Mini 2000: A Robust Miniature Mass Spectrometer with Continuous Atmospheric Pressure Interface
Abstract
:1. Introduction
2. Experimental Section
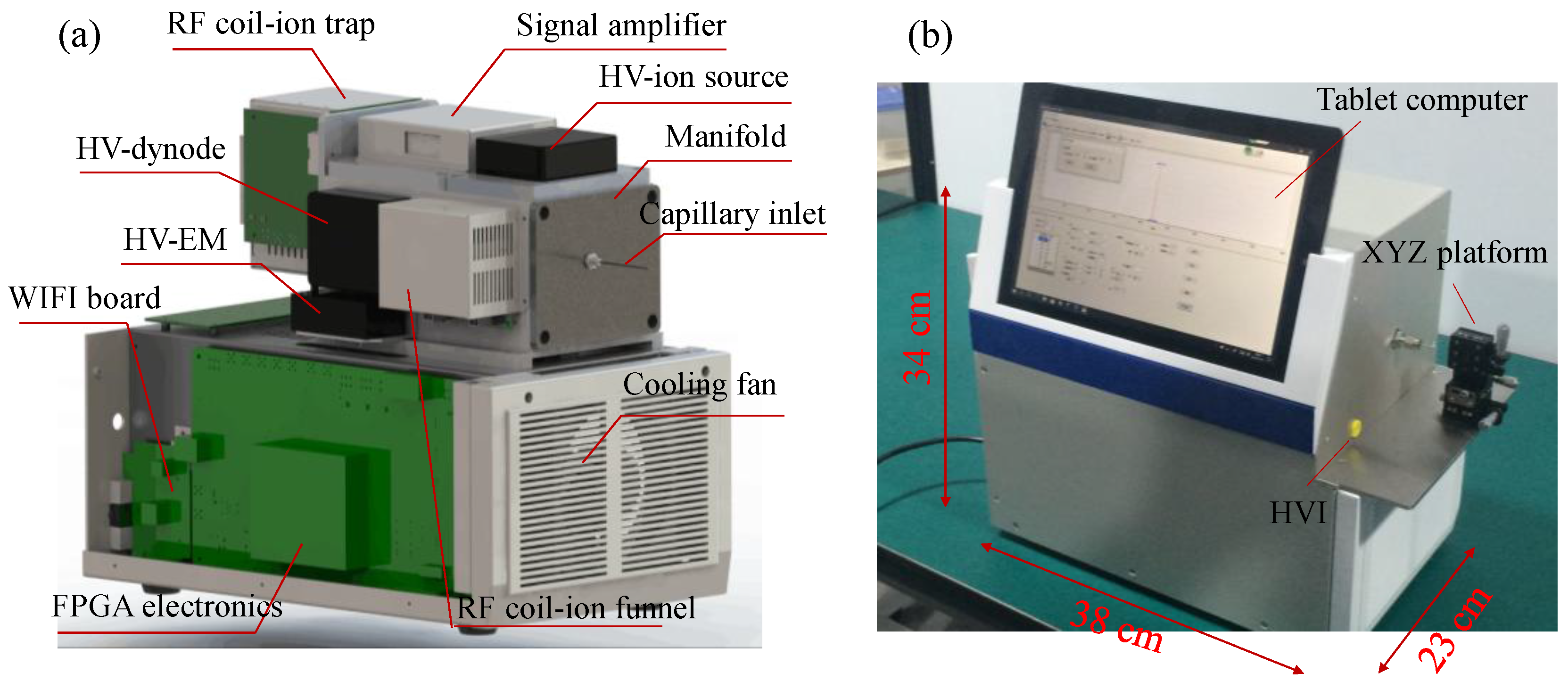
2.1. Instrumentation
2.2. Chemical Samples
3. Results and Discussion
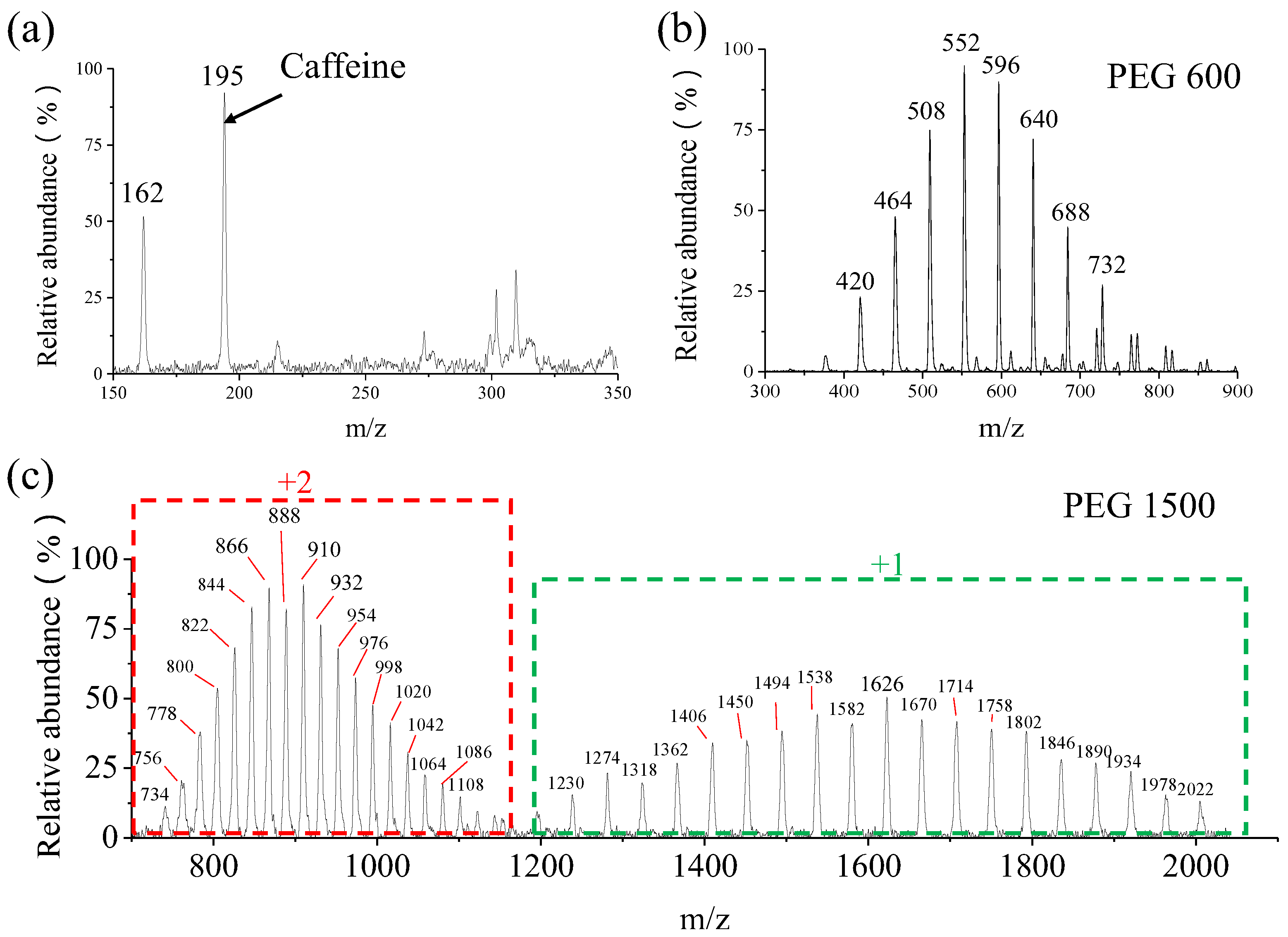
3.1. Instrument Stability
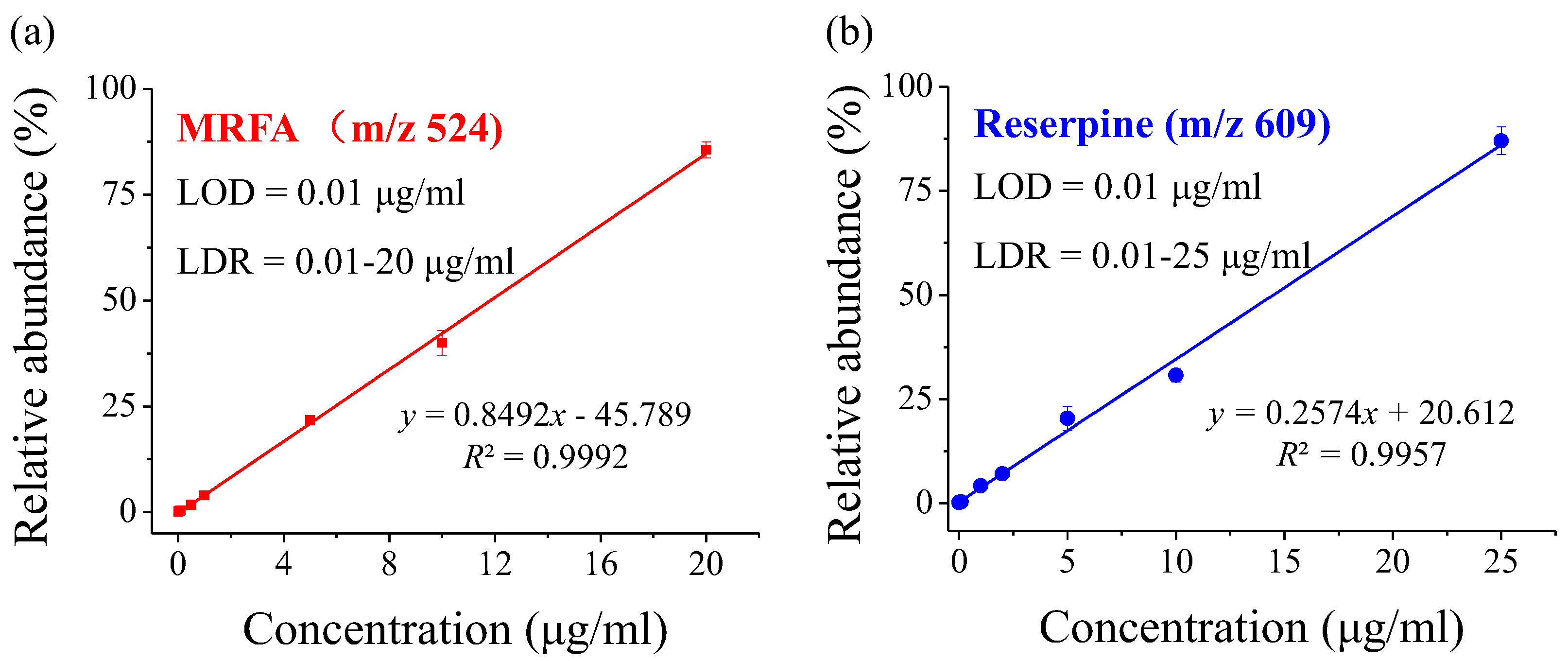
3.2. Sensitivity
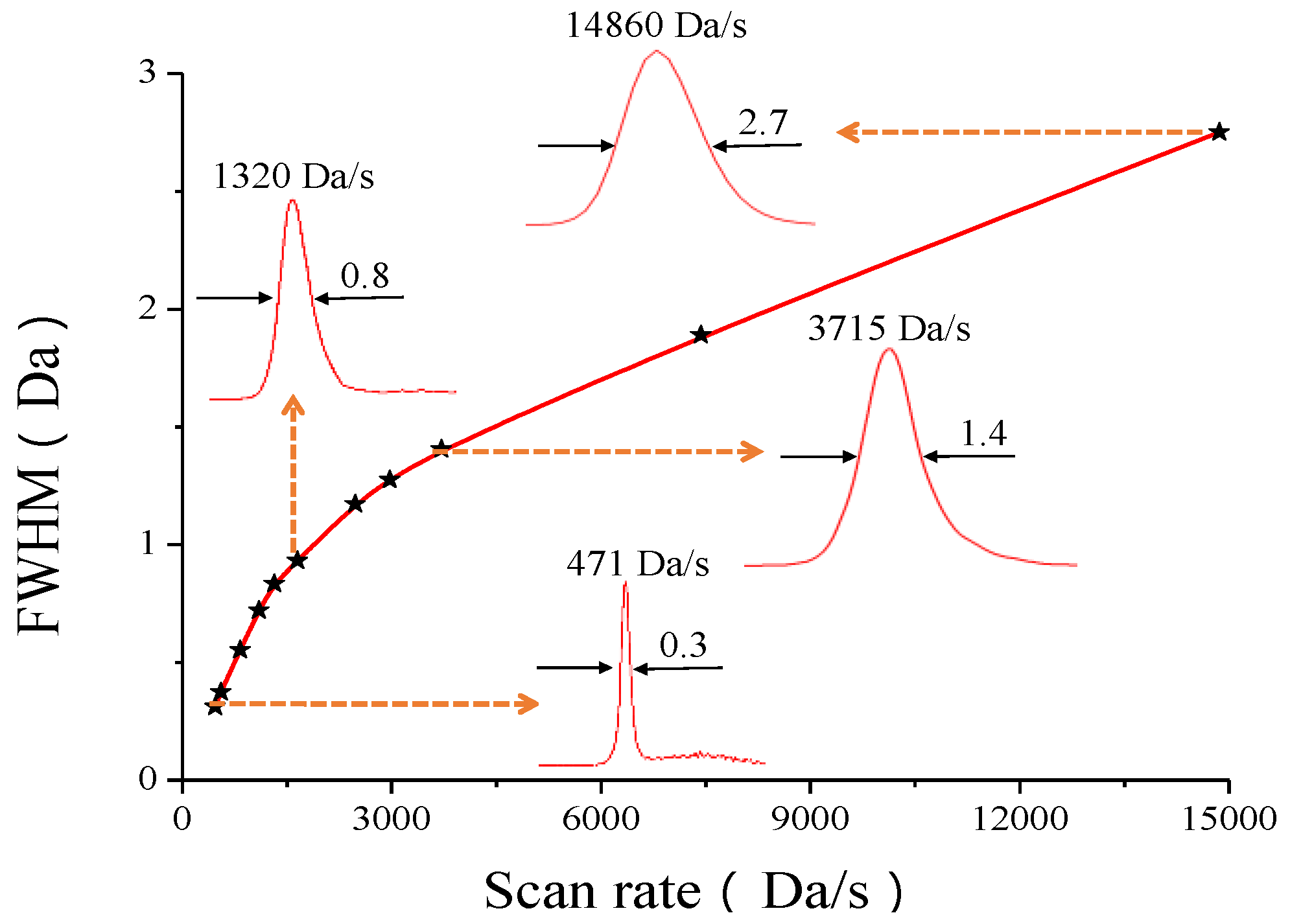
3.3. MS Resolution
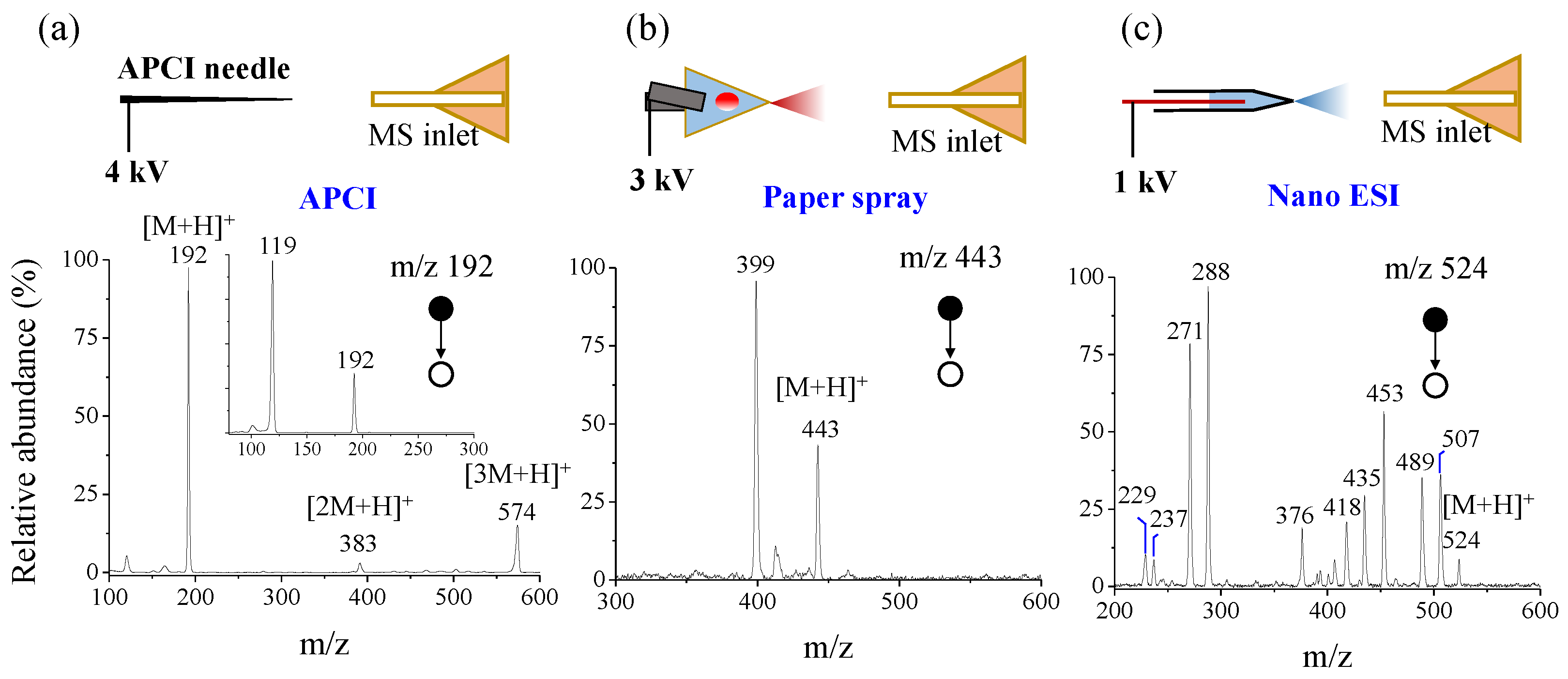
3.4. Coupling with Different Ambient Ionization Sources
3.5. Mass Range
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lebedev, A.T. Environmental mass spectrometry. Rev. Anal. Chem. 2013, 6, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.S. Instrumentation for trace detection of high explosives. Rev. Sci. Instrum. 2004, 75, 2499–2512. [Google Scholar] [CrossRef]
- Nilles, J.M.; Connell, T.R.; Durst, H.D. Quantitation of Chemical Warfare Agents Using the Direct Analysis in Real Time (DART) Technique. Anal. Chem. 2009, 81, 6744–6749. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.H.; Hodges, R.R.; Wright, W.W.; Blevins, V.A.; Duerksen, K.D.; Brooks, L.D. Pioneer Venus Sounder Probe Neutral Gas Mass Spectrometer. IEEE Trans. Geosci. Remote Sens. 1980, 18, 80–84. [Google Scholar] [CrossRef]
- Niemann, H.B.; Atreya, S.K.; Bauer, S.J.; Biemann, K.; Block, B.P.; Carignan, G.R.; Donahue, T.M.; Frost, R.L.; Gautier, D.; Haberman, J.A.; et al. The Gas Chromatograph Mass Spectrometer for the Huygens Probe. Space Sci. Rev. 2002, 104, 553–591. [Google Scholar] [CrossRef]
- Von Zahn, U.; Fricke, K.H.; Hunten, D.M.; Krankowsky, D.; Mauersberger, K.; Nier, A.O. The upper atmosphere of Venus during morning conditions. J. Geophys. Res. Space Phys. 1980, 85, 7829–7840. [Google Scholar] [CrossRef]
- Hofer, L.; Wurz, P.; Buch, A.; Cabane, M.; Coll, P.; Coscia, D.; Gerasimov, M.; Lasi, D.; Sapgir, A.; Szopa, C.; et al. Prototype of the gas chromatograph–mass spectrometer to investigate volatile species in the lunar soil for the Luna-Resurs mission. Planet. Space Sci. 2015, 111, 126–133. [Google Scholar] [CrossRef]
- Meyer, S.; Tulej, M.; Wurz, P. Mass spectrometry of planetary exospheres at high relative velocity: Direct comparison of open-and closed-source measurements. Geosci. Instrum. Methods Data Syst. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Riedo, A.; Bieler, A.; Neuland, M.; Tulej, M.; Wurz, P. Performance evaluation of a miniature laser ablation time-of-flight mass spectrometer designed for in situ investigations in planetary space research. J. Mass Spectrom. 2013, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, T.; Ren, Y.; Hendricks, P.I.; Cooks, R.G.; Ouyang, Z. Mini 12, miniature mass spectrometer for clinical and other applications—Introduction and characterization. Anal. Chem. 2014, 86, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Cooks, R.G.; Manicke, N.E.; Dill, A.L.; Ifa, D.R.; Eberlin, L.S.; Costa, A.B.; Wang, H.; Huang, G.; Ouyang, Z. New ionization methods and miniature mass spectrometers for biomedicine: DESI imaging for cancer diagnostics and paper spray ionization for therapeutic drug monitoring. Faraday Discuss. 2011, 149, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Cooks, R.G. Miniature mass spectrometers. Annu. Rev. Anal. Chem. 2009, 2, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.T.; Pulliam, C.J.; Ouyang, Z.; Cooks, R.G. Miniature and fieldable mass spectrometers: Recent advances. Anal. Chem. 2015, 88, 2–29. [Google Scholar] [CrossRef] [PubMed]
- Ketola, R.A.; Kotiaho, T.; Cisper, M.E.; Allen, T.M. Environmental applications of membrane introduction mass spectrometry. J. Mass Spectrom. 2002, 37, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Janfelt, C.; Frandsen, H.; Lauritsen, F.R. Characterization of a mini membrane inlet mass spectrometer for on-site detection of contaminants in both aqueous and liquid organic samples. Rapid Commun. Mass Spectrom. 2006, 20, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Cooks, R.; Allen, T.; Cisper, M.; Hemberger, P. Membrane introduction mass spectrometry: Trends and applications. Mass Spectrom. Rev. 2000, 19, 1–37. [Google Scholar] [CrossRef]
- Bell, R.J.; Davey, N.G.; Martinsen, M.; Collin-Hansen, C.; Krogh, E.T.; Gill, C.G. A field-portable membrane introduction mass spectrometer for real-time quantitation and spatial mapping of atmospheric and aqueous contaminants. J. Am. Soc. Mass Spectrom. 2015, 26, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, P.I.; Dalgleish, J.K.; Shelley, J.T.; Kirleis, M.A.; Mcnicholas, M.T.; Li, L.; Chen, T.; Chen, C.; Duncan, J.; Boudreau, F.J.; et al. Autonomous in Situ Analysis and Real-Time Chemical Detection Using a Backpack Miniature Mass Spectrometer: Concept, Instrumentation Development, and Performance. Anal. Chem. 2014, 86, 2900–2908. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cooks, R.G.; Ouyang, Z. Breaking the pumping speed barrier in mass spectrometry: Discontinuous atmospheric pressure interface. Anal. Chem. 2008, 80, 4026–4032. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Feng, Y.; Wei, Y.; Wang, Y.; Xu, W. Development of a miniature mass spectrometer with continuous atmospheric pressure interface. Analyst 2015, 140, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, H.; Yang, T.; Zhai, Y.; Wei, X.; Xu, H.; Zhao, X.; Li, D.; Wei, X. A “Brick Mass Spectrometer” Driven by a Sinusoidal Frequency Scanning Technique. Anal. Chem. 2017, 89, 5578–5584. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xue, Z.; Zhang, Y.; Huang, Z.; Fang, X.; Qu, F.; Ouyang, Z.; Xu, W. Development and Characterizations of a Miniature Capillary Electrophoresis Mass Spectrometry System. Anal. Chem. 2015, 87, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Jjunju, F.P.M.; Maher, S.; Li, A.; Syed, S.U.A.H.; Smith, B.; Heeren, R.M.A.; Taylor, S.K.; Cooks, R.G. Hand-held portable desorption atmospheric pressure chemical ionization ion source for in situ analysis of nitroaromatic explosives. Anal. Chem. 2015, 87, 10047–10055. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Ouyang, Z. Direct Analysis of Nonvolatile Chemical Compounds on Surfaces Using a Hand-Held Mass Spectrometer with Synchronized Discharge Ionization Function. Anal. Chem. 2016, 88, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ouyang, Z. Synchronized discharge ionization for analysis of volatile organic compounds using a hand-held ion trap mass spectrometer. Anal. Chem. 2013, 85, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.D.; Charipar, N.; Mulligan, C.C.; Zhang, X.; Cooks, R.G.; Ouyang, Z. Low-temperature plasma probe for ambient desorption ionization. Anal. Chem. 2008, 80, 9097–9104. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Manicke, N.E.; Lin, J.; Cooks, R.G.; Ouyang, Z. Development, characterization, and application of paper spray ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Jiang, T.; Huang, G.; Wei, Y.; Xu, W. An aerodynamic assisted miniature mass spectrometer for enhanced volatile sample analysis. Analyst 2016, 141, 5404–5411. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhang, X.; Xu, H.; Zheng, Y.; Yuan, T.; Xu, W. Mini Mass Spectrometer Integrated with a Miniature Ion Funnel. Anal. Chem. 2017, 89, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Hu, L.; Xu, W.; Li, H.; Li, C. A Dual-Source Miniature Mass Spectrometer with Improved Sensitivity. Int. J. Mass Spectrom. 2017, 423, 15–19. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Zhang, X.; Zhai, Y.; Xu, W. Mini 2000: A Robust Miniature Mass Spectrometer with Continuous Atmospheric Pressure Interface. Instruments 2018, 2, 2. https://doi.org/10.3390/instruments2010002
Meng X, Zhang X, Zhai Y, Xu W. Mini 2000: A Robust Miniature Mass Spectrometer with Continuous Atmospheric Pressure Interface. Instruments. 2018; 2(1):2. https://doi.org/10.3390/instruments2010002
Chicago/Turabian StyleMeng, Xiangzhi, Xiaohua Zhang, Yanbing Zhai, and Wei Xu. 2018. "Mini 2000: A Robust Miniature Mass Spectrometer with Continuous Atmospheric Pressure Interface" Instruments 2, no. 1: 2. https://doi.org/10.3390/instruments2010002
APA StyleMeng, X., Zhang, X., Zhai, Y., & Xu, W. (2018). Mini 2000: A Robust Miniature Mass Spectrometer with Continuous Atmospheric Pressure Interface. Instruments, 2(1), 2. https://doi.org/10.3390/instruments2010002




