The Synthesis of C70 Fullerene Nanowhiskers Using the Evaporating Drop Method
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ogawa, K.; Kato, T.; Ikegami, A.; Tsuji, H.; Aoki, N.; Ochiai, Y.; Bird, J.P. Electrical properties of field-effect transistors based on C60 nanowhiskers. Appl. Phys. Lett. 2006, 88, 112109. [Google Scholar] [CrossRef]
- Larsen, C.; Barzegar, H.R.; Nitze, F.; Wagberg, T.; Edman, L. On the fabrication of crystalline C60 nanorod transistors from solution. J. Nanotechnol. 2012, 23, 344015. [Google Scholar] [CrossRef]
- Joyce, H.J.; Gao, Q.; Tan, H.H.; Jagadish, C.; Kim, Y.; Zou, J.; Smith, L.M.; Jackson, H.E.; Yarrison-Rice, J.M.; Parkinson, P.; et al. III–V semiconductor nanowires for optoelectronic device applications. Prog. Quantum. Electron. 2011, 35, 23–75. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.H.; Bocchetta, P. Polymer/Fullerene Nanocomposite for Optoelectronics-Moving toward Green Technology. J. Compos. Sci. 2011, 6, 393. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis and properties of fullerene nanowhiskers and fullerene nanotubes. J. Nanosci. Nanotechnol. 2009, 9, 41–50. [Google Scholar] [CrossRef]
- Salhi, B.; Hossain, M.; Mukhaimer, A.; Al-Sulaiman, F.A. Nanowires: A new pathway to nanotechnology-based applications. J. Electroceram. 2016, 37, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Yang, P. Semiconductor Nanowires for Energy Conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef]
- Zhang, G.; Finefrock, S.; Liang, D.; Yadav, G.G.; Yang, H.; Fang, H.; Wu, Y. Semiconductor nanostructure-based photovoltaic solar cells. Nanoscale 2011, 3, 2430–2443. [Google Scholar] [CrossRef]
- Sun, K.; Kargar, A.; Park, N.; Madsen, K.N.; Naughton, P.W.; Bright, T.; Jing, Y.; Wang, D. Compound Semiconductor Nanowire Solar Cells. IEEE J. Sel. Top. Quantum Electron. 2011, 17, 1033–1049. [Google Scholar] [CrossRef]
- Okuda-Shimazaki, J.; Nudejima, S.; Takaku, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effects of fullerene nanowhiskers on cytotoxicity and gene expression. Health 2010, 2, 1456–1459. [Google Scholar] [CrossRef]
- Akiyama, T. Development of Fullerene Thin-Film Assemblies and Fullerene-Diamine Adducts towards Practical Nanocarbon-Based Electronic Materials. Bull. Chem. Soc. Jpn. 2019, 92, 1181–1199. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis of fullerene nanowhiskers using the liquid–liquid interfacial precipitation method and their mechanical, electrical and superconducting properties. Sci. Technol. Adv. Mater. 2015, 16, 013502. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Polymeric nanocomposites reinforced with nanowhiskers: Design, development, and emerging applications. J. Plast. Film. Sheeting 2020, 36, 312–333. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, W.H. Carbon Nanotubes as Active Components for Gas Sensors. J. Sens. 2009, 2009, 160698. [Google Scholar] [CrossRef]
- Naumova, O.V.; Nastaushev, Y.V.; Svitasheva, S.N.; Sokolov, L.V.; Zakharov, N.D.; Werner, P.; Gavrilova, T.A.; Dultsev, F.N.; Aseev, A.L. Molecular-beam epitaxy-grown Si whisker structures: Morphological, optical and electrical properties. Nanotechnology 2008, 19, 225708. [Google Scholar] [CrossRef]
- Zhang, X.; Dubrovskii, V.G.; Sibirev, N.V.; Cirlin, G.E.; Sartel, C.; Tchernycheva, M.; Harmand, J.C.; Glas, F. Growth of Inclined GaAs Nanowires by Molecular Beam Epitaxy: Theory and Experiment. Nanoscale Res. Lett. 2010, 5, 1692–1697. [Google Scholar] [CrossRef]
- Xia, M.; Guo, H.Y.; Hussain, M.I. Controllable Combustion Synthesis of SiC Nanowhiskers in a Si–C–N System: The Role of the Catalyst. Appl. Sci. 2020, 10, 252. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K.; Hill, J.P.; Ariga, K. Solvent Engineering for Shape-Shifter Pure Fullerene (C60). J. Am. Chem. Soc. 2009, 131, 6372–6373. [Google Scholar] [CrossRef]
- Makhmanov, U.K.; Kokhkharov, A.M.; Bakhramov, S.A.; Erts, D. The formation of self-assembled structures of C60 in solution and in the volume of an evaporating drop of a colloidal solution. Lith. J. Phys. 2020, 60, 194–204. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O. Fullerenes in molecular liquids. Solutions in “good” solvents: Another view. J. Mol. Liq. 2011, 161, 1–12. [Google Scholar] [CrossRef]
- Xu, T.; Shen, W.; Huang, W.; Lu, X. Fullerene micro/nanostructures: Controlled synthesis and energy applications. Mater. Today Nano 2020, 11, 100081. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Sabirov, D.S.; Terentyev, A.O.; Bulgakov, R.G. Polarizability of fullerene [2+2]-dimers: A DFT study. Phys. Chem. Chem. Phys. 2014, 16, 14594–14600. [Google Scholar] [CrossRef] [PubMed]
- Itaka, K.; Yamashiro, M.; Yamaguchi, J.; Haemori, M.; Yaginuma, S.; Matsumoto, Y.; Kondo, M.; Koinuma, H. High-Mobility C60 Field-Effect Transistors Fabricated on Molecular-Wetting Controlled Substrates. Adv. Mater. 2006, 18, 1713–1716. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, G.; Jiang, B.; Ji, D.; Kong, H.; Riehemann, K.; Ji, Q.; Fuchs, H. Self-assembled fullerene (C60)-pentacene superstructures for photodetectors. SmartMat 2021, 2, 109–118. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Nakanishi, W.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Hierarchically Structured Fullerene C70 Cube for Sensing Volatile Aromatic Solvent Vapors. ACS Nano 2016, 10, 6631–6637. [Google Scholar] [CrossRef]
- Roy, J.K.; Kar, S.; Leszczynski, J. Optoelectronic Properties of C60 and C70 Fullerene Derivatives: Designing and Evaluating Novel Candidates for Efficient P3HT Polymer Solar Cells. Materials 2019, 12, 2282. [Google Scholar] [CrossRef]
- Grebinyka, A.; Grebinyk, S.; Prylutska, S.; Rittere, U.; Matyshevska, O.; Dandekar, T.; Frohm, M. C60 fullerene accumulation in human leukemic cells and perspectives of LED-mediated photodynamic therapy. Free Radic. Biol. Med. 2018, 124, 319–327. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Miyazawa, K.I.; Obayashi, A.; Kuwabara, M. C60 nanowhiskers in a mixture of lead zirconate titanate sol–C60 toluene solution. J. Am. Ceram. Soc. 2001, 84, 3037–3039. [Google Scholar] [CrossRef]
- Hotta, K.; Miyazawa, K. Synthesis and growth investigation of C60 fullerene nanowhiskers. J. Phys. Conf. Ser. 2009, 159, 012021. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tachibana, M.; Kojima, K. Photo-assisted growth of C60 nanowhiskers from solution. J. Cryst. Growth 2005, 274, 617–621. [Google Scholar] [CrossRef]
- Miyazawa, K. C70 Nanowhiskers Fabricated by Forming Liquid/Liquid Interfaces in the Systems of Toluene Solution of C70 and Isopropyl Alcohol. J. Am. Ceram. Soc. 2002, 85, 1297–1299. [Google Scholar] [CrossRef]
- Bao, L.; Xu, T.; Guo, K.; Huang, W.; Lu, X. Supramolecular Engineering of Crystalline Fullerene Micro-/Nano-Architectures. Adv. Mater. 2022, 34, 2200189. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.V.; Benzigar, M.R.; Talapaneni, S.N.; Singh, G.; Karakoti, A.S.; Yi, J.; Al-Muhtaseb, A.H.; Ariga, K.; Ajayan, P.M.; Vinu, A. Self-Assembled Fullerene Nanostructures: Synthesis and Applications. Adv. Funct. Mater. 2022, 32, 2106924. [Google Scholar] [CrossRef]
- Ariga, K.; Shresth, L.K. Fullerene Nanoarchitectonics with Shape-Shifting. Materials 2020, 13, 2280–2295. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.; Choi, H.C. Selective growth of a C70 crystal in a mixed solvent system: From cube to tube. Chem. Mater. 2015, 27, 2408–2413. [Google Scholar] [CrossRef]
- Cui, D.; MacLeod, J.M.; Rosei, F. Planar Anchoring of C70 Liquid Crystals Using a Covalent Organic Framework Template. Small 2019, 15, 1903294. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Terentyev, A.O.; Cataldo, F. Bisadducts of the C60 and C70 fullerenes with anthracene: Isomerism and DFT estimation of stability and polarizability. Comput. Theor. Chem. 2016, 1081, 44–48. [Google Scholar] [CrossRef]
- Bakhramov, S.A.; Makhmanov, U.K.; Kokhkharov, A.M. Synthesis of nanoscale fullerene C60 filaments in the volume of an evaporating drop of a molecular solution and preparation of thin nanostructured coatings on their basis. Appl. Sol. Energy 2019, 55, 309–314. [Google Scholar] [CrossRef]
- Makhmanov, U.K.; Kokhkharov, A.M.; Bakhramov, S.A.; Esanov, S.A. Synthesis of fullerene C60 nanotubes in the volume of an evaporating drop of colloidal solution. Rom. J. Phys. 2022, 67, 601–609. [Google Scholar]
- Shao-Horn, Y.; Sheng, W.C.; Chen, S.; Ferreira, P.J.; Holby, E.F.; Morgan, D. Instability of Supported Platinum Nanoparticles in Low-Temperature Fuel Cells. Top. Catal. 2007, 46, 285–305. [Google Scholar] [CrossRef]

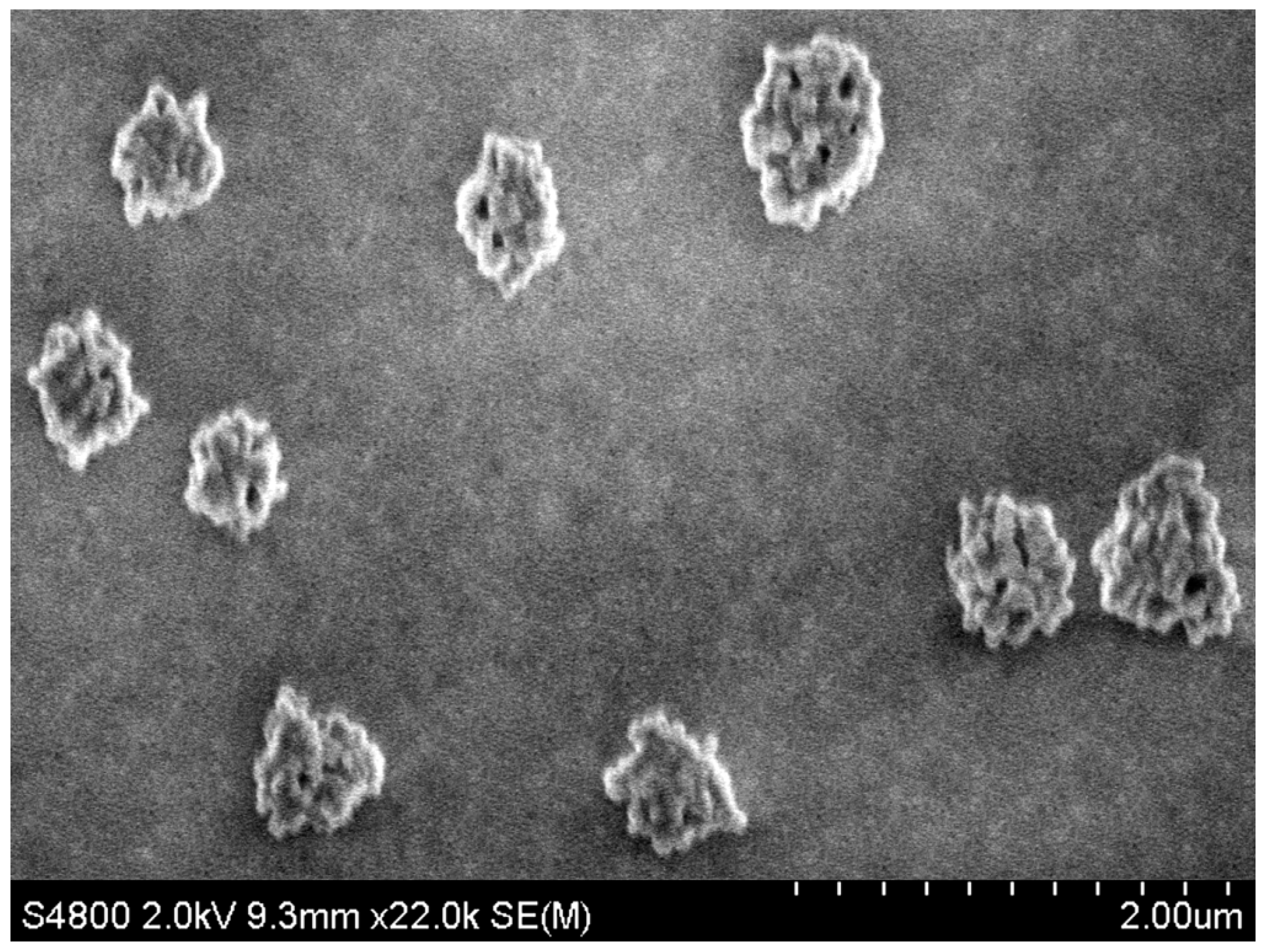
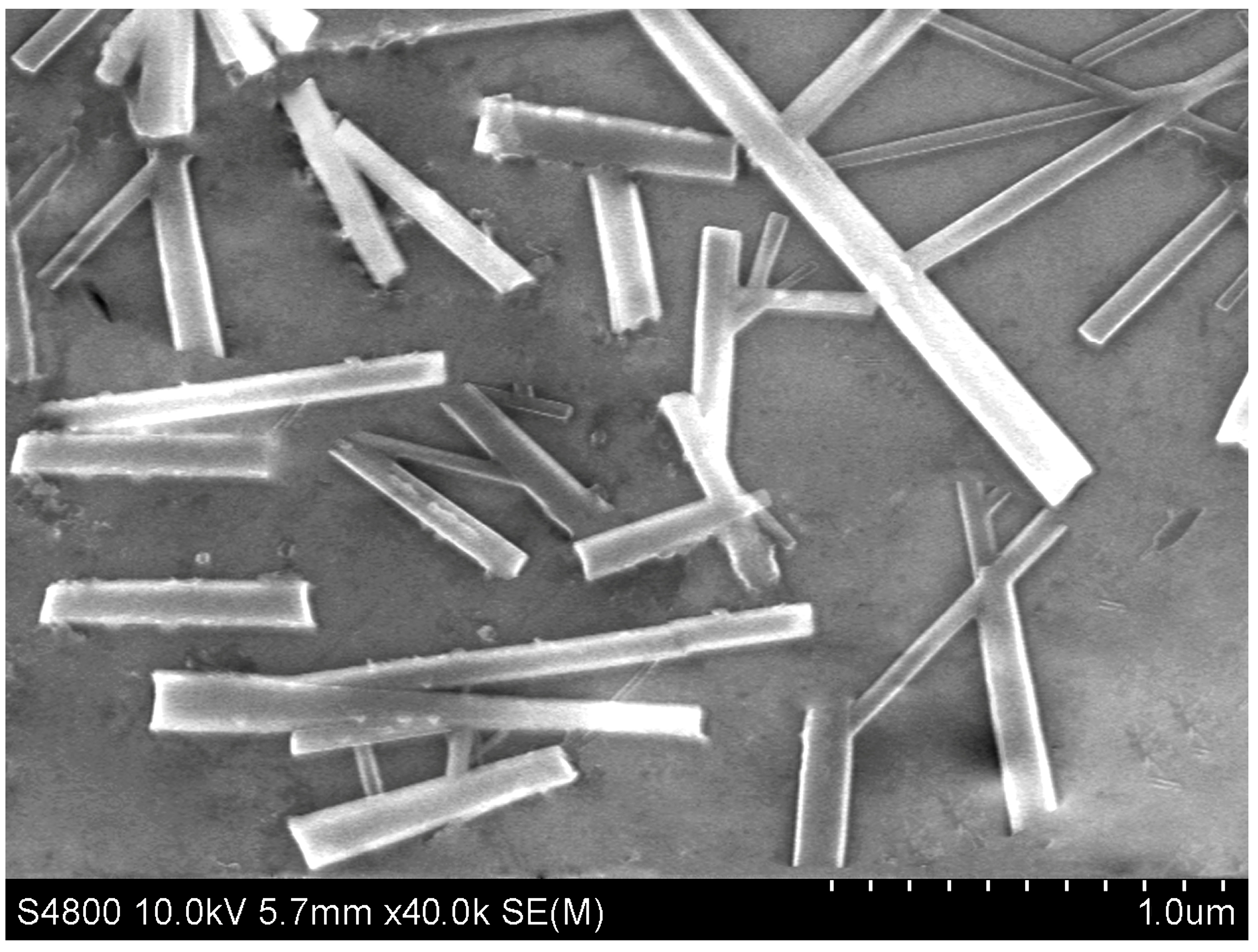


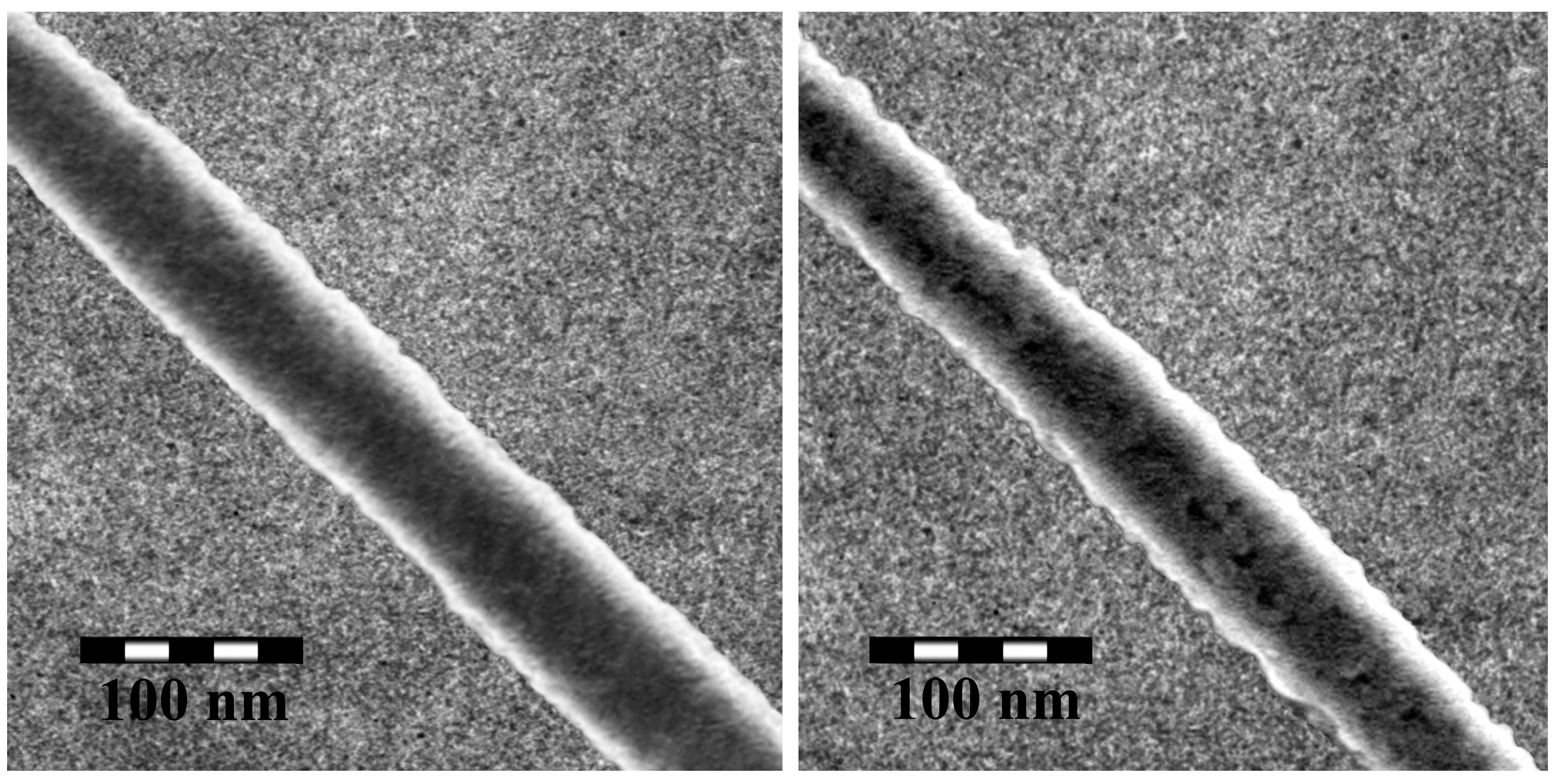
| C/(mol·L−1) a | T/(°C) b | Average Length/μm | Average Width/nm |
|---|---|---|---|
| ~1.1 × 10−3 | 28 | 0.75 | 105 |
| 32 | 1.35 | 152 | |
| 36 | 1.8 | 175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhramov, S.A.; Makhmanov, U.K.; Aslonov, B.A. The Synthesis of C70 Fullerene Nanowhiskers Using the Evaporating Drop Method. Condens. Matter 2023, 8, 62. https://doi.org/10.3390/condmat8030062
Bakhramov SA, Makhmanov UK, Aslonov BA. The Synthesis of C70 Fullerene Nanowhiskers Using the Evaporating Drop Method. Condensed Matter. 2023; 8(3):62. https://doi.org/10.3390/condmat8030062
Chicago/Turabian StyleBakhramov, Sagdulla A., Urol K. Makhmanov, and Bobirjon A. Aslonov. 2023. "The Synthesis of C70 Fullerene Nanowhiskers Using the Evaporating Drop Method" Condensed Matter 8, no. 3: 62. https://doi.org/10.3390/condmat8030062
APA StyleBakhramov, S. A., Makhmanov, U. K., & Aslonov, B. A. (2023). The Synthesis of C70 Fullerene Nanowhiskers Using the Evaporating Drop Method. Condensed Matter, 8(3), 62. https://doi.org/10.3390/condmat8030062








