Abstract
The extent to which estradiol, progesterone, and testosterone influence mating behavior across the menstrual cycle is unclear. The Proactive and Responsive Mating Strategies Scales (PARMSS) were developed to separately examine two specific components of sexuality and were used here to test divergent hormonal associations. Preliminary psychometric data (N = 364 females) suggest that both scales consist of one factor and demonstrate strong psychometric properties (internal consistency, test–retest reliability, and construct and convergent validity). The PARMSS were used in a repeated-measures observational study to examine the relationships between changes in endogenous hormone levels and both proactive and responsive mating intentions with potential new short-term or long-term partners in healthy pre-menopausal participants (N = 38). At two points in their cycle, participants provided salivary hormone samples in the laboratory and reported the likelihood of engaging in proactive and responsive behaviors with men in photos and vignettes. Participants reported greater responsive than proactive intentions. Increases in estradiol and testosterone across the cycle were associated with increases in short-term mating intentions, particularly responsivity to potential short-term relationship partners. No associations were found for intentions that were proactive or that involved potential long-term partners or for progesterone. Changes in the three hormones explained changes in short-term responsive mating intentions (22% of the variance). The results suggest (a) cyclical changes in estradiol and testosterone are differentially associated with changes in responsive vs. proactive mating intentions and (b) context-dependent changes (i.e., short-term vs. long-term mating intentions and possibly relationship status). The findings require replication with larger and diverse samples.
1. Introduction
Research in behavioral endocrinology has been slow in understanding how hormones affect sexual behavior across the menstrual cycle [1,2], but recent meta-analyses suggest hormonal links to sexual desire and sexual functioning—e.g., [3,4]. Previous studies also suggest that mating preferences differ by menstrual cycle phase and context—e.g., [5], but few studies have directly examined whether endogenous estradiol, progesterone, and testosterone modulate mating tactics across the cycle. Hormonal mechanisms may differ depending on relationship status [6,7] or current intentions (e.g., short-term [ST] versus long-term [LT] relationship tactics) [5]. These contextual factors may help explain variability in mating strategies/patterns. Researchers have recommended that individual components of female sexuality be examined instead of the historical tendency to conceptualize it in a unitary or broad simplistic way (e.g., as sexual desire generally) [8,9,10]. These issues have likely limited research on female mating behaviors such that findings are often not directly comparable between studies, and different types of mating behaviors (e.g., proactive and responsive mating behavior) are put in the same categories. As a result, previous research may have failed to capture nuances in female sexual behavior. There is a need for studies examining the relationship between hormones, the menstrual cycle, and sexual behavior to explicitly define aspects of female mating behavior.
In 1976, Beach proposed three primary classifications for female sexual behavior: attractivity, proceptivity, and receptivity [11]. While primarily applied to animal behavior, this model of sexual behavior may have value for informing research on human sexuality (e.g., [12]). In animals, proceptive and receptive sexual behaviors are influenced by endogenous hormones [11,13], tend to co-occur during periods of estrus and, thus, peak fertility [11,13,14,15,16,17,18,19,20], and strongly suggest a role for gonadal hormones in nonhuman female sexual behavior. Animal research on proceptivity indicates stimulatory effects of estradiol [21,22,23], progesterone enhancing estrogenic effects [24,25,26,27], and stimulatory effects of androgens [21,28,29,30]. Research on receptivity indicates stimulatory effects of estradiol [20] that may be independent of androgens [30,31], stimulatory effects of progesterone that are not independent of estradiol [14,20,32], and both low-dose physiological range stimulatory effects of exogenous testosterone [24] and high-dose inhibitory effects [19,30].
Explicit behavioral definitions of human proceptive and receptive behavior rarely appear in studies of human female sexual behavior and are inconsistent across studies—e.g., [33,34,35]. Furthermore, no scale exists that measures both constructs in an equivalent comparable manner. Previous studies have utilized items that get at one of these constructs or that diffusely measure both together. The present study suggests and delineates two components of human female sexual behavior (i.e., proactive and responsive mating behaviors), and examines hormonal mechanisms underlying these mating tactics. These terms overlap, yet differ, from definitions of proceptive and receptive mating behaviors examined in animal research. Our terms relate to measurable human behavior that can be used when examining mating thoughts, intentions, and potentially physical actions. We define proactivity as behavior involving a potential mate and visible energy expenditure. Relevant behaviors would typically increase the likelihood of intercourse and range from approaching potential mates to initiating any type of sexual interaction. We define responsivity as consummatory behaviors that involve little to no energy expenditure and the absence of approach behaviors. These behaviors typically occur in response to mating efforts put forward by the potential partner. Examples of responsivity include agreeing to go on a date when asked or engaging in sexual activity when a potential mate initiated it. Some concepts and behaviors may not fit into either category because they are vague and may overlap with both constructs at different times (e.g., sexual desire) or may be both effortful and suggestive of behavior signaling responsivity (e.g., going out to potentially meet a mate, dressing in a “sexy” way). Our differentiation between responsive and proactive mating tactics is similar to Basson’s (2000) proposal that female sexual responses can be either responsive/triggered or spontaneous [36]. Responsivity is more reactive, and proactivity is more spontaneous or self-initiated. Proactivity and responsivity may differ qualitatively and quantitatively and likely represent different mating tactics that may be adaptive in different contexts. There is a need for psychometrically sound scales that measure these constructs separately and to examine hormonal mechanisms involved in these two sexual strategies.
Previous human menstrual cycle research has not explicitly examined whether proactive and responsive mating tactics/behaviors change across the cycle but has largely focused on mate preferences and sexual desire/interest [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. For example, some studies suggest that the frequency of proactive behaviors such as flirtation and female-initiated sexual activity peaks around ovulation [38,39,40,41,42], when circulating estradiol and testosterone levels are highest [43,44]. Around ovulation, some studies report a greater frequency of total and self-initiated sexual encounters [40,45,46] and opportunistic mating (i.e., “trading up” from a primary partner in favor of a more attractive mate with better resources) [47]. Further, some large-scale studies and meta-analyses [5,42] suggest changes in reproductive hormones may affect mating intentions.
Only a few studies appear to have utilized hormonal assay data to examine associations between endogenous hormones and proactive behavior. Bancroft and colleagues found that higher free testosterone (FT) was associated with reported increases in the frequency of sexual initiation and more permissive sexual morality towards extra-pair relationships, though FT was not associated with their measure of proceptivity/proactivity, and the authors did not control for other hormones [33]. Marcinkowska and colleagues found that neither average levels nor within-subject changes in estradiol or progesterone across the cycle significantly predicted sexual initiation [40]. However, a change in estradiol was a positive predictor and a change in progesterone was a negative predictor of sexual desire. Further, although wearing red does not fit clearly within our definitions of either proactivity or responsivity, Blake and colleagues found no robust association between the estradiol-to-progesterone ratio and the likelihood of wearing red [34]. While a number of studies examined associations between hormones and desire or sex drive across the cycle (e.g., [57]), more research is needed to examine relationships between estradiol and progesterone and female sexual proactivity as defined above.
In terms of responsivity, one study examined the relationship between endogenous hormone levels and female sexual responsivity, although they did not use this terminology [58]. Estradiol was positively associated with self-reports of extra-pair desire, and progesterone was positively associated with in-pair sexual desire across the cycle. In contrast, another study found a negative association between progesterone and general sexual desire [57]. No studies explicitly looked at testosterone and sexual responsivity in naturally cycling individuals. However, cycle phase was not associated with sexual responsivity to sexual stimuli or with sexual activity in naturally cycling females [59].
Overall, the limited research on hormonal associations with female sexual proactivity and responsivity suggests that estradiol and testosterone may be positively associated with general female mating intentions. The influence of progesterone on proactive mating tactics is less clear, with some hormonal studies suggesting an excitatory role in proactive mating tactics in partnered females (e.g., [58]), whereas others (e.g., [60]) suggest an inhibitory role or no role. There is a gap in terms of studies that explicitly examine if changes in estradiol, progesterone, and testosterone differentially affect proactive and responsive mating behavior. This may be related to there being a gap in measurement tools that reliably differentiate aspects of female sexuality. Such research is important to understand the effects of hormonal fluctuations within women.
There is a need to consider individual differences in hormonal sensitivity, and not just cross-sectional hormone levels, when examining relationships between hormones and behavior—e.g., [61,62]. This need has been highlighted in research on female sexuality [10,61,63]. Examining the extent to which hormonal change across the cycle within participants is associated with concurrent change in sexual behavior using a within-subjects design is one way to consider hormone levels while controlling for some aspects of both hormonal sensitivity and individual differences in hormonal change. Recent studies suggest that changes in hormone concentrations may be most predictive of fluctuations in psychological variables such as stress or mood, including premenstrual symptoms—e.g., [40,64]. Basic science research suggests mechanisms whereby pulsatile steroid hormone release (i.e., initial hormonal change vs. continuous high or low hormone levels) can activate time- and rate-dependent mechanisms that affect gene transcription and behavior [64]. Thus, the present study examined how changes in hormones across the menstrual cycle are associated with changes in mating tactics (proactivity and responsivity) with new potential mates in four different contexts (ST vs. LT relationship contexts and written scripts versus visual pictures of the men). Examining mating intentions with potential new mates, as opposed to intentions in LT relationships, allows measurement in everyone regardless of relationship status, relationship interest, or attractiveness. Given that estradiol and testosterone tend to covary together around ovulation, analyses with statistical control for other hormones were also conducted.
The aims of the present paper were (a) to present information and initial psychometric data on the development of a measure of proactive and receptive mating intentions and (b) to examine whether changes in intentions across the menstrual cycle are associated with changes in estradiol, progesterone, and testosterone. We hypothesized that female mating intentions with new or unfamiliar potential mates are positively associated with estradiol and testosterone and negatively associated with progesterone. No directional hypotheses were made about relationships between hormones and mating intentions as a function of the different contexts.
2. Materials and Methods
2.1. Participants
Participants were recruited within a larger project examining “hormones and sociosexuality” [65]. An initial sample of 629 completed a screening questionnaire, 364 participants met inclusion criteria for the larger study and completed at least one testing session, and 310 completed both sessions online or in the laboratory. Psychometric analyses on the mating intentions measures were completed using data from those who completed one testing session (N = 364). Participant flow is reflected in the EQUATOR STROBE flow chart in Figure 1. Those selected to participate in the two sessions across their menstrual cycle were of reproductive age (i.e., 16 to 44 years); had not had an abortion/miscarriage or used emergency contraception in the past three months; were neither pregnant, in the post-partum period, nor menopausal; had some attraction to men; and completed questions to assess these eligibility criteria. Overall, 47 participants living in northwestern Ontario, Canada participated in the laboratory portion of the present two-session cross-sectional observational study and met inclusion criterion [i.e., a regular menstrual cycle and free-cycling (not using hormonal contraceptives) and, thus, were eligible to provide saliva samples for hormone assay]. For inclusion in these analyses, participants were further screened with the following five post-hoc exclusion criteria: (1) missing hormone data at one of the two time points (n = 8); (2) no sexual attraction to men (n = 1); (3) pregnant (n = 0); (4) lactating (n = 1); and (5) menopausal (n = 0). As the visual stimuli included photos of men, and this study examined hormonal mating mechanisms from an evolutionary perspective (i.e., with reproduction as the goal), all participants had menstrual cycles and some attraction to men.
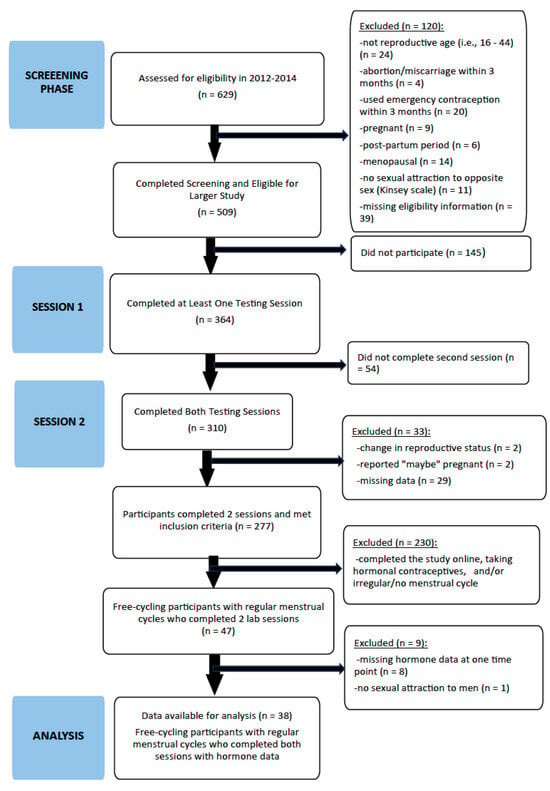
Figure 1.
EQUATOR STROBE participant flow diagram. Note: Excluded participants at each stage are reflected by underlined text.
The final sample included 38 female participants (assigned female at birth) recruited from a university campus and the local community who completed the screening portion of the larger study and attended two laboratory sessions between October 2012 and August 2014. They ranged in age from 18 to 41 (M = 22.62, SD = 5.36) and were of predominantly Caucasian/European descent (76.3%). Fifteen (39.5%) participants were single, 15 (39.5%) were in a relationship, and 8 (21.1%) changed relationship status over the course of the study.
2.2. Measures
2.2.1. Demographics
Demographic information was collected within a screening questionnaire (e.g., ethnicity, age, relationship status, menstrual cycle regularity, sexual orientation, sociosexuality). A menstrual cycle item developed in our lab assessed cycle regularity (see Appendix A), with the last two responses reflecting a regular cycle. The Kinsey scale was used to measure sexual orientation ranging from 1 (exclusively heterosexual) to 6 (exclusively homosexual) [66] as well as the option of asexual. It was used when determining inclusion criteria. The Multidimensional Model of Sociosexuality (MDSOI) [67] assesses sociosexuality with three subscales (i.e., ST mating orientation (STMO), LT mating orientation (LTMO), and sociosexual behavior), reflecting individual differences in motivations to engage in both types of sexual/romantic relationships, separately from actual behavior. Internal consistencies have been found to range from 0.83 to 0.95 [67]. Scores were examined as potential covariates and used to examine construct validity.
2.2.2. Proactive and Responsive Mating Strategies Scales (PARMSS)
The PARMSS were developed in our lab by the third and fourth authors and include two scales that measure proactive and responsive mating intentions in a theoretical encounter with a new potential mate using two similar sets of eight items (see Appendix B). Each pair of items differs only in terms of the type of mating intentions: (1) proactive (e.g., asking a person for their phone number) or (2) responsive (e.g., giving a person your phone number if asked). The PARMSS utilize a common metric across proactive and responsive behaviors, posing the same questions in a proactive manner (i.e., How likely would you be to buy him a drink?) and a responsive manner (i.e., How likely would you be to allow him to buy you a drink?). Items are rated on a 9-point scale from 1 (not at all likely) to 9 (extremely likely). In order to capture current hormonal context, participants were asked to complete the ratings based “on how you feel now as well as your feelings and behavior in the past 48 h (i.e., over the past 2 days)”. The scales can be used with photo or video stimuli or vignettes. Initial psychometric work was completed in two dissertations [65,68].
Test development involved first specifying and defining the two domains as discussed above. Next, item generation involved both deductive and inductive methods to ensure content validity. A literature review was performed, and items in existing scales and studies were inspected. Next, item generation took place along with discussion and review with four researchers in the area. Independent review and discussion focused on ensuring items fit with scales and that no salient content was missed. Items were then pre-tested on members of the laboratory to again ensure that the items reflected the domains of interest, the response options were meaningful, and salient items had not been missed. Respondents verbalized their mental process when providing answers. As reported below, cross-sectional data were collected for exploratory factor analyses and possible item reduction, factors were extracted, and eigenvalues and inter-item and item-total correlations were examined. Evidence of one factor per scale was considered as support for content validity. Internal consistency (Cronbach’s alpha) estimates and test–retest reliability data were calculated. Finally, initial estimates of concurrent and divergent validity were calculated by looking at correlations with measures of ST and LT mating orientation, previous sexual behaviors, masturbation frequency, and history of infidelity.
2.2.3. Mating Vignettes
The PARMSS were completed for three contexts (i.e., with a potential ST relationship vignette, with a potential LT relationship vignette, and with photographs of attractive men; see vignettes in Appendix C). The two vignettes were adapted from previous research [69]. The photographs (n = 4) of men were selected based on high ratings of attractiveness in a previous study [68]. Participants were asked to read each vignette or look at each photo and then respond to the 16 PARMSS items.
For each of the three contexts (the two vignettes and the picture-rating task), proactivity and responsivity scores were calculated by aggregating the eight relevant items. Thus, there were six main scores: (1) ST proactivity, (2) LT proactivity, (3) picture proactivity, (4) ST responsivity, (5) LT responsivity, and (6) picture responsivity. Higher scores reflect greater intentions to engage in the particular mating behavior. Six higher level scores were also calculated: total proactivity (ST proactivity + LT proactivity); total responsivity (ST responsivity + LT responsivity); total picture mating intentions (picture proactivity + picture responsivity); total ST mating intentions (ST proactivity + ST responsivity); total LT mating intentions (LT proactivity + LT responsivity); and total PARMSS mating intentions (all six main scale scores aggregated).
2.2.4. Hormone Assays
Saliva samples were collected during two laboratory sessions. Participants were asked to refrain from eating, exercising, smoking, brushing their teeth, wearing chapstick/lipstick, or drinking anything but water for one hour prior to attending the lab session. Prior to providing the sample, participants rinsed their mouths with chilled water. Participants were provided with verbal instructions to fill the test tube with roughly 2 mL of saliva over the course of completing the laboratory questionnaire. Samples were collected in 10 mL polypropylene test tubes and stored in a laboratory freezer at no less than −34 °C until shipment to a Salimetrics laboratory at Penn State University for immunoassay with the Salimetrics® 17β-Estradiol, Progesterone, and Testosterone Enzyme Immunoassay Kits. Saliva samples were immunoassayed for estradiol, progesterone, and testosterone at sensitivities of 0.1, 5, and 1 pg/nL, respectively. Intra-assay coefficients of variation (CV) were 3.39% (estradiol), 2.02% (testosterone), and 4.28% (progesterone). Inter-assay CV ranges were 5.12%, 5.69%, and 3.51%, respectively. All participants provided a saliva sample in both lab sessions. Testosterone could not be assayed in one sample because the quantity of saliva was not sufficient.
2.3. Procedure
The project was part of a larger study [68] that received approval from the Lakehead University Research Ethics Board, and biosafety approval was obtained for all procedures involving saliva collection. The design best fits with a repeated-measures observational study. Questionnaire data were collected via Survey Monkey, a secure online database, and enhanced SSL security encrypted responses. The EQUATOR STROBE Guidelines for reporting observational studies were followed in the report of this study [70].
2.3.1. Recruitment and Screening
Potential participants were directed to an online screening questionnaire. Participants were then contacted to schedule two lab sessions.
2.3.2. Lab Sessions: Time 1 and Time 2
Laboratory sessions were scheduled between 8:30 and 10:30 am to control for diurnal fluctuation of hormones and sexual interest. Measures of menstrual cycle phase were initially used to schedule the two laboratory sessions at two specific cycle phases (i.e., the periovulatory and luteal phases) using backwards counting or reverse counting and in a counterbalanced manner. An attempt was made to schedule sessions to correspond with days 12 (−17) for the periovulatory phase (i.e., the estimated highest fertility day) and either 22 (−7) or 26 (−3) for the premenstrual phase of the estimated menstrual cycles to capture both the early and late premenstrual luteal phase. Further, LH testing was used to attempt to schedule periovulatory sessions on days 0 or +1 from the LH surge, with an acceptable range being two days before the LH surge and within three days after the LH surge (i.e., −2 to +3 when 0 represents the day of the positive LH test). Volunteers were provided with a kit of at least five LH strips and instructions. If they obtained a positive result more than two days before their scheduled periovulatory laboratory session, their appointment was changed to occur within two days of the positive test. These phases were targeted because they differ in conception likelihood; patterns of sexual functioning; as well as relative hormonal concentrations of estradiol, progesterone, and testosterone. However, participant data were included in the present study as long as they had data at two time points, regardless of cycle phase, given the goal of examining a full range of hormonal variability and how hormonal change is associated with within-subject mating intentions. For both lab sessions, participants provided a saliva sample and completed the PARMSS for the three contexts (i.e., the ST and LT vignettes and the picture-rating task).
2.3.3. Statistical Analyses
Change scores were computed between the lab sessions (Time 1 minus Time 2) for the 3 hormones (estradiol, progesterone, and testosterone) and the 12 PARMSS scores. The relationships between change in hormones and change in PARMSS scores were examined using: (a) Pearson correlations, (b) partial correlations where changes in the other two hormones were statistically controlled, and (c) linear regression analyses with three predictor variables (changes in estrogen, progesterone, and testosterone) where appropriate. The latter two sets of analyses were run with the understanding that hormones change across the cycle in a correlated manner such that it may be difficult to capture the effects of one hormone independently from the others.
To explore potential covariates, initial correlations and t-tests examined relationships between the change scores and age, sociosexuality, sexual orientation, and relationship status. Sociosexuality, sexual orientation, and age were not associated with changes in any of the PARMSS scores or hormone levels (all p < 0.05). Thus, these variables were not used as covariates. However, changes in 10 of 12 PARMSS full and subscale scores differed as a function of relationship status, with singles showing less change or variability in their mating intentions between sessions compared to partnered participants (e.g., total responsivity change: single M = 0.56, SD = 0.98; partnered M = 0.82, SD = 1.71; t(28) = 2.67, p = 0.013). Thus, while subsamples were small, significant effects were followed up with exploratory analyses on single versus partnered participants to examine whether the relationship between hormones and mating intentions differs in these two contexts.
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), versions 26 and 29.020. The alpha level was set at p < 0.05. Given the large effect sizes in the current project, it was deemed appropriate to designate probability levels between 0.05 and 0.07 as non-significant trends. Pearson correlation effect sizes were interpreted as per Cohen’s (1988) recommendations (0.10 = a weak or small effect; 0.30 = a moderate effect; 0.50 or larger = a strong or large effect) [71]. Prior to each of the main analyses, the data were screened for errors and missing values, and distributions were examined for normality and univariate outliers (±z ≥ 3.29) [72]. Normality was assessed visually and statistically (skewness divided by the standard error of skewness <3; kurtosis divided by the standard error of kurtosis <3). Missing values were missing completely at random and occurred for less than 5% of values. They could not be replaced, as the scales had less than 10 items, and replacement would violate the 10% rule [72]. Thus, pairwise deletion was used, and sample sizes for the primary correlations ranged from 27 to 38. Statistical test assumptions were examined, and violations were noted. Data screening revealed two outliers (one for total PARMSS change score and one for progesterone change score). Relevant analyses were run both with and without outliers. Taking into consideration that these reflect real within-subject change scores, analyses based on the full sample are reported because there were no substantive differences in results when the outliers were removed. As the main analyses in this study identified significant medium-large correlations ranging from 0.41 to 0.48, we conducted a post-hoc power analysis with these effect sizes, alpha (two-tailed) = 0.05, and power = 0.80, using an online sample size calculator [73]. The required sample size to identify these effect sizes ranged from 31 to 44. Our sample sizes of 27 to 38 overlap the required sample range to identify these effect sizes, but our study is underpowered to identify small or medium effect sizes (i.e., correlations below 0.44).
3. Results
3.1. Factor Analyses, Reliability, and Validity of the PARMSS
Data for factor analyses and reliability and validity tests were collected within two dissertations [65,68]. Given the hypothesis of one factor per scale and the reasonably large number of items per factor (eight), the sample size (N = 364) was more than adequate for factor analysis. Exploratory factor analyses were completed here for data collected in five contexts (ST and LT vignettes, three photos) separately for both the proactive and responsive scales (see Table 1 and Table 2). Both scree plots and the eigenvalue >1 criterion were used to determine the number of factors to extract [74]. All factor analyses indicated that the proactive scale consisted of only one factor, as did the responsive scale, contributing content validity evidence. The Kaiser–Meyer–Olkin (KMO) measure verified the sampling adequacy for all 10 factor analyses, and KMO ranged from 0.875 to 0.912, which is considered great to superb [74]. For the proactive scale (see Table 1), across the five contexts, the eigenvalues for factor 1 ranged from 5.16 to 5.98, all items loaded highly on factor 1 (factor loadings ranged from 0.671 to 0.924), and the factor accounted for 64.47% to 74.80% of the variance. Similarly, across the five contexts, the responsive scale (see Table 2) had eigenvalues for factor 1 that ranged from 5.41 to 5.85, all items loaded highly on factor 1 (factor loadings ranged from 0.716 to 0.929), and the factor accounted for 67.56% to 73.10% of the variance.

Table 1.
Proactive mating intentions scale: item factor loadings, eigenvalues, and internal consistency across five contexts.

Table 2.
Responsive mating intentions scale: item factor loadings, eigenvalues, and internal consistency in five contexts.
Internal consistency has been examined in two projects using Cronbach’s alpha. There was evidence of good internal consistency: alpha = 0.92 for both the proactive (N = 64) and responsive (N = 63) scales across vignettes and photos [68]. In the present study, the proactive and responsive scales demonstrated good internal consistency across contexts for both proactive (0.91 to 0.95) and responsive (0.93 to 0.95) scales (N = 364) (see Table 1 and Table 2). In the first study, proactive and responsive scales had good test–retest reliability with vignettes [r(45) = 0.76, p < 0.01 and r(45) = 0.78, p < 0.01, respectively] and photos [both r(45) = 0.88, p < 0.01] over a mean of 23.15 (SD = 13.17) days [68]. In the present study, test–retest reliability ranged from r = 0.76 to 0.77 for the proactive scale and r = 0.80 to 0.82 for the responsive scale over a mean of 22.30 days (SD = 41.49) days (N = 295) (see Table 1 and Table 2). It should be noted that these test–retest reliability coefficients are likely underestimates since participants were purposely assessed at different menstrual cycle phases in both studies [65,68].
The mean responsive scale scores were significantly higher than the proactive scale scores in both ST [M = 31.36, SD = 17.03 vs. M = 21.88, SD = 13.90; t(342) = 19.628, p < 0.001] and LT [M = 39.74, SD = 20.44 vs. M =31.17, SD = 19.47; t(349) = 18.60, p < 0.001] vignette contexts. Proactive and responsive scales were also highly correlated [r(343) = 0.85, p < 0.01]. Thus, females who report more proactive mating intentions also generally report being more responsive to the sexual/romantic advances of new men.
The PARMSS show preliminary evidence of convergent and divergent validity [68]. The proactive and responsive scales were positively correlated with ST mating orientation [r(60) = 0.42, p < 0.01; r(60) = 0.49, p < 0.01, respectively] but not LT mating orientation [r(60) = 0.15, p = 0.27; r(60) = 0.12, p = 0.36, respectively] or previous sexual behavior [r(52) = 0.21, p = 0.142; r(52) = 0.18, p = 0.198, respectively] scales from the MDSOI [67]. As the PARMSS were designed to measure mating intentions in new or potential relationships, it is not surprising that they provide information on mating motivations specific to potential ST relationships. The proactive and responsive scales were not related to masturbation frequency [r(50) = 0.13, p = 0.372; r(50) = 0.02, p = 0.867, respectively], suggesting that they measure something other than sex drive (divergent validity). While the proactive scale was not related to a history of infidelity [r(51) = 0.11, p = 0.46], the responsive scale was positively associated [r(51) = 0.34, p = 0.016], suggesting that responsivity is more relevant to extra-pair sex than proactivity.
3.2. Descriptive Data
The absolute values of hormonal change between the two testing sessions were as follows: estradiol (M = 0.73 pg/mL, SD = 0.58), progesterone (M = 94.44 pg/mL, SD = 109.04), and testosterone (M = 16.00 pg/mL, SD = 11.69). Absolute values of the mating intention change scores were as follows: ST proactivity (M = 0.83, SD = 0.80), LT proactivity (M = 1.64, SD = 1.65), picture proactivity (M = 0.74, SD = 0.81), ST responsivity (M = 1.20, SD = 1.25), LT responsivity (M = 1.34, SD = 1.54), picture responsivity (M = 0.69, SD = 0.75), total proactivity (M = 1.00, SD = 1.07), total responsivity (M = 1.00, SD = 1.12), total picture (M = 1.43, SD = 1.52), total ST (M = 1.94, SD = 1.91), total LT (M = 2.91, SD = 3.17), and total PARMSS (M = 1.01, SD = 1.16).
3.3. Correlations and Partial Correlations
3.3.1. Estradiol
Correlations and partial correlations between change scores for estradiol and change scores for the twelve PARMSS scores for the full sample are found in the left panel of Table 3 (see Table 3). For the full sample, a change in estradiol level was moderately positively correlated with a change in ratings of ST responsivity (r = 0.48, p = 0.006, n = 32) and a change in total ST mating intentions (r = 0.44, p = 0.013, n = 32) (see Figure 2). Partial correlations did not reveal any unique effects of estradiol that were independent of other hormonal changes.

Table 3.
Correlations (and partial correlations controlling for change in the other two hormones) between changes in each hormone (estradiol, progesterone, and testosterone) and changes in mating intention scores on the Proactive and Responsive Mating Strategies Scales (PARMSS) across contexts for all participants across the menstrual cycle a.
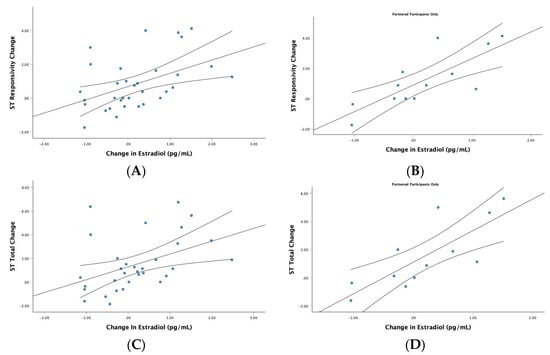
Figure 2.
Change in estradiol and female short-term (ST) mating intentions. Scatterplots reflect correlations between changes in estradiol and changes in ST mating intentions across the menstrual cycle. The top row reflects these correlations for responsive mating intentions when evaluating men for a potential ST relationship in (A) the full female sample (r = 0.48, p = 0.006, n = 32), and (B) partnered participants (r = 0.81, p = 0.002, n = 12). The bottom row reflects bivariate correlations for all ST mating intentions when evaluating men for a potential ST relationship for (C) the full female sample (r = 0.44, p = 0.013, n = 32), and (D) partnered participants (r = 0.79, p = 0.002, n = 12). Each graph includes the line of best fit with the 95% CIs.
3.3.2. Progesterone
3.3.3. Testosterone
Correlations and partial correlations between the change scores for testosterone and change scores for the twelve PARMSS scores are found in the right column of Table 3 (see Table 3). For the full sample, changes in testosterone were positively correlated with changes in ST responsivity (r = 0.44, p = 0.013, n = 31) and changes in total ST mating intentions (r = 0.41, p = 0.021, n = 31) (see the left side of Figure 3). Partial correlations on the full sample did not reveal any unique effects of testosterone.
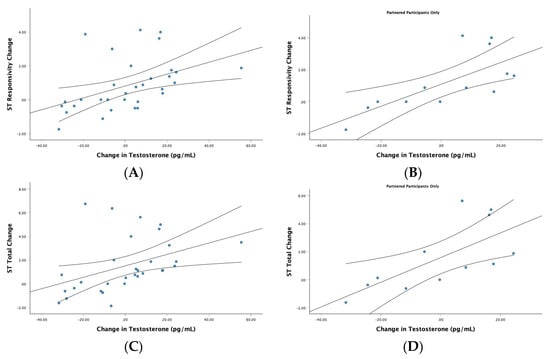
Figure 3.
Change in testosterone and female short-term (ST) mating intentions. Scatterplots reflect correlations between changes in testosterone and changes in ST mating intentions across the menstrual cycle. The top row reflects these correlations for responsive mating intentions when evaluating men for a potential ST relationship in (A) the full female sample (r = 0.44, p = 0.013, n = 31), and (B) partnered participants (r = 0.73, p = 0.007, n = 12). The bottom row reflects these bivariate correlations for all ST mating intentions when evaluating men for a potential ST relationship for (C) the full female sample (r = 0.41, p = 0.021, n = 31), and (D) partnered participants (r = 0.69, p = 0.013, n = 12). Each graph includes the line of best fit with the 95% CIs.
3.3.4. Exploratory Examination of Relationship Status
Given that the PARMSS scores differed as a function of relationship status, singles showed less change in mating intentions between sessions compared to partnered participants, and past studies suggested that hormonal mechanisms may differ with relationship status [6,7], we explored whether relationship status influenced the strength and direction of the significant associations between hormones and ST mating intention scores found above. While the sample sizes were small, some large effect sizes were noted.
The simple correlations between estradiol and ST mating intentions were only significant in partnered participants (i.e., ST responsivity: r = 0.81, p = 0.002, n = 12; total ST: r = 0.79, p = 0.002, n = 12; see right side of Figure 2) and not in single people (i.e., ST responsivity: r = 0.14, p = 0.636, n = 14; total ST: r = 0.05, p = 0.875, n = 14). The partial correlations were not significant for single (negative correlations: ST responsivity: r = −0.07, p = 0.821, n = 10; total ST: r = −0.23, p = 0.474, n = 10) or partnered participants (positive correlations: ST responsivity: r = 0.53, p = 0.116, n = 8; total ST: r = 0.53, p = 0.113, n = 8). However, the latter large effect sizes suggested possible unique effects of estradiol on ST mating intention scores for partnered participants.
For testosterone, simple correlations with ST mating intentions were also only significant in partnered participants: ST responsivity (r = 0.73, p = 0.007, n = 12) and total ST mating intentions (r = 0.69, p = 0.013, n = 12; see the right side of Figure 3). No significant simple correlational effects were found for singles, although the correlations were positive and of medium effect size (ST responsivity: r = 0.33, p = 0.254, n = 14; total ST: r = 0.37, p = 0.195, n = 14). Partial correlations were not significant, suggesting no unique effect of testosterone on ST mating intention scores in single participants (ST responsivity: r = 0.26, p = 0.407, n = 10; total ST: r = 0.38, p = 0.219, n = 10) or partnered participants (ST responsivity: r = 0.25, p = 0.492, n = 8; total ST: r = 0.14, p = 0.693, n = 8).
3.4. Regressions
To follow up on the above findings, we ran regressions to examine the cumulative proportion of variance that changes in estradiol, progesterone, and testosterone could account for in (a) total ST and (b) ST responsive mating intention changes across the cycle. There were no issues with multicollinearity. Changes in the three hormones explained a significant amount of variance in ST responsivity change scores [F (3, 27) = 3.82, p = 0.02, R2 = 0.22], and there was a nonsignificant trend for total ST change scores [F (3, 27) = 2.96, p = 0.05, R2 = 0.16]. The changes in hormones across the menstrual cycle best explained changes in ST responsive mating intentions. (Exploratory regressions on all 12 PARMSS change scores can be found in the Supplementary Materials).
While acknowledging the small samples and low power, we explored whether relationship status was relevant to the significant effect for ST responsive mating intentions. A follow-up regression was run on single participants and another on those in a relationship. Changes in the three hormones significantly explained the change in ST responsivity scores for partnered participants [F (3, 8) = 5.72, p = 0.022, R2 = 0.56] but not in those who were single [F (3, 10) = 0.45, p = 0.721, R2 = 0.15].
4. Discussion
4.1. Preliminary Psychometric Support for the PARMSS
Initial development work on the PARMSS as a measure of proactive and responsive mating intentions with new potential mates was supported here. The results from the exploratory factor analyses with women indicated that each scale was composed of one internally consistent reliable factor when used in all three contexts and that both scale scores have reasonable test–retest reliability over a mean 23-day period even though fluctuation was expected due to cyclical hormone change. There was a higher endorsement of responsive than proactive mating intentions, which makes sense given the social risk and energy expenditure involved in proactive behavior. Evidence of convergent validity came from both scales having strong positive correlations with the ST mating orientation scale from the MDSOI [67]. Evidence of divergent validity came from low nonsignificant correlations with the MDSOI LT mating orientation and previous sexual behavior scales and a measure of masturbation frequency. This was expected, as the PARMSS measure mating motivations with potential new mates as opposed to actual behavior. The latter are affected by attractiveness, opportunity, and relationship status.
4.2. A Stimulatory Effect of Estradiol on ST Mating Intentions across the Cycle
Estradiol was positively associated with overall ST mating intentions and responsive ST Mating Intentions with a medium-large effect size. This is somewhat consistent with hypothesis one. However, exploratory analyses revealed that these effects were driven by participants in a relationship. While partial correlations did not reveal unique effects of estradiol, this may be related to low power or the possibility that estradiol works interactively with other hormones to stimulate responsive mating behaviors in ST contexts. Our findings fit with some previous studies—e.g., [58,75], suggesting that relationship status or type of partner (in-pair vs. extra-pair) is an important contextual factor in understanding hormonal mechanisms or menstrual cycle shifts in mating preferences. These findings also suggest the possibility that studies with a higher proportion of participants in relationships may be more likely to find significant associations between estradiol and sexual behavior—e.g., [40].
Estradiol, a hormone that peaks with fertility across the menstrual cycle, may increase female ST mating intentions. The results are consistent with animal research indicating stimulatory effects of estradiol on female receptivity—e.g., [30,31]. Our human findings were strongest for those with a partner. From an evolutionary perspective, this effect likely reflects an adaptation that increases reproductive success. This finding is consistent with previous studies suggesting that females display increases in opportunistic mating during periods of higher conception likelihood—e.g., [47], show increases in unrestricted sociosexuality, unrestricted desire (e.g., for extra-pair partners) with increases in estradiol [58,76], and report greater responsivity to both in-pair and extra-pair partners at peak fertility [41,42,77]. The findings also fit with aspects of the dual mating hypothesis and the good genes sexual selection theories [78] where females pursue a mating strategy in which they secure paternal investment through an LT partner and pursue ST extra-pair relationships to gain access to genetic fitness—e.g., [79]. The fact that we found a positive relationship between increases in estradiol and increases in ST mating intentions for partnered but not single participants may fit with a finding that partnered (vs. single) females showed larger cyclic shifts in their ratings of men’s attractiveness in the context of potential ST relationships [80]. It is noteworthy that we also found that partnered individuals showed larger changes in mating intentions between sessions than single people. Given that the current study focused on intentions with potential new mates, increases in estradiol may stimulate extra-pair mating motivation, which is consistent with previous research underscoring estradiol’s role in processes central to mate acquisition [81].
It is noteworthy that when each type and combination of mating intention was examined (e.g., proactive, responsive, ST, LT), changes in the three hormones (estradiol, testosterone, progesterone) across the cycle best predicted a change in ST responsivity, and particularly so for partnered individuals. While we did not examine in-pair mating intentions, our findings suggest that the hormonal modulation of mating intentions appears strongest for ST mating intentions when one already has a partner. Similarly, the finding that responsive rather than proactive behaviors are more strongly affected by change in estradiol is consistent with previous research—e.g., [82] suggesting that shifts in ST fertile-phase proactivity and responsivity may function to enhance access to extra-pair resources when conception likelihood is highest. The observed increases in ST responsive mating intentions in partnered females at this time suggest that these mating strategies may afford access to a larger gene pool without the higher associated risk of losing a relationship due to any obvious proactive pursuit of an extra-pair partner. The passive and low-risk nature of responsive (versus proactive) behaviors are less likely to compromise the stability of a LT relationship and may function to enhance access to a larger pool of genetic resources with potential ST extra-pair partners.
4.3. A Stimulatory Effect of Testosterone on Female ST Mating Intentions
Increases in testosterone were associated with increased responsive ST, and overall ST, mating intentions in the full sample (medium-large effect size), which partially supports hypothesis two. These effects were strongest for those in a relationship. However, correlations were no longer significant upon statistically controlling for estradiol and progesterone, possibly suggesting that the testosterone effect may be due to confounding by a stimulatory effect of estradiol or that estradiol and testosterone act together in an interdependent manner (e.g., aromatization).
A stimulatory effect of testosterone on female mating intentions should be viewed along with past research suggesting a stimulatory role of androgens in female sexual behavior. First, animal research indicates a testosterone stimulatory effect on female proceptivity—e.g., [28,30]. Second, higher androgen sensitivity (but not higher androgen levels) has been associated with a greater tendency for visual proximal sexual cues to activate sexual desire in human females [10]. Third, Costa and colleagues reported a stimulatory effect of testosterone on the sexual desire of single but not partnered females (while we found stronger effects for partnered participants and for ST mating intentions) [83]. Fourth, Shirazi and colleagues found a positive relationship between testosterone and both unrestricted sociosexuality and sexual desire [84]. Fifth, Tzalazidis and Oinonen found positive associations between androgenic symptoms [i.e., hirsutism and other symptoms of polycystic ovary syndrome (PCOS)] and unrestricted sociosexuality, masturbation frequency, and attraction to women [63]. Finally, testosterone has been associated with female masturbation, orgasm, and sexual self-esteem [54,55]; flirting with extra-pair men during the luteal phase [58]; and general sexual desire and functioning [4]. These studies suggest a role of endogenous androgens in female sexuality, particularly in terms of both sexual interest/appetite and ST mating intentions.
Bancroft and Graham [1] hypothesized the existence of a subgroup of females who, similar to males, have some dependence on testosterone for their sexual behavior. The present findings suggest the possibility that there may be both testosterone-dependent subgroups of females and testosterone-dependent types of mating intentions. Testosterone may play a role in activating female mating behavior (i.e., responsive mating intentions) in response to the proactive actions of potential ST partners. While subsamples were small, the findings also suggest that relationship status may be a moderating factor worthy of future research.
There are two reasons why it might make sense for testosterone (in addition to estradiol) to stimulate mating intentions. First, there are high levels of estradiol at both the high-fertile (late follicular) and low-fertile (mid-luteal) phases of the cycle, while testosterone peaks only during the former phase. Thus, a testosterone stimulatory effect could maximally direct mating behavior to the more fertile portion of the cycle. Second, female testosterone levels increase when they see an attractive man [85] or have sexual thoughts [86], providing an adaptive mechanism and ideal opportunity for this increase in testosterone to stimulate mating intentions. A stimulatory effect of testosterone would be the most efficient and focused mechanism to orient female mating intentions towards attractive men (i.e., good genes) both when conception is most likely and when the opportunity (i.e., an attractive man) presents itself. While their results require replication, a finding by Shirazi and colleagues is worth considering in this context [76]. They found that the testosterone increase during the viewing of sexual stimuli occurred only during the follicular and not the luteal phase. Thus, females may have testosterone surges in response to male/sexual stimuli only during more fertile times of the cycle.
These findings add to the literature on female sexuality, which suggests associations between testosterone and relationship status [87], relationship commitment [88], and engagement in new relationships. Ellison suggested that testosterone plays an important role in the initiation and establishment of sexual relationships [89], and the present findings are consistent.
4.4. No Association between Progesterone and Mating Intentions
The most obvious explanation for our progesterone findings is that there are indeed no relationships between progesterone and either proactive or responsive mating intentions. This fits with past research. It fits with animal studies suggesting that progesterone on its own does not stimulate sexual behavior but requires estrogen priming [14,32]. Our finding is also consistent with human studies indicating that progesterone is not associated with general sexual interest [90], extra-pair sexual interest [56], or vaginal–penile intercourse frequency in sexually active menstruating individuals [49]. However, the current finding is inconsistent with a few studies suggesting that progesterone negatively predicts female sexual desire and behavior [40,41,76]. It may be noteworthy that we found nonsignificant small–medium negative correlations between changes in progesterone and mating intentions with the vignettes when changes in estradiol and testosterone were statistically controlled. These nonsignificant negative partial correlations raise the possibility that increases in progesterone may inhibit mating intentions, but the co-occurring changes in estradiol and testosterone counteract these effects across the menstrual cycle. While we have attempted to statistically control for the other hormones using partial correlations, hormone interactions are complicated by the fact that hormones can have both agonistic and antagonistic effects on each other (e.g., progesterone decreases the number of estrogen receptors [91]). Thus, we acknowledge that this statistical control has limitations.
4.5. Strengths, Limitations, and Future Directions
This study is the first to examine how cyclical changes in endogenous hormones are related to female sexual behavior as defined by proactive and responsive mating intentions in both ST and LT mating contexts. The use of hormonal assay rather than cycle phase estimation afforded a more precise understanding of the relationship between these variables as several concerns have arisen about the consistency, precision, and misclassification of cycle-phase estimation data [92]. The use of the PARMSS to examine intentions as opposed to actual behavior provided a clearer picture of female sexual intentions by circumventing the potential confounding variables of mate availability and female attractivity. It also allowed for a direct comparison of proactive and responsive mating intentions to find that responsive mating intentions are much stronger. The focus on state-related sexual intentions as opposed to longer-term trait-like sexual experiences is also a strength, as some studies on sexuality examine associations between one hormone sample and sexual traits (e.g., one’s experiences over the past 30 days [55]). Further, the exclusion of hormonal contraception users eliminated the potential confounding variable of exogenous hormone use, e.g., [4]. The use of hormonal change scores (a) is more powerful/sensitive than between-subject analyses of hormone levels [64] and (b) takes into account hormonal sensitivity and change as opposed to just hormone level.
A few limitations are worth noting and suggest areas for future research. First, we provided preliminary psychometric data on the PARMSS, but further research will increase confidence in any findings using the measure. The next steps include confirmatory factor analysis, additional research on validity (e.g., predictive), and determining minimal important change and minimal clinically important difference values. It would also be useful to develop a similar measure to assess proactive and responsive mating intentions in the context of established relationships. Second, as participants were of young reproductive age (i.e., early 20s) and all reported some attraction to men, the hormonal results may not be generalized for participants in their 30s and 40s or individuals without sexual attraction to men. Third, the range of estradiol change scores was restricted, suggesting that stronger effects may be found if a larger range of estradiol change is captured. Fourth, salivary immunoassays may be limited by cross-reactivities to other substances and may overestimate hormone levels [93,94], although the latter issue may be less problematic when evaluating change. Fifth, we did not examine or include recent/prior sexual behavior as a covariate. Sixth, while medium to large effect sizes were observed, the study included small samples for relationship status subgroups and our study is underpowered to identify small or medium effect sizes (i.e., correlations below 0.44). We consider the relationship status group findings to be worthy of inclusion, yet it is important to emphasize that these findings are exploratory and preliminary. Finally, the findings reflect associations, and causality cannot be implied due to the study design. All findings require replication with larger samples and exploration with samples that include other sexual orientations.
Research on proactive and responsive mating intentions may have implications for improving the treatment of sexual desire disorders, for understanding how hormonal contraceptives may affect sexual behavior, and for understanding female mating strategies. Differentiating between different aspects of female sexual behavior and desire (e.g., proactivity and responsivity components) allows for a more sensitive and powerful measurement of specific unique aspects of female sexuality. This better allows researchers to detect the effects of hormones when they exist. When different aspects of sexual desire/interest/intentions are measured together within larger constructs or are not measured at all, it reduces the sensitivity of the measures and reduces the likelihood of finding an effect when it exists for one component. The present findings suggest that small increases in endogenous estradiol and testosterone may enhance female receptivity or responsivity to new potential partners. This is relevant to the role of hormones in behaviors involved in initiating relationships. While it is unclear if these findings generalize proactive and responsive behaviors with existing partners or exogenous estradiol and testosterone, future research is warranted. Given that oral contraceptives inhibit endogenous hormonal cyclicity across the menstrual cycle, it is important to understand how oral contraceptives affect these aspects of sexuality. Future repeated-measures studies could compare free-cycling and triphasic oral contraceptive groups on changes in proactive and responsive sexual intentions across the cycle in studies where triphasic oral contraceptive users are examined in two or more phases with differing estradiol doses. Finally, the findings suggest that people searching for a sexual/relationship partner should be proactive about it. Participants reported being much more likely to say “yes” to an advance from a potential mate than to make that advance themselves.
5. Conclusions
Our results provide initial pilot data suggesting (a) psychometric support for the PARMSS, and (b) that endogenous hormones (i.e., estradiol and testosterone) may function to elicit ST responsive mating behaviors, as compared to mating behaviors that are proactive or involve potential new LT partners. This is the first study to separate proactive and responsive mating intentions, quantify differences in these types of intentions, and examine how changes in these intentions across the menstrual cycle covary with changes in estradiol, progesterone, and testosterone. The findings underscore the potential value of the following methodological suggestions for future research examining hormones and female sexual behavior: (a) delineating proactive and responsive behaviors, (b) examining the ST and LT mating contexts separately, and (c) using within-subject designs to control for between-subject variability in hormonal sensitivity. Replication studies with larger sample sizes should also examine relationship status, and possibly sociosexuality, as modulating variables. These findings contribute to the effort to develop psychometrically sound measures of individual components of female sexuality instead of using unitary or broad simplistic measures. Such measures can be used to identify hormonal mechanisms in specific aspects of female sexuality to help understand how natural cyclical hormonal fluctuations are associated with normal and abnormal sexual experiences. Understanding hormonal mechanisms in female sexual behavior also has implications for individualizing treatment for sexual desire disorders and understanding the effects of hormonal contraceptives on sexual behavior.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sexes5040034/s1, Table S1: Multiple Regressions Examining the Extent to Which Changes in Estradiol, Progesterone, and Testosterone Together Predict Change in Proactive and Responsive Mating Strategies Scale (PARMSS) Scores in the Full Sample of Female Participants.
Author Contributions
Conceptualization, K.A.O., M.L.T., M.P. and K.E.Z.; methodology, M.L.T., K.A.O. and M.P.; formal analysis, K.E.Z., M.L.T., M.P. and K.A.O.; investigation, M.L.T. and M.P.; resources, K.A.O.; data curation, M.L.T., K.E.Z., M.P. and K.A.O.; writing—original draft preparation, K.E.Z., K.A.O. and M.L.T.; writing—review and editing, K.E.Z., K.A.O., M.L.T. and M.P.; visualization, K.E.Z., K.A.O. and M.L.T.; supervision, K.A.O. and M.L.T.; project administration, M.L.T. and K.A.O.; funding acquisition, K.A.O. and M.L.T. All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a doctoral award from the Canadian Institutes of Health Research (CIHR; Gender and Health Institute) in 2011 to the second author (#237398; #249718); and an internal university Social Sciences and Humanities Research Council of Canada (SSHRC) Research Development Fund Award (R#1463178) to the last author. Funding sources did not have any involvement in the study design; the collection, analysis, or interpretation of data; the writing of the report; or in the decision to submit the article for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Lakehead University Research Ethics Board [REB Project # 046 12-13 (Romeo File Number 1462780); 27 September 2012].
Informed Consent Statement
Written informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data that support the findings of this study have been deposited and are available upon approved request in Borealis, the Canadian Dataverse Repository (https://borealisdata.ca/; accessed on 7 October 2024) [95]. Due to the sensitive nature of some questions (e.g., sexual behavior), participants were not asked to provide consent to share the data publicly. However, access to data that support the findings is available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Cycle Regularity Item
Check the statement that best describes your menstrual cycle:
- [ ]
- I have gone through menopause and do not get a period
- [ ]
- I am not currently menstruating because I am currently lactating or breast feeding
- [ ]
- I never have my period
- [ ]
- I have not had my period in the last three months
- [ ]
- Some months I get my period and some months I don’t
- [ ]
- I usually get my period every month, but it is irregular and I cannot predict when it will start
- [ ]
- I usually get my period within two to three days of when I expect it
- [ ]
- My period is like clockwork; the same number of days elapse between periods
Appendix B
Proactive and Responsive Mating Strategies Scale (PARMSS)
Instructions:
Please use the following scale ranging from 1 (not at all likely) to 9 (extremely likely) to rate how likely you would be to do each of the following:
- Give this person your phone number if asked
- Ask this person for their number
- Return a smile or eye contact from this person
- Smile, or initiate eye contact with, this person
- Dance with this person if asked
- Ask this person to dance with you
- Allow this person to buy you a drink
- Buy this person a drink or ask this person to buy you a drink
- Allow this person to kiss you
- Initiate kissing with this person
- Accept a ride home from this person if offered
- Offer this person a ride home or ask this person for a ride home
- Allow this person to initiate any sexual activity
- Initiate any sexual activity with this person
- Allow this person to initiate sex
- Initiate sex with this person
Scoring: Odd-numbered items make up the responsive subscale. Even numbered items make up the proactive subscale. Items are aggregated for three possible scores: proactive subscale, responsive subscale, and the total PARMSS.
Appendix C
Mating Vignettes
Short-term Vignette:
Consider the following hypothetical situation. Your life is exactly as it is right now. Imagine that you just met someone new that you think would be ideal or attractive for a short-term relationship. A short-term relationship is primarily sexual in nature and tends not to last very long. Examples of this type of relationship include someone you may have a sexual affair with and someone you may have a one-night stand with. Based on how you feel now as well as your feelings and behavior in the past 48 h (i.e., over the past 2 days), how likely are you to…
Long-term Vignette:
Consider the following hypothetical situation. Your life is as it is now. Imagine that you just met someone new that you think would be ideal or attractive for a long-term relationship. A long-term relationship involves commitment as well as an emotional connection. Examples of this type of relationship would include someone you may want to move in with, someone you may consider leaving a current partner to be with, and someone you may wish to marry at some point. Based on how you feel now as well as your feelings and behavior in the past 48 h (i.e., over the past 2 days), how likely are you to…
Pictures:
For each of the following four images, please imagine that the person pictured is now around your age and complete the ratings that follow based on how you feel now as well as your feelings and behaviors in the past 48 h (i.e., over the past 2 days).
References
- Bancroft, J.; Graham, C.A. The varied nature of women’s sexuality: Unresolved issues and a theoretical approach. Horm. Behav. 2011, 59, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Regan, P.C. Rhythms of desire: The association between menstrual cycle phases and female sexual desire. Can. J. Hum. Sex. 1996, 5, 145–156. [Google Scholar]
- Islam, R.M.; Bell, R.J.; Green, S.; Page, M.J.; Davis, S.R. Safety and efficacy of testosterone for women: A systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol. 2019, 7, 754–766. [Google Scholar] [CrossRef]
- Maseroli, E.; Vignozzi, L. Are endogenous androgens linked to female sexual function? A systemic review and meta-analysis. J. Sex. Med. 2022, 19, 553–568. [Google Scholar] [CrossRef]
- Gildersleeve, K.; Haselton, M.G.; Fales, M.R. Do women’s mate preferences change across the ovulatory cycle? A meta-analytic review. Psychol. Bull. 2014, 140, 1205–1259. [Google Scholar] [CrossRef]
- Barrett, E.S.; Tran, V.; Thurston, S.W.; Frydenberg, H.; Lipson, S.F.; Thune, I.; Ellison, P.T. Women who are married or living as married have higher salivary estradiol and progesterone than unmarried women. Am. J. Hum. Biol. 2015, 27, 501–507. [Google Scholar] [CrossRef]
- Kuzawa, C.; Gettler, L.T.; Huang, Y.; McDade, T.W. Mothers have lower testosterone than non-mothers: Evidence from the Philippines. Horm. Behav. 2010, 57, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.B. Reexploring the concept of sexual desire. J. Sex. Marital. Ther. 2002, 28, 39–51. [Google Scholar] [CrossRef]
- Levine, S.B. The nature of sexual desire: A clinician’s perspective. Arch. Sex. Behav. 2003, 32, 279–285. [Google Scholar] [CrossRef]
- Rellini, A.H.; Stratton, N.; Tonani, S.; Santamaria, V.; Brambilla, E.; Nappi, R.E. Differences in sexual desire between women with clinical versus biochemical signs of hyperandrogenism in polycystic ovarian syndrome. Horm. Behav. 2013, 63, 65–71. [Google Scholar] [CrossRef]
- Beach, F.A. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm. Behav. 1976, 7, 105–138. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Kippin, T.E.; Coria-Avila, G.A. What can animal models tell us about human sexual response? Annu. Rev. Sex. Res. 2003, 14, 1–63. [Google Scholar] [PubMed]
- de Jonge, F.H.; Eerland, E.M.; Van de Poll, N.E. The influence of estrogen, testosterone and progesterone on partner preference, receptivity and proceptivity. Physiol. Behav. 1986, 37, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.A.; Pfeifle, J.K. Hormonal control of receptivity, proceptivity and sexual motivation. Physiol. Behav. 1983, 30, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.R. Sexual behavior of free ranging rhesus monkeys (Macaca mulatta). I. Specimens, procedures and behavioral characteristics of estrus. J. Comp. Psychol. 1942, 33, 113–142. [Google Scholar] [CrossRef]
- Wallen, K. Influence of female hormonal state on rhesus sexual behavior varies with space for social interaction. Science 1982, 217, 375–376. [Google Scholar] [CrossRef]
- Young, W.C.; Orbison, W.D. Changes in selected features of behaviour in pairs of oppositely sexed chimpanzees during the sexual cycle and after ovariectomy. J. Comp. Physiol. Psychol. 1944, 37, 107–143. [Google Scholar] [CrossRef]
- Everitt, B.J.; Herbert, J.; Hamer, J.D. Sexual receptivity of bilaterally adrenalectomized female rhesus monkeys. Physiol. Behav. 1972, 8, 409–415. [Google Scholar] [CrossRef]
- Johnson, D.F.; Phoenix, C.H. Hormonal control of female sexual attractiveness, proceptivity and receptivity in rhesus monkeys. J. Comp. Physiol. Psychol. 1976, 90, 473–483. [Google Scholar] [CrossRef]
- Young, W.C. The hormones and mating behaviour. In Sex and Internal Secretions; Young, W.C., Ed.; Bailliere, Tindall and Cox: London, UK, 1961; pp. 1173–1239. [Google Scholar]
- Michael, R.P.; Keverne, E.B.; Zumpe, D.; Bonsall, R.W. Neuroendocrine factors in the control of primate behaviour. Rec. Prog. Horm. Res. 1972, 28, 665–706. [Google Scholar]
- Wallen, K.; Winston, L.A.; Gaventa, S.; Davis-DaSilva, M.; Collins, D.C. Periovulatory changes in female sexual behavior and patterns of ovarian steroid secretion in group-living rhesus macaques. Horm. Behav. 1984, 18, 431–450. [Google Scholar] [CrossRef]
- Warner, L.H. A study of sex behaviour in the white rat by means of the obstruction method. Comp. Psychol. Mong. 1927, 4, 1–68. [Google Scholar]
- Baum, M.J.; Everitt, B.J.; Herbert, J.; Keverne, E.B. Hormonal basis of proceptivity and receptivity in female primates. Arch. Sex. Behav. 1977, 6, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Rowell, T.E. Female reproduction cycles and social behaviour in primates. Adv. Stud. Behav. 1972, 4, 69–105. [Google Scholar]
- Frye, C.A.; Bayon, L.E.; Pursnani, N.K.; Purdy, R.H. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998, 808, 72–83. [Google Scholar] [CrossRef]
- Tennent, B.J.; Smith, E.R.; Davidson, J.M. The effects of estrogen and progesterone on female rat proceptive behavior. Horm. Behav. 1980, 14, 65–75. [Google Scholar] [CrossRef]
- Beyer, C.; Vidal, N.; Mijares, A. Probable role of aromatization in the induction of estrous behaviour by androgens in the ovariectomized rabbit. Endocrinology 1970, 87, 1386–1389. [Google Scholar] [CrossRef]
- Keverne, E.B. Sexual receptivity and attractiveness in the female rhesus monkey. In Advances in the Study of Behaviour; Rosenblatt, J.S., Ed.; Academic Press: New York, NY, USA, 1976; Volume 7, pp. 155–200. [Google Scholar]
- Trimble, M.R.; Herbert, J. The effect of testosterone or oestradiol upon the sexual and associated behaviour of the adult female rhesus monkey. J. Endocrinol. 1968, 42, 171–185. [Google Scholar] [CrossRef]
- Beach, F.A.; Leboeuf, B.J. Coital behaviour in dogs: I. Preferential mating in the bitch. Anim. Behav. 1967, 15, 546–558. [Google Scholar] [CrossRef]
- Cochran, C.G. Proceptive patterns of behavior throughout the menstrual cycle in female rhesus monkeys. Behav. Neural Biol. 1979, 27, 342–353. [Google Scholar] [CrossRef]
- Bancroft, J.; Sherwin, B.B.; Alexander, G.M.; Davidson, D.W.; Walker, A. Oral contraceptives, androgens, and the sexuality of young women: I. A comparison of sexual experience, sexual attitudes, and gender role in oral contraceptive users and nonusers. Arch. Sex. Behav. 1991, 20, 105–120. [Google Scholar] [CrossRef]
- Blake, K.R.; Dixson, B.J.W.; O’Dean, S.M.; Denson, T.F. No compelling positive association between ovarian hormones and wearing red clothing when using multinomial analyses. Horm. Behav. 2017, 90, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Grebe, N.M.; Gangestad, S.W.; Garver-Apgar, C.E.; Thornhill, R. Women’s luteal phase sexual proceptivity and the functions of extended sexuality. Psychol. Sci. 2013, 24, 2106–2110. [Google Scholar] [CrossRef] [PubMed]
- Basson, R. The female sexual response: A different model. J. Sex. Marital. Ther. 2000, 26, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.; Kressel, L.; Joshi, P.D.; Louie, B. Meta-analysis of menstrual cycle effects on women’s mate preferences. Emot. Rev. 2014, 6, 229–249. [Google Scholar] [CrossRef]
- Adams, D.B.; Gold, A.R.; Burt, A.D. Rise in female-initiated sexual activity at ovulation and its suppression by oral contraceptives. N. Engl. J. Med. 1978, 299, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Cantú, S.M.; Simpson, J.A.; Griskevicius, V.; Weisberg, Y.J.; Durante, K.M.; Beal, D.J. Fertile and selectively flirty: Women’s behavior toward men changes across the ovulatory cycle. Psychol. Sci. 2013, 25, 431–438. [Google Scholar] [CrossRef]
- Marcinkowska, U.M.; Shirazi, T.; Mijas, M.; Roney, J.R. Hormonal underpinnings of the variation in sexual desire, arousal and activity throughout the menstrual cycle—A multifaceted approach. J. Sex. Res. 2023, 60, 1297–1303. [Google Scholar] [CrossRef]
- Roney, J.R.; Simmons, Z.L. Hormonal predictors of sexual motivation in natural menstrual cycles. Horm. Behav. 2013, 63, 636–645. [Google Scholar] [CrossRef]
- Arslan, R.C.; Schilling, K.M.; Gerlach, T.M.; Penke, L. Using 26,000 diary entries to show ovulatory changes in sexual desire and behavior. J. Pers. Soc. Psychol. 2021, 121, 410–431, Erratum in J. Pers. Soc. Psychol. 2023, 125, 1238. [Google Scholar] [CrossRef]
- Atukorala, K.R.; Silva, W.; Amarasiri, L.; Fernando, D.M.S. Changes in serum testosterone during the menstrual cycle—An integrative systematic review of published literature. Gynecol. Reprod. Endocrinol. Metab. 2020, 3, 9–20. [Google Scholar] [CrossRef]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation. Endotext. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279054 (accessed on 7 June 2024).
- Bullivant, S.B.; Sellegren, S.A.; Stern, K.; Spencer, N.A.; Jacob, S.; Mennella, J.A.; McClintock, M.K. Women’s sexual desire during the menstrual cycle: Identification of the sexual phase by noninvasive measurement of luteinizing hormone. J. Sex. Res. 2004, 41, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Matteo, S.; Rissman, E.F. Increased sexual activity during the midcycle portion of the human menstrual cycle. J. Sex. Res. 1984, 18, 249–255. [Google Scholar] [CrossRef]
- Durante, K.M.; Li, N.P. Oestradiol level and opportunistic mating in women. Biol. Lett. 2009, 5, 179–182. [Google Scholar] [CrossRef]
- PPersky, H.; Dreisbach, L.; Miller, W.R.; O’brien, C.P.; Khan, M.A.; Lief, H.I.; Charney, N.; Strauss, D. The relation of plasma androgen levels to sexual behaviors and attitudes of women. Psychosom. Med. 1982, 44, 305–319. [Google Scholar] [CrossRef]
- Prasad, A.; Mumford, S.L.; Louis, G.M.B.; Ahrens, K.A.; Sjaarda, L.A.; Schliep, K.C.; Schisterman, E.F. Sexual activity, endogenous reproductive hormones and ovulation in premenopausal women. Horm. Behav. 2014, 66, 330–338. [Google Scholar] [CrossRef]
- Wåhlin-Jacobsen, S.; Pedersen, A.T.; Kristensen, E.; Læssøe, N.C.; Lundqvist, M.; Cohen, A.S.; Hougaard, D.M.; Giraldi, A. Is there a correlation between androgens and sexual desire in women? J. Sex. Med. 2015, 12, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Wallen, K. Desire and ability: Hormones and the regulation of female sexual behavior. Neurosci. Biobehav. Rev. 1990, 14, 233–241. [Google Scholar] [CrossRef]
- van Anders, S.M.; Hamilton, L.D.; Schmidt, N.; Watson, N.V. Associations between testosterone secretion and sexual activity in women. Horm. Behav. 2007, 51, 477–482. [Google Scholar] [CrossRef]
- Tao, L.; Duan, Z.; Liu, Y.; Hou, H.; Zhang, X. Correlation of sexual dysfunction with sex hormone and estrogen receptor gene polymorphism in Chinese Han women with epilepsy. Epilepsy Res. 2021, 169, 106527. [Google Scholar] [CrossRef]
- Macdowall, W.G.; Clifton, S.; Palmer, M.J.; Tanton, C.; Copas, A.J.; Lee, D.M.; Mitchell, K.R.; Mercer, C.H.; Sonnenberg, P.; Johnson, A.M.; et al. Salivary testosterone and sexual function and behavior in men and women: Findings from the Third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3). J. Sex. Res. 2022, 59, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Islam, R.M.; Skiba, M.A.; Bell, R.J.; Davis, S.R. Associations between androgens and sexual function in premenopausal women: A cross-sectional study. Lancet Diabetes Endocrinol. 2020, 8, 693–702. [Google Scholar] [CrossRef] [PubMed]
- van Stein, K.R.; Strauß, B.; Brenk-Franz, K. Ovulatory shifts in sexual desire but not mate preferences: An LH-test-confirmed, longitudinal study. Evol. Psychol. 2019, 17, 1474704919848116. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.C.; Hahn, A.C.; Fisher, C.I.; Wang, H.; Kandrik, M.; DeBruine, L.M. General sexual desire, but not desire for uncommitted sexual relationships, tracks changes in women’s hormonal status. Psychoneuroendocrinology 2018, 88, 153–157. [Google Scholar] [CrossRef]
- Grebe, N.M.; Emery Thompson, M.; Gangestad, S.W. Hormonal predictors of women’s extra-pair vs. in-pair sexual attraction in natural cycles: Implications for extended sexuality. Horm. Behav. 2016, 78, 211–219. [Google Scholar] [CrossRef]
- Elaut, E.; Buysse, A.; De Sutter, P.; Gerris, J.; De Cuypere, G.; T’Sjoen, G. Cycle-related changes in mood, sexual desire, and sexual activity in oral contraception-using and nonhormonal-contraception-using couples. J. Sex. Res. 2016, 53, 125–136. [Google Scholar] [CrossRef]
- Bancroft, J.; Sanders, D.; Davidson, D.; Warner, P. Mood, sexuality, hormones, and the menstrual cycle: III. Sexuality and the role of androgens. Psychosom. Med. 1983, 45, 509–516. [Google Scholar] [CrossRef]
- Graham, C.A.; Bancroft, J.; Doll, H.A.; Greco, T.; Tanner, A. Does oral contraceptive induced reduction in free testosterone adversely affect the sexuality or mood of women? Psychoneuroendocrinology 2007, 32, 246–255. [Google Scholar] [CrossRef]
- Oinonen, K.A. Putting a finger on potential predictors of oral contraceptive side effects: 2D:4D and middle-phalangeal hair. Psychoneuroendocrinology 2009, 34, 713–726. [Google Scholar] [CrossRef]
- Tzalazidis, R.; Oinonen, K.A. Continuum of symptoms in Polycystic Ovary Syndrome (PCOS): Links with sexual behavior and unrestricted sociosexuality. J. Sex. Res. 2021, 58, 532–544. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Martinez, P.E.; Nieman, L.K.; Koziol, D.E.; Thompson, K.D.; Schenkel, L.; Wakim, P.G.; Rubinow, D.R. Premenstrual Dysphoric Disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am. J. Psychiatry 2017, 174, 980–989. [Google Scholar] [CrossRef]
- Teatero, M. Women’s Reproductive Experiences (REP) and Hormones: Patterns in Affective, Sexual, and Physical Well-Being. Doctoral Dissertation, Lakehead University, Thunder Bay, ON, Canada, 2016. [Google Scholar]
- Kinsey, A.C.; Pomeroy, W.R.; Martin, C.E. Sexual behavior in the human male. Am. J. Public Health 1948, 93, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.J.; Kirkpatrick, L.A. The structure and measurement of human mating strategies: Toward a multidimensional model of sociosexuality. Evol. Hum. Behav. 2007, 28, 382–391. [Google Scholar] [CrossRef]
- Phillips, M. The Ovulatory Shift: Proceptive and Receptive Mating Behaviours Across the Menstrual Cycle. Doctoral Dissertation, Lakehead University, Thunder Bay, ON, Canada, 2015. [Google Scholar]
- Little, A.C.; Jones, B.C.; Burriss, R.P. Preferences for masculinity in male bodies change across the menstrual cycle. Horm. Behav. 2007, 51, 633–639. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 5th ed.; Allyn and Bacon: New York, NY, USA, 2007. [Google Scholar]
- Arifin, W.N. Sample Size Calculator. 2024. Available online: http://wnarifin.github.io (accessed on 7 June 2024).
- Field, A. Uncovering Statistics Using SPSS, 3rd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2009. [Google Scholar]
- Little, A.C.; Jones, B.C.; Penton-Voak, I.S.; Burt, D.M.; Perrett, D.I. Partnership status and the temporal context of relationships influence human female preferences for sexual dimorphism in male face shape. Proc. Biol. Sci. 2002, 269, 1095–1100. [Google Scholar] [CrossRef]
- Shirazi, T.N.; Bossio, J.A.; Puts, D.A.; Chivers, M.L. Menstrual cycle phase predicts women’s hormonal responses to sexual stimuli. Horm. Behav. 2018, 103, 45–53. [Google Scholar] [CrossRef]
- Roney, J.R.; Simmons, Z.L. Within-cycle fluctuations in progesterone negatively predict changes in both in-pair and extra-pair desire among partnered women. Horm. Behav. 2016, 81, 45–52. [Google Scholar] [CrossRef]
- Gangestad, S.W.; Garver-Apgar, C.; Simpson, J.A.; Cousins, A.J. Changes in women’s mate preferences across the ovulatory cycle. J. Pers. Soc. Psychol. 2007, 92, 151–163. [Google Scholar] [CrossRef]
- Larson, C.M.; Pillsworth, E.G.; Haselton, M.G. Ovulatory shifts in women’s attractions to primary partners and other men: Further evidence of the importance of primary partner sexual attractiveness. PLoS ONE 2012, 7, e44456. [Google Scholar] [CrossRef]
- Fink, B.; Penton-Voak, I. Evolutionary psychology of facial attractiveness. Curr. Dir. Psychol. Sci. 2002, 11, 154–158. [Google Scholar] [CrossRef]
- Wardecker, B.M.; Smith, L.K.; Edelstein, R.S.; Loving, T.J. Intimate relationships then and now: How old hormonal processes are influenced by our modern psychology. Adapt. Human. Behav. Physiol. 2015, 1, 150–176. [Google Scholar] [CrossRef]
- Pillsworth, E.G.; Haselton, M.G. Male sexual attractiveness predicts differential ovulatory shifts in female extra-pair attraction and male mate retention. Evol. Hum. Behav. 2006, 27, 247–258. [Google Scholar] [CrossRef]
- Costa, R.M.; Oliveira, G.; Pestana, J.; Costa, D.; Oliveira, R.F. Do psychosocial factors moderate the relation between testosterone and female sexual desire? The role of interoception, alexithymia, defense mechanisms, and relationship status. Adapt. Human. Behav. Physiol. 2019, 5, 13–30. [Google Scholar] [CrossRef]
- Shirazi, T.N.; Self, H.; Dawood, K.; Rosenfield, K.A.; Penke, L.; Carré, J.M.; Ortiz, T.; Puts, D.A. Hormonal predictors of women’s sexual motivation. Evol. Hum. Behav. 2019, 40, 336–344. [Google Scholar] [CrossRef]
- López, H.H.; Hay, A.C.; Conklin, P.H. Attractive men induce testosterone and cortisol release in women. Horm. Behav. 2009, 56, 84–92. [Google Scholar] [CrossRef]
- Goldey, K.L.; van Anders, S.M. Sexy thoughts: Effects of sexual cognitions of testosterone, cortisol, and arousal in women. Horm. Behav. 2011, 59, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, R.S.; Chopik, W.J.; Kean, E.L. Sociosexuality moderates the association between testosterone and relationship status in men and women. Horm. Behav. 2011, 60, 248–255. [Google Scholar] [CrossRef]
- van Anders, S.M.; Watson, N.V. Social neuroendocrinology: Effects of social contexts and behaviors on sex steroids in humans. Hum. Nat. 2006, 17, 212–237. [Google Scholar] [CrossRef]
- Ellison, P.T. Reproductive Ecology and Human Evolution, 1st ed.; Routledge: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Dennerstein, L.; Gotts, G.; Brown, J.B.; Morse, C.A.; Farley, T.M.; Pinol, A. The relationship between the menstrual cycle and female sexual interest in women with PMS complaints and volunteers. Psychoneuroendocrinology 1994, 19, 293–304. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Peck, E.J.; Clark, J.H. Progesterone antagonism of the oestrogen receptor and oestrogen-induced uterine growth. Nature 1975, 254, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; McRae-Clark, A.L.; Carlson, S.; Saladin, M.E.; Gray, K.M.; Wetherington, C.L.; McKee, S.A.; Allen, S.S. Determining menstrual phase in human biobehavioral research: A review with recommendations. Exp. Clin. Psychopharmacol. 2016, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, O.C.; Dlugash, G.; Mehta, P.H. Hormone measurement in social neuroendocrinology: A comparison of immunoassay and mass spectroscopy methods. In Routledge International Handbook of Social Neuroendocrinology; Schultheiss, O.C., Mehta, P.H., Eds.; Routledge: New York, NY, USA, 2019; pp. 239–255. [Google Scholar]
- Welker, K.M.; Lassetter, B.; Brandes, C.M.; Prasad, S.; Koop, D.R.; Mehta, P.H. A comparison of salivary testosterone measurement using immunoassays and tandem mass spectrometry. Psychoneuroendocrinology 2016, 71, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Teatero, M.; Oinonen, K. The Proactive and Responsive Sexual Strategies Scales (PARMSS) and Changes in Estradiol, Progesterone, and Testosterone Across the Menstrual Cycle: Two Data Sets. 2024. Available online: https://borealisdata.ca/dataset.xhtml?persistentId=doi:10.5683/SP3/VOJUDS (accessed on 7 June 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).