL, and effectively represents most of what is classically understood as the CHL. (E,F): The deepest macroscopic layer is a similar bundle that extends between the deep fascicles (arrows) of both columns, therefore reinforcing the deep attachment of the muscular tendons at the boundaries of the rotator interval. The scale bars represent 1 cm.
![Jfmk 10 00149 g007]()
Figure 8.
The multi-layered structure of the humeral insertion of the CHL. (A,B): The most superficial layer corresponds to the most lateral aspect of the CHL, where fibers around the apex of the V-shaped insertion along the caudal surface of the coracoid limb follow a superomedial-to–caudolateral route towards the rotator interval, before merging with the capsuloligamentous tissue of the shoulder in a superficial plane. (C,D): The main body of both the anterior and posterior columns of the CHL is inserted laterally in the rotator interval, partially overlapping (arrows) with the tendinous insertions of both the SScM (anterior column) and the SSpM (posterior column), effectively forming a single arch (dotted line in D) which links both muscles. The scale bars represent 1 cm.
Figure 8.
The multi-layered structure of the humeral insertion of the CHL. (A,B): The most superficial layer corresponds to the most lateral aspect of the CHL, where fibers around the apex of the V-shaped insertion along the caudal surface of the coracoid limb follow a superomedial-to–caudolateral route towards the rotator interval, before merging with the capsuloligamentous tissue of the shoulder in a superficial plane. (C,D): The main body of both the anterior and posterior columns of the CHL is inserted laterally in the rotator interval, partially overlapping (arrows) with the tendinous insertions of both the SScM (anterior column) and the SSpM (posterior column), effectively forming a single arch (dotted line in D) which links both muscles. The scale bars represent 1 cm.
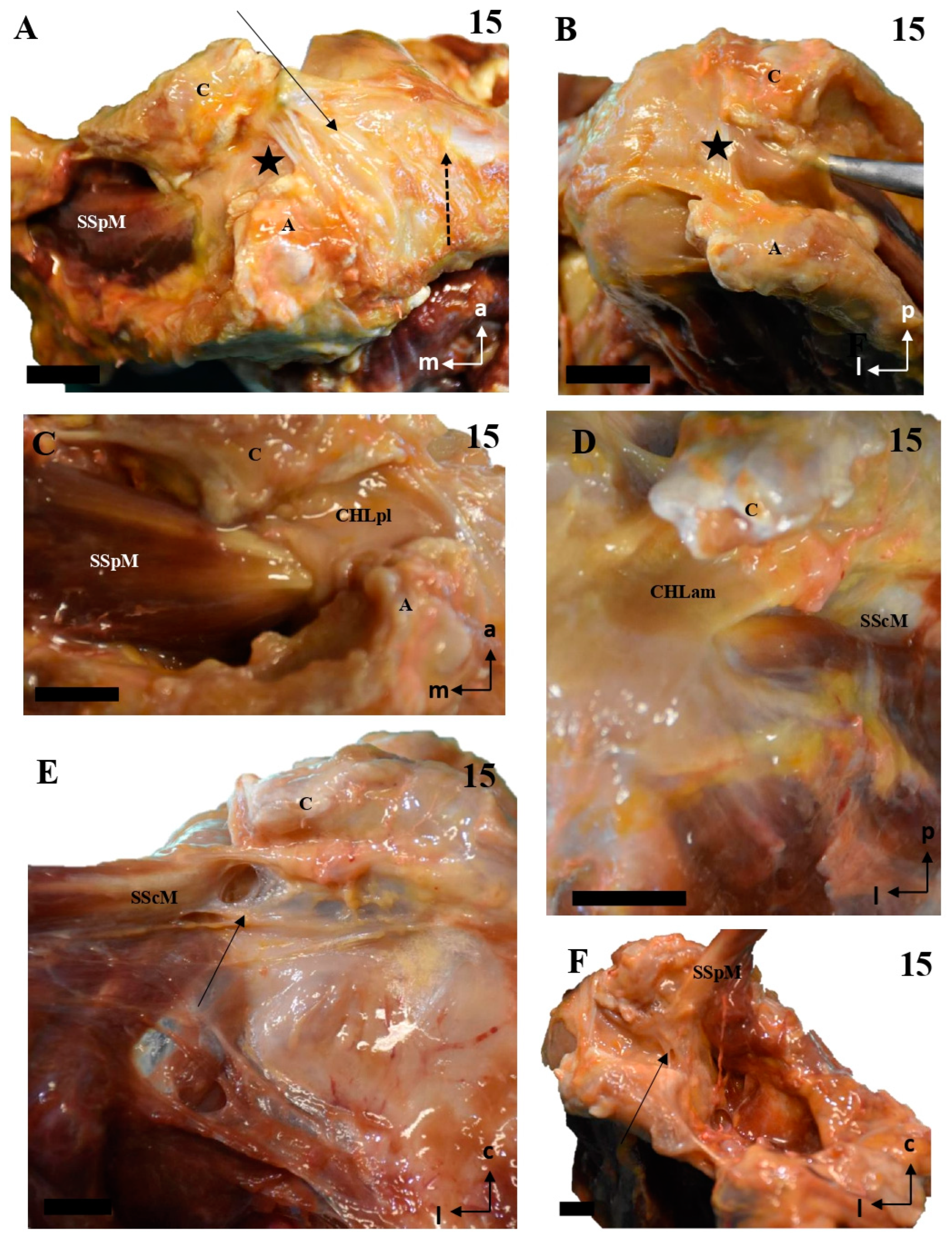
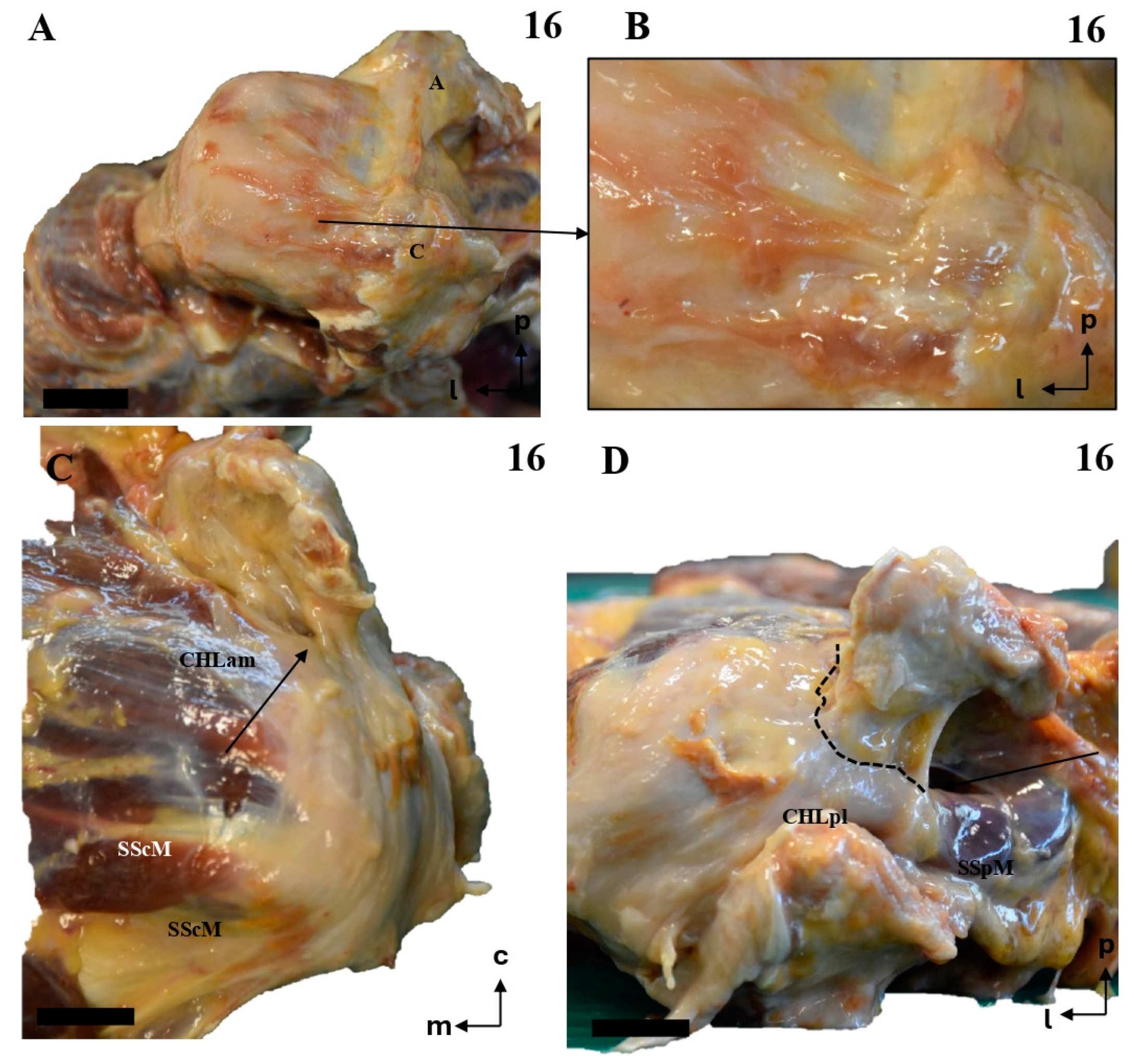
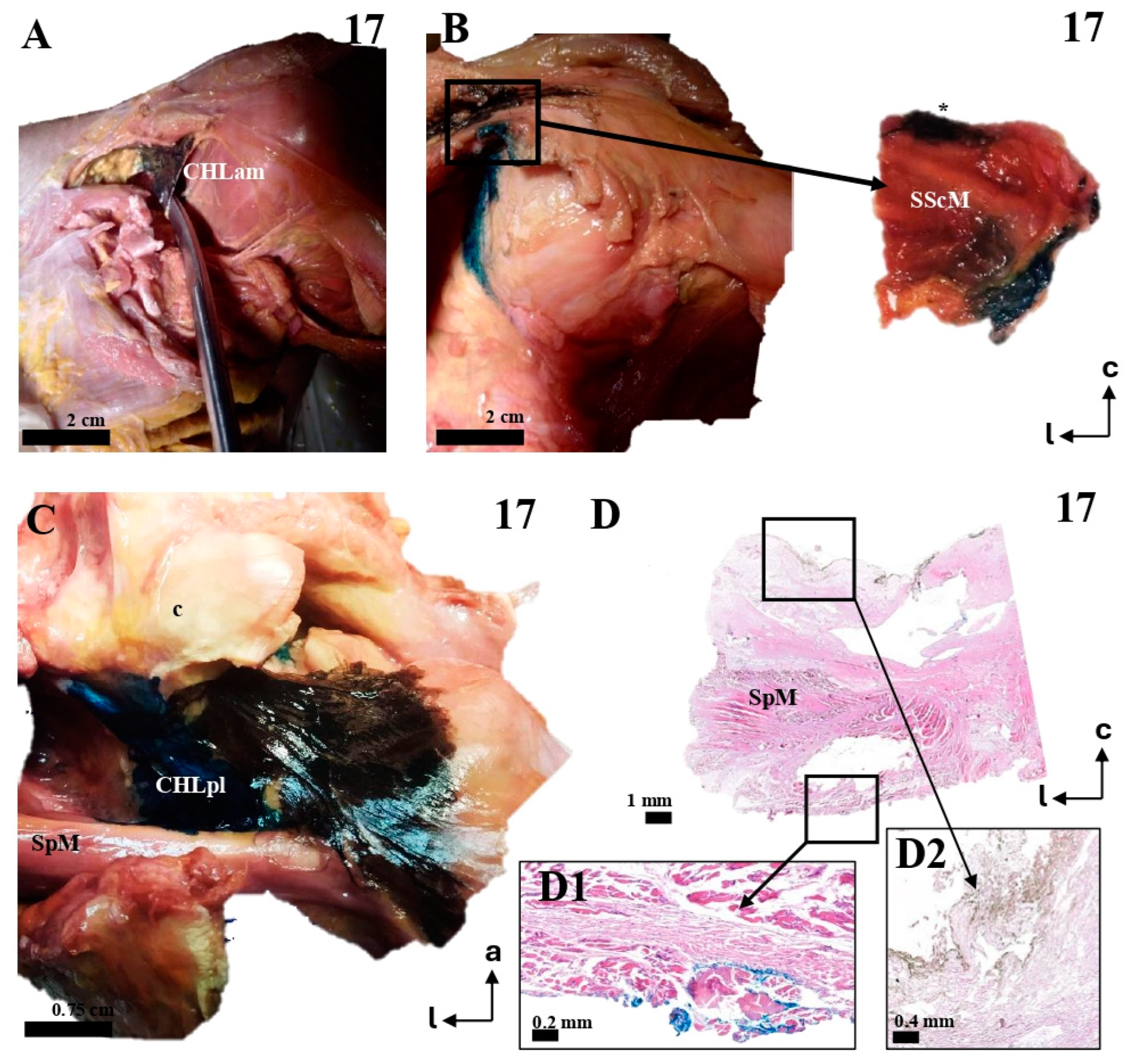
Figure 9.
The multi-layered insertion humeral insertion of the CHL. (A,B): A frontal view of the subscapular region, with the superficial fascicle of the CHLam inked in black (A) and the deep fascicle inked in blue (B). The detail shows a macroscopic picture of a section of the SScM, demonstrating the distribution of the fascicles along its superficial (asterisk) and deep surfaces. (C): a zenithal view of the coracoid process (c) and the suprascapular fossa, with the SpM displaced posteriorly in order to view the disposition of the posterior fascicles (superficial in black, see D2, and deep in blue, see D1) around its myotendinous insertion. (D): A histological picture of the deep (D1) and superficial (D2) fascicles of the CHLpl around the SpM lateral portion.
Figure 9.
The multi-layered insertion humeral insertion of the CHL. (A,B): A frontal view of the subscapular region, with the superficial fascicle of the CHLam inked in black (A) and the deep fascicle inked in blue (B). The detail shows a macroscopic picture of a section of the SScM, demonstrating the distribution of the fascicles along its superficial (asterisk) and deep surfaces. (C): a zenithal view of the coracoid process (c) and the suprascapular fossa, with the SpM displaced posteriorly in order to view the disposition of the posterior fascicles (superficial in black, see D2, and deep in blue, see D1) around its myotendinous insertion. (D): A histological picture of the deep (D1) and superficial (D2) fascicles of the CHLpl around the SpM lateral portion.
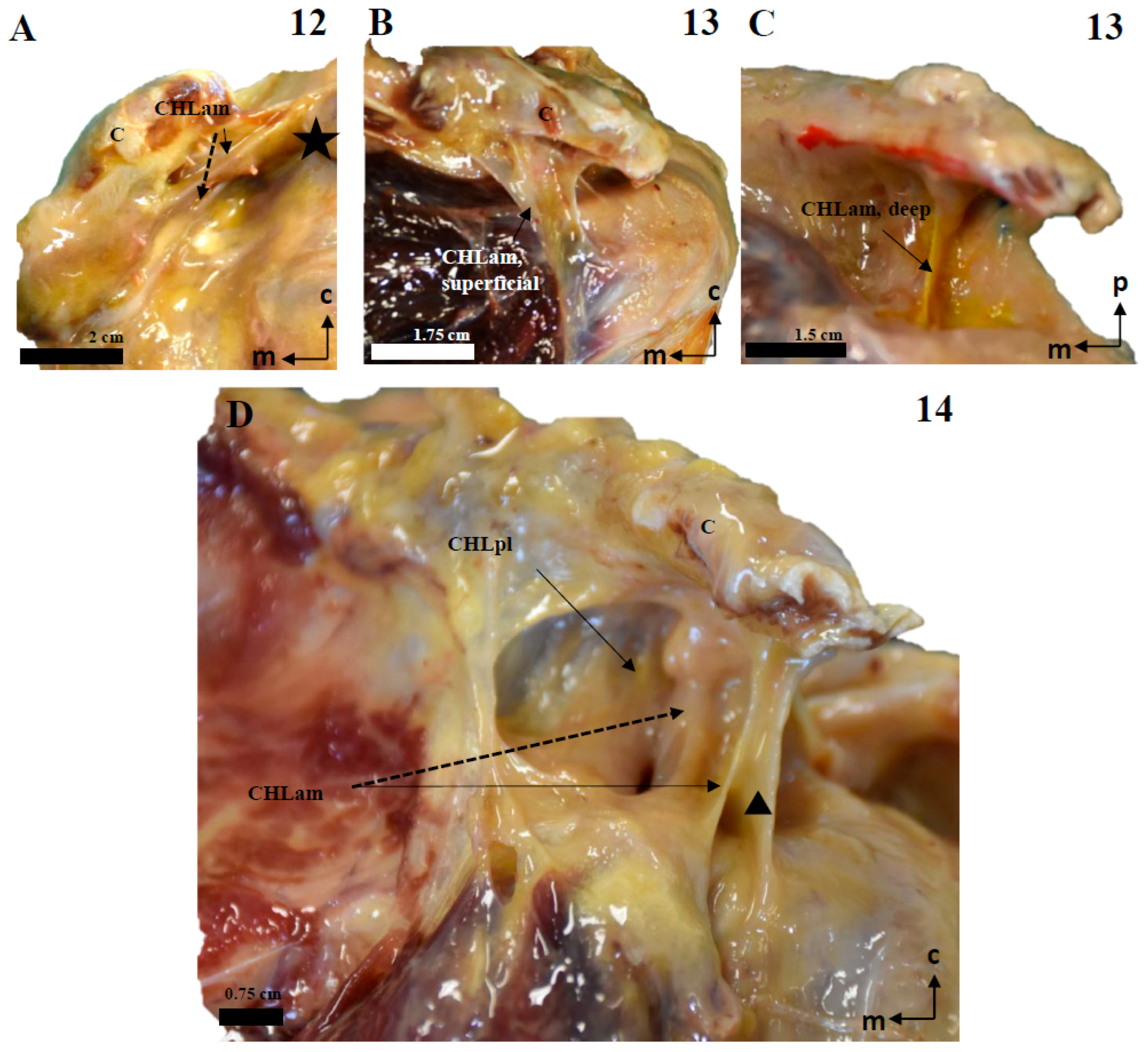
Figure 10.
The multi-layered structure of the scapular insertion of the CHL. (A): The insertion of the superficial fascicle (red) of the anterior column (CHLam) overlaps mediolaterally with the insertion of the deep fascicle (yellow), which occupies the medial and inferior aspect of the coracoid base (see B). Both fascicles of the posterolateral column (CHLpl) are inserted in the posterior edge of the coracoid process (B). The rectangle in A depicts the process of liberating the deep fascicle of the CHLam during the dissection. The scale bars represent 0.5 cm.
Figure 10.
The multi-layered structure of the scapular insertion of the CHL. (A): The insertion of the superficial fascicle (red) of the anterior column (CHLam) overlaps mediolaterally with the insertion of the deep fascicle (yellow), which occupies the medial and inferior aspect of the coracoid base (see B). Both fascicles of the posterolateral column (CHLpl) are inserted in the posterior edge of the coracoid process (B). The rectangle in A depicts the process of liberating the deep fascicle of the CHLam during the dissection. The scale bars represent 0.5 cm.
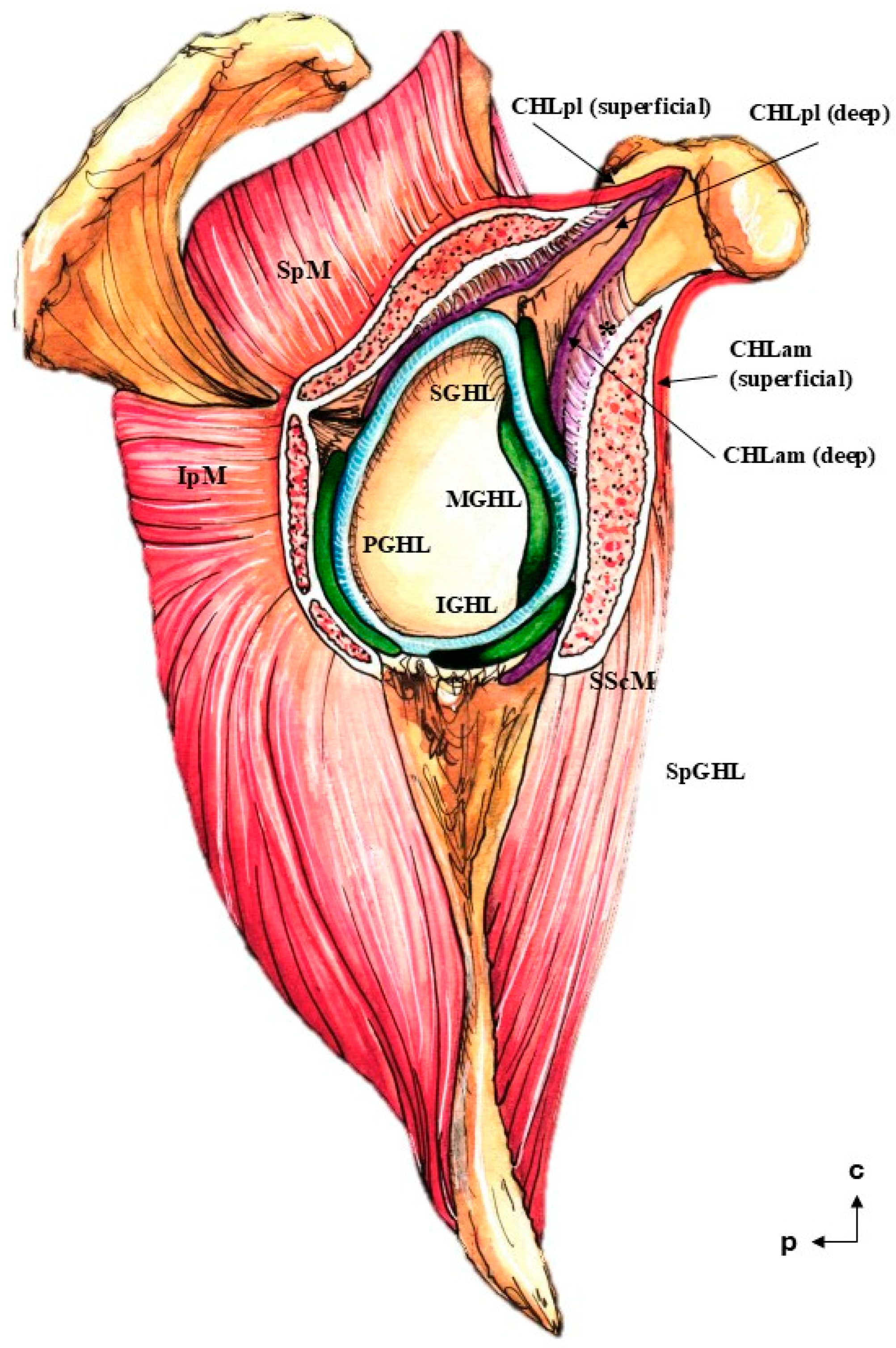
Figure 11.
The putative distribution of the reinforcement of the shoulder joint capsule. The most superficial plane is composed of the superficial fascicles of both the CHLam and the CHLpl (red). The intermediate level (purple) is formed by the deep fascicles of both columns of the CHL, as well as the spiral glenohumeralligament (SpCHL). The deepest level is formed by the canonical glenohumeral ligaments: superior (SGHL), middle (MGHL), anteroinferior (IGHL), and posteroinferior (PGHL). Note the clamp formed by the fascicles of the CHL around the subscapularis (SScM) and supraspinatus muscles (SpM). The relationship between the CHL and the infraspinatus muscle (IpM) is unclear as far as the authors are concerned. Regarding the deep column of the CHLam, its set of proximal insertions is variable, and only one typology is depicted; also note its double function/overlap as a suspensory ligament of the subscapular bursa (asterisk).
Figure 11.
The putative distribution of the reinforcement of the shoulder joint capsule. The most superficial plane is composed of the superficial fascicles of both the CHLam and the CHLpl (red). The intermediate level (purple) is formed by the deep fascicles of both columns of the CHL, as well as the spiral glenohumeralligament (SpCHL). The deepest level is formed by the canonical glenohumeral ligaments: superior (SGHL), middle (MGHL), anteroinferior (IGHL), and posteroinferior (PGHL). Note the clamp formed by the fascicles of the CHL around the subscapularis (SScM) and supraspinatus muscles (SpM). The relationship between the CHL and the infraspinatus muscle (IpM) is unclear as far as the authors are concerned. Regarding the deep column of the CHLam, its set of proximal insertions is variable, and only one typology is depicted; also note its double function/overlap as a suspensory ligament of the subscapular bursa (asterisk).
Table 1.
Linear measurements of the CHL insertions in centimeters. Note that the distal measurements do not represent true insertions, but linear measurements of the convergence between the fascicle of the CH and the capsuloligamentous complex of the shoulder. The fascicles of the posterior column are difficult to discriminate, and in many cases, separate measurements are not reported (‘Incl.’ for ‘included’). n.e in case 1 stands for not evaluated, as it was either non-existent or artifacted during dissection ‘None’ was reserved for a case (8) where some of the expected structures were clearly absent.
Table 1.
Linear measurements of the CHL insertions in centimeters. Note that the distal measurements do not represent true insertions, but linear measurements of the convergence between the fascicle of the CH and the capsuloligamentous complex of the shoulder. The fascicles of the posterior column are difficult to discriminate, and in many cases, separate measurements are not reported (‘Incl.’ for ‘included’). n.e in case 1 stands for not evaluated, as it was either non-existent or artifacted during dissection ‘None’ was reserved for a case (8) where some of the expected structures were clearly absent.
| Case | Anterior Column | Posterior Column |
|---|
| | Superficial | Deep | Superficial | Deep |
|---|
| | Proximal | Distal | Proximal | Distal | Proximal | Distal | Proximal | Distal |
|---|
| 1 | 1.2 | 1.4 | n.e | 0.7 | 2.2 | 0.8 | Incl | Incl |
| 2 | 1 | 2.2 | 0.5 | 0.4 | 1.18 | 2.23 | Incl | Incl |
| 3 | 1.6 | 1.3 | 0.45 | 0.3 | 1.25 | 1.1 | 0.2 | 0.2 |
| 4 | 0.3 | 0.5 | 0.5 | 0.56 | 1.75 | 1.5 | Incl | Incl |
| 5 | 1 | 1 | 0.7 | 0.3 | 1.1 | 0.3 | 0.6 | 0.4 |
| 6 | 1.2 | 0.8 | 0.5 | 0.6 | 1.7 | 1.5 | 0.3 | 0.4 |
| 7 | 1.2 | 1.6 | 0.6 | 1.5 | 2.3 | 2 | 0.6 | 0.2 |
| 8 | 1.5 | 1.3 | 0.7 | 1 | 1.7 | 1.3 | None | None |
| 9 | 1 | 1.1 | 0.6 | 0.5 | 1.3 | 1.2 | Incl | Incl |
| 10 | 0.7 | 1.2 | 1 | 0.9 | 1.6 | 1.6 | 0.9 | 0.9 |
| 11 | 1.4 | 1 | 0.9 | 0.7 | 1.6 | 1.4 | Incl | Incl |
| 12 | 1.8 | 0.8 | 1.6 | 0.9 | 1.4 | 1.6 | Incl | Incl |
| 13 | 1.4 | 1.2 | 0.7 | 1 | 2.8 | 1.55 | Incl | Incl |
| 14 | 1.5 | 1.1 | 0.5 | 0.6 | 1.7 | 1.2 | Incl | 0.7 |
| 15 | 1.4 | 1.8 | 0.8 | 0.3 | 1.8 | 1.6 | Incl | Incl |
| 16 | 1.5 | 1.7 | 0.6 | 0.5 | 2.1 | 1.2 | Incl | Incl |
| Total | 1.23 ± 0.37 | 1.25 ± 0.42 | 0.71 ± 0.29 | 0.67 ± 0.32 | 1.78 ± 0.45 | 1.38 ± 0.44 | 0.52 ± 0.27 | 0.46 ± 0.28 |