Weak Ferromagnetism in a One-Orbital Double-Exchange Model with Ising Spins for Cerium Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Magnetic Properties of Free Ce or Ce Ions
- Rule 1: Maximize , where is the spin quantum number of a single electron.
- Rule 2: Maximize consistent with electron occupancy of the orbitals, where etc. is the orbital quantum number.
- Rule 3: Use (if the shell is less than half full) or (if the shell is more than half full) to calculate the total angular momentum.
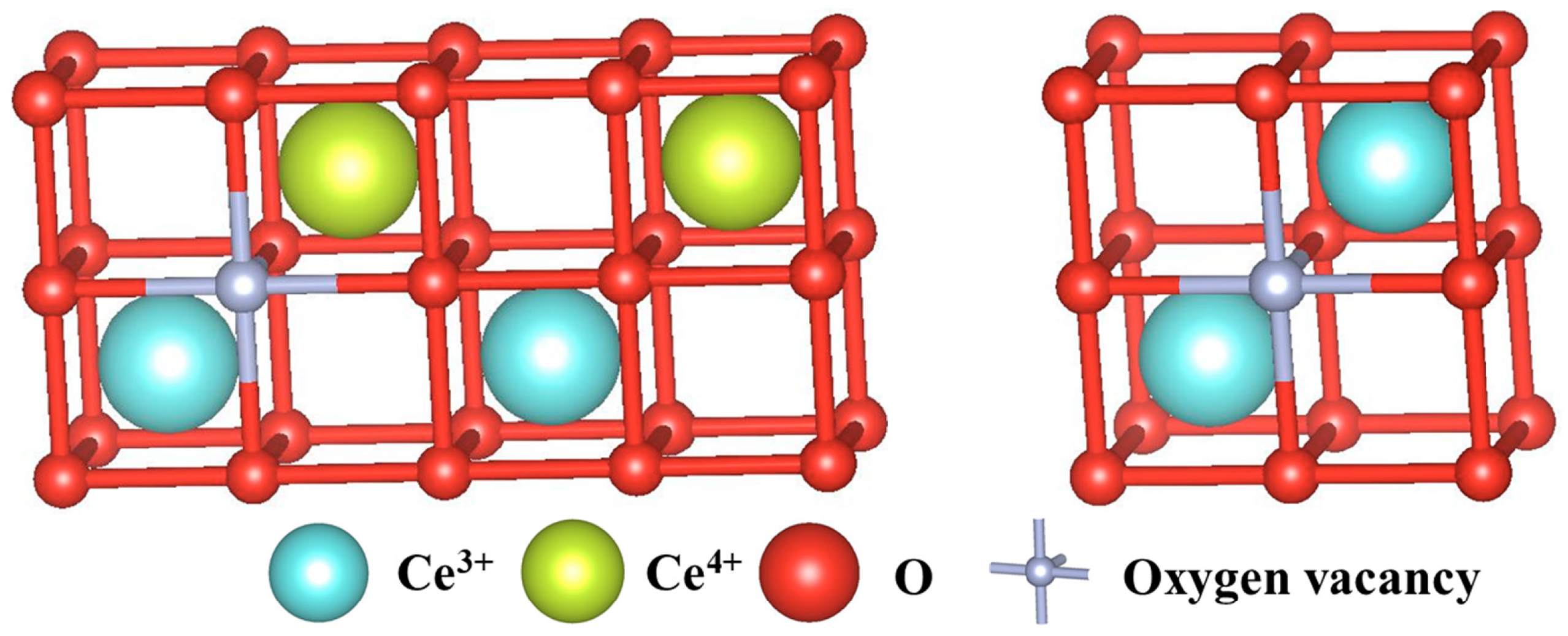
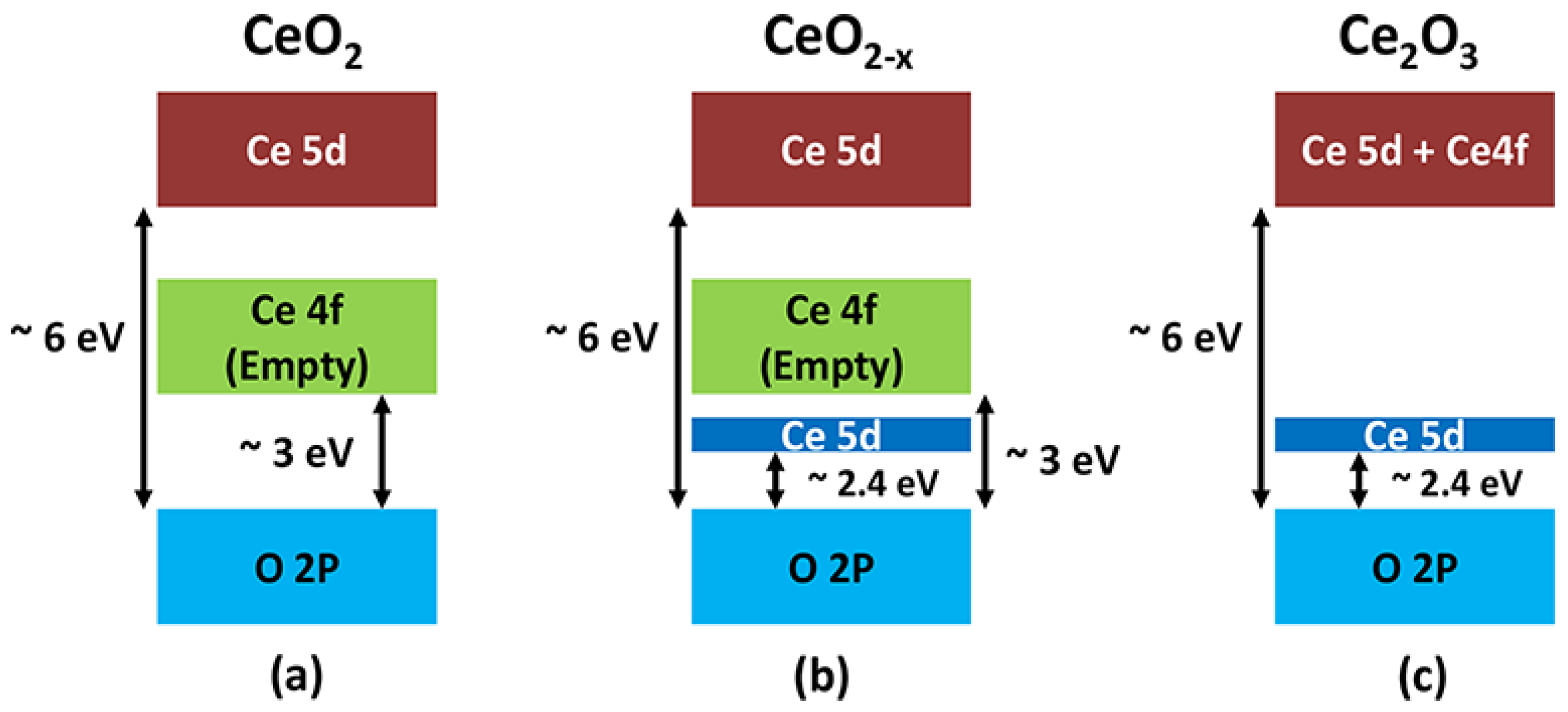
2.2. Crystal and Electronic Structure of Cerium Oxides
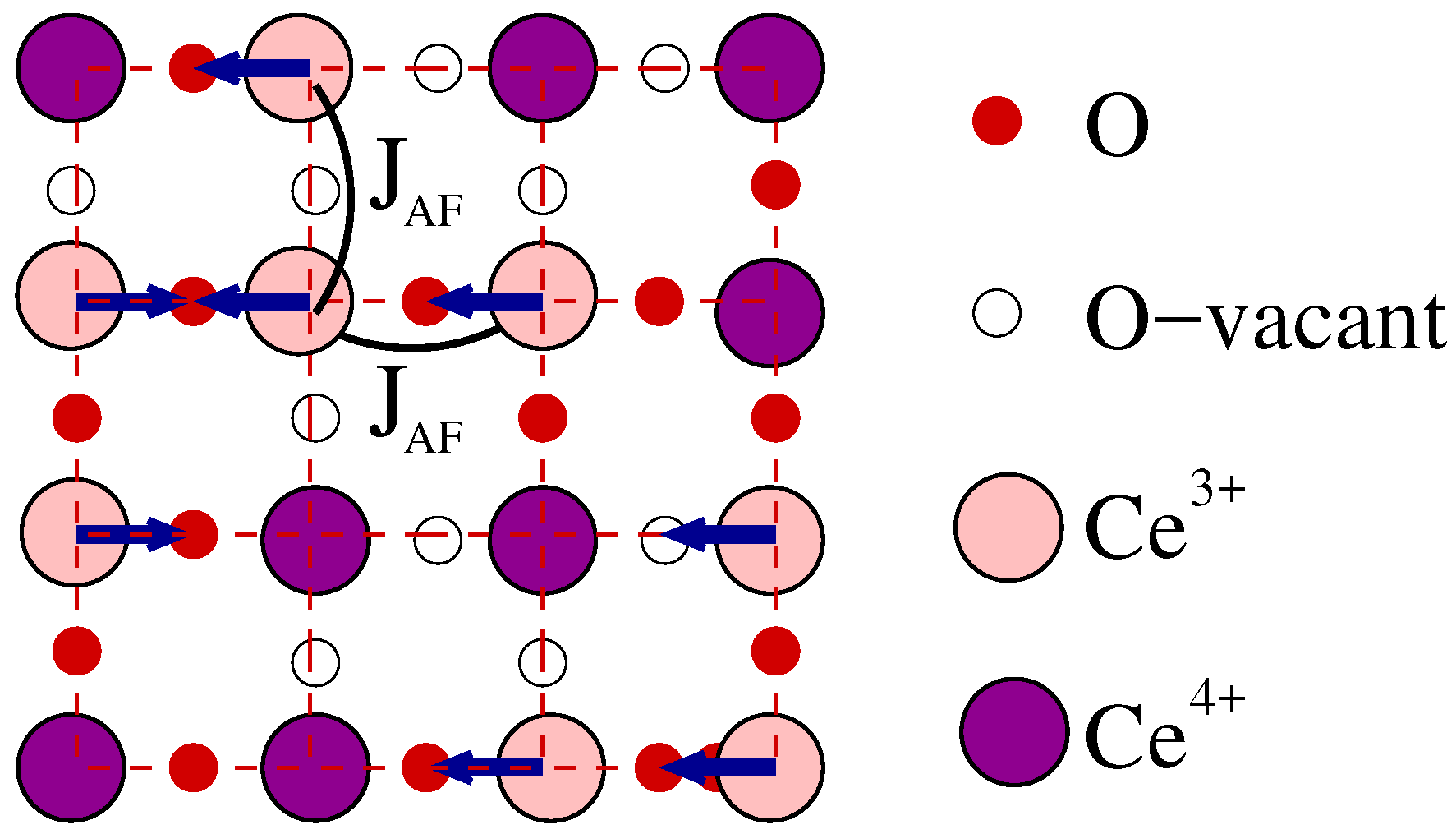
2.3. One-Orbital Model
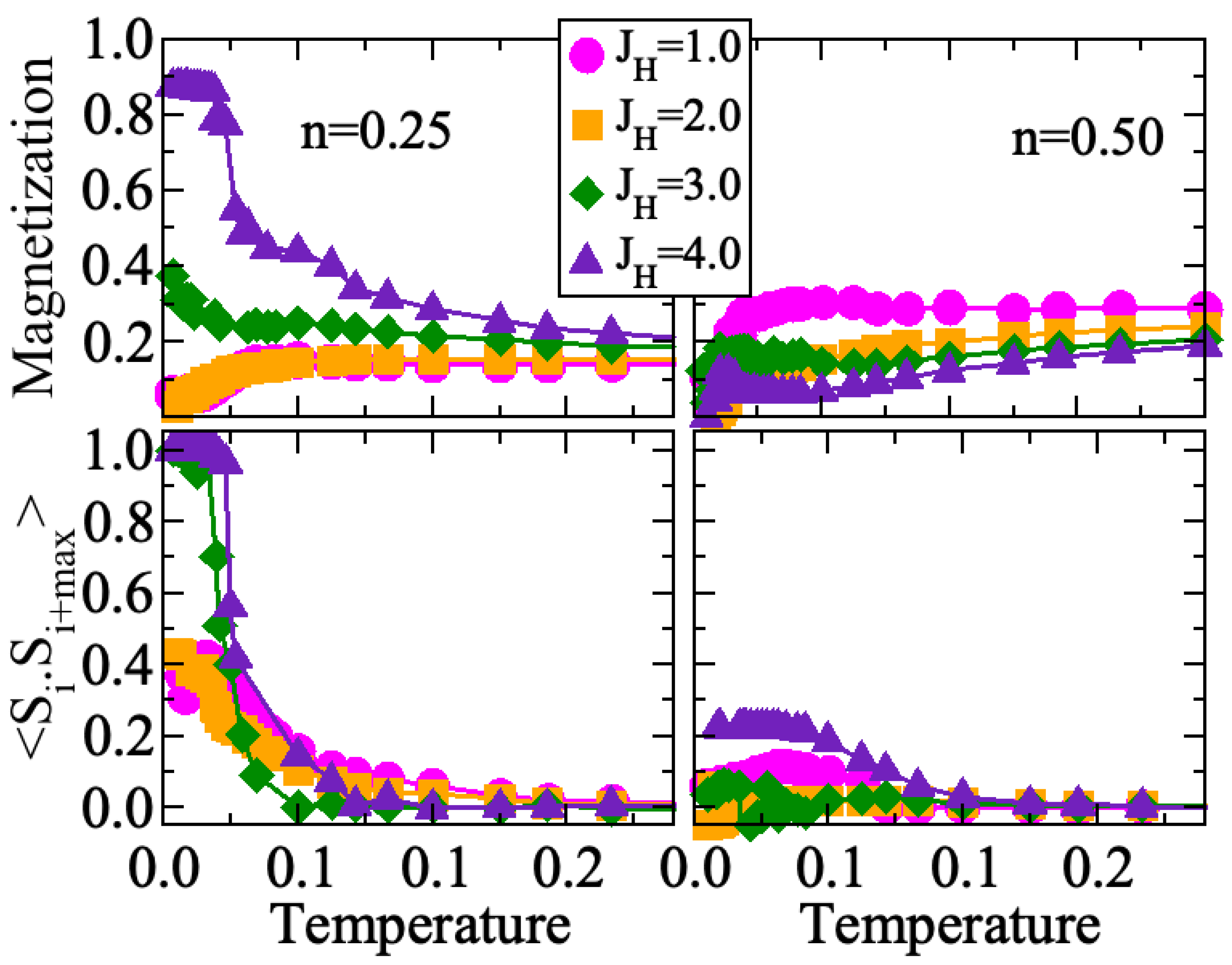
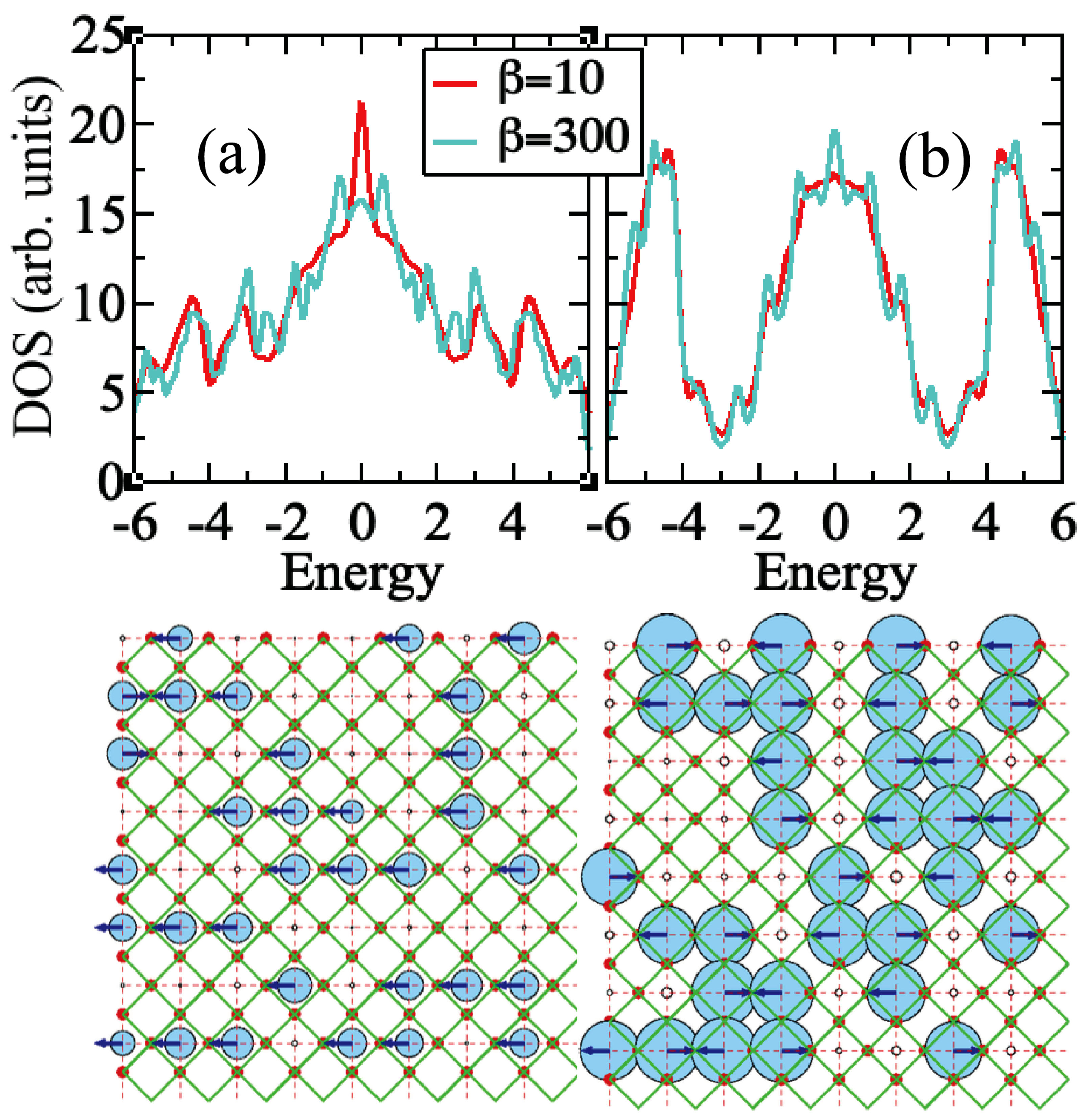
3. Results
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bednorz, J.G.; Müller, K.A. Possible high-Tc superconductivity in the Ba-La-Cu-O system. Z. Phys. Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Dagotto, E. Nanoscale Phase Separation and Colossal Magnetoresistance; Cardona, M., Fulde, P., von Klitzing, K., Merlin, R., Quessier, H.-J., Störmer, H., Eds.; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Ohno, H.; Munekata, H.; Penney, T.; von Molnár, S.; Chang, L.L. Magnetotransport properties of p-type (In,Mn)As diluted magnetic III-V semiconductors. Phys. Rev. Lett. 1992, 68, 2664–2667. [Google Scholar] [CrossRef] [PubMed]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Jasinski, P.; Suzuki, T.; Anderson, H.U. Nanocrystalline undoped ceria oxygen sensor. Sensors Actuators B 2003, 95, 73–77. [Google Scholar] [CrossRef]
- Stoukides, M. Solid-electrolyte membrane reactors: Current experience and future outlook. Catal. Rev. 2000, 42, 1–70. [Google Scholar] [CrossRef]
- Gorte, R.J.; Park, S.; Vohs, J.M. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar]
- Esposito, V.; Traversa, E. Design of electroceramics for solid oxide fuel cell applications: Playing with ceria. J. Am. Ceram. Soc. 2008, 91, 1037–1051. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 91, 1411–1420. [Google Scholar] [CrossRef]
- Caputo, F.; De Nicola, M.; Ghibelli, L. Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol. 2014, 92, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, A.; Bhargavi, R.; Rangarajan, N.; Siddesh, U.; Rao, C.N.R. Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys. Rev. B 2006, 74, 161306. [Google Scholar] [CrossRef]
- Coey, J.M.D. d0 ferromagnetism. Sol. Stat. Sci. 2005, 76, 660–667. [Google Scholar] [CrossRef]
- Wen, Q.-Y.; Zhang, H.-W.; Song, Y.-Q.; Yang, Q.-H.; Zhu, H.; Xiao, J.Q. Room-temperature ferromagnetism in pure and Co doped CeO2 powders. J. Phys. Condens. Matter 2007, 19, 246205. [Google Scholar] [CrossRef]
- Xia, C.; Hu, C.; Chen, P.; Wan, B.; He, X.; Tian, Y. Magnetic properties and photoabsorption of the Mn-doped CeO2 nanorods. Mater. Res. Bull. 2010, 45, 794–798. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lu, Y.-H.; Huang, T.-W.; Yan, D.-C.; Dong, C.-L. Oxygen Vacancy Dependent Magnetism of CeO2 Nanoparticles Prepared by Thermal Decomposition Method. J. Phys. Chem. C 2010, 114, 19576–19581. [Google Scholar] [CrossRef]
- Shah, L.R.; Ali, B.; Zhu, H.; Wang, W.G.; Song, Y.Q.; Zhang, H.W.; Shah, S.I.; Xiao, J.Q. Detailed study on the role of oxygen vacancies in structural, magnetic and transport behavior of magnetic insulator: Co-CeO2. J. Phys. Condens. Matter 2009, 21, 486004. [Google Scholar] [CrossRef] [Green Version]
- Shah, L.R.; Wang, W.; Zhu, H.; Ali, B.; Song, Y.Q.; Zhang, H.W.; Shah, S.I.; Xiao, J.Q. Role of dopant, defect, and host oxide in the observed room temperature ferromagnetism: Co-ZnO versus Co-CeO2. J. Appl. Phys. 2009, 105, 07C515. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.-Y.; Zhang, H.-W.; Yang, Q.-H.; Song, Y.-Q.; Xiao, J.Q. Effects of Fe doping and the dielectric constant on the room temperature ferromagnetism of polycrystalline CeO2 oxides. J. Appl. Phys. 2010, 107, 09C307. [Google Scholar] [CrossRef]
- Ackland, K.; Coey, J.M.D. Room temperature magnetism in CeO2—A review. Phys. Rep. 2018, 746, 1–39. [Google Scholar] [CrossRef]
- García, M.A.; Merino, J.M.; Fernández Pinel, E.; Quesada, A.; de la Venta, J.; Ruíz González, M.L.; Castro, G.R.; Crespo, P.; Llopis, J.; González-Calbet, J.M.; et al. Magnetic Properties of ZnO Nanoparticles. Nano Lett. 2007, 7, 1489–1494. [Google Scholar] [CrossRef] [Green Version]
- Chaboy, J.; Boada, R.; Piquer, C.; Laguna-Marco, M.A.; García-Hernández, M.; Carmona, N.; Llopis, J.; Ruíz-González, M.L.; González-Calbet, M.; Fernández, J.F.; et al. Evidence of intrinsic magnetism in capped ZnO nanoparticles. Phys. Rev. B 2010, 82, 064411. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Rossier, J.; Palacios, J.J. Magnetism in Graphene Nanoislands. Phys. Rev. Lett. 2007, 99, 177204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, Y.; Song, Y.; Zhang, X.; Ma, Y.; Liang, J.; Chen, Y. Room-Temperature Ferromagnetism of Graphene. Nano Lett. 2009, 9, 220–224. [Google Scholar] [CrossRef]
- Hong, N.H.; Sakai, J.; Poirot, N.; Brizé, V. Room-temperature ferromagnetism observed in undoped semiconducting and insulating oxide thin films. Phys. Rev. B 2006, 73, 132404. [Google Scholar] [CrossRef]
- Rumaiz, A.K.; Ali, B.; Ceylan, A.; Boggs, M.; Beebe, T.; Shah, S.I. Experimental studies on vacancy induced ferromagnetism in undoped TiO2. Sol. Stat. Commun. 2007, 144, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Ramazanov, S.; Dobola, D.; Orudzhev, F.; Knápek, A.; Polčák, J.; Potoček, M.; Kaspar, P.; Dallaev, R. Surface modification and enhancement of ferromagnetism in BiFeO3 nanofilms deposited on HOPG. Nanomaterials 2020, 10, 1990. [Google Scholar] [CrossRef]
- Loh, E.Y.; Gubernatis, J.E.; Scalettar, R.T.; White, S.R.; Scalapino, D.J.; Sugar, R.L. Sign problem in the numerical simulation of many-electron systems. Phys. Rev. B 1990, 41, 9301. [Google Scholar] [CrossRef]
- dos Santos, R.R. Introduction to quantum Monte Carlo simulations for fermionic systems. Braz. J. Phys. 2003, 33, 36–54. [Google Scholar] [CrossRef] [Green Version]
- Coey, J.M.D.; Venkatesan, M.; Xu, H. Introduction to Magnetic Oxides; Functional Metal Oxides: New Science and Novel Applications; Ogale, S.B., Venkatesan, T.V., Blamire, M.G., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013; Chapter 1; pp. 1–49. [Google Scholar]
- Eyring, L. Handbook on the Physics and Chemistry of Rare Earths; Gschneider, K.A., Jr., Eyring, L., Eds.; Metals: Amsterdam, The Netherlands, 1979; Volume 1. [Google Scholar]
- Hayes, W.; Stoneham, A.M. Defects and Defect Processes in Nonmetallic Solids; Hayes, W., Stoneham, A.M., Eds.; Dover: New York, NY, USA, 1979. [Google Scholar]
- Gangopadhyay, S.; Frolov, D.D.; Masunov, A.E.; Seal, S. Structure and properties of cerium oxides in bulk and nanoparticulate forms. J. Alloys Compd. 2014, 584, 199–208. [Google Scholar] [CrossRef]
- Kato, T.; Tsunazawa, Y.; Liu, W.; Tokoro, C. Structural change analysis of cerianite in weathered residual rare earth ore by mechanochemical reduction using X-ray absorption fine structure. Minerals 2019, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states - I-Orbital degeneracy. Proc. R. Soc. A 1937, 161, 220–235. [Google Scholar]
- Khomskii, D.I.; Sawatzky, G.A. Interplay between spin, charge and orbital degrees of freedom in magnetic oxides. Solid State Commun. 1997, 102, 87–99. [Google Scholar] [CrossRef]
- Dorenbos, P. A review on how lanthanide impurity levels change with chemistry and structure of inorganic compounds. ECS J. Sol. Stat. Sci. Technol. 2013, 2, R3001–R3011. [Google Scholar] [CrossRef] [Green Version]
- Yoca, S.E.; Pamleri, P.; Quinet, P.; Jumet, G.; Biémot, É. Radiative properties and core-polarization effects in the W5+ ion. J. Phys. B At. Mol. Opt. Phys. 2012, 45, 035002. [Google Scholar] [CrossRef] [Green Version]
- Younis, A.; Chou, D.; Li, S. Cerium Oxide Nanostructures and Their Applications; Functionalized Nanomaterials; Farrukh, M.A., Ed.; IntechOpen: London, UK, 2016; Chapter 3. [Google Scholar]
- Goodenough, J.B. Theory of the Role of Covalence in the Perovskite-Type Manganites [La,M(II)]MnO3. Phys. Rev. 1955, 100, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87–98. [Google Scholar] [CrossRef]
- Anderson, P.W. New approach to the theory of superexchange interactions. Phys. Rev. 1959, 115, 2–115. [Google Scholar] [CrossRef]
- Anderson, P.W. Theory of Magnetic Exchange Interactions: Exchange in Insulators and Semiconductors. Solid State Phys. 1963, 14C, 99–214. [Google Scholar]
- Riyadi, S.; Giriyapura, S.; de Groot, R.A.; Caretta, A.; van Loosdrecht, P.H.M.; Palstra, T.T.M.; Blake, G.R. Ferromagnetic order from p-electrons in Rubidium Oxide. Chem. Mater. 2011, 23, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Labhart, M.; Raoux, D.; Känzig, W.; Bösch, M.A. Magnetic order in 2p-electron systems: Electron paramagnetic resonance and antiferromagnetic resonance in the alkali hyperoxides KO2, RbO2, and CsO2. Phys. Rev. B 1979, 20, 53–70. [Google Scholar] [CrossRef]
- Dagotto, E.; Hotta, T.; Moreo, A. Colossal Magnetoresistant Materials: The Key Role of Phase Separation. Phys. Rep. 2001, 344, 1. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, G.; Mayr, M.; Dagotto, E. Phase diagram of a model for diluted magnetic semiconductors beyond mean-field approximations. Phys. Rev. Lett. 2002, 89, 277202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayr, M.; Alvarez, G.; Dagotto, E. Global versus local ferromagnetism in a model for diluted magnetic semiconductors studied with Monte Carlo techniques. Phys. Rev. B 2002, 65, 241202. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, Y.; Alvarez, G.; Moreo, A.; Dagotto, E. Large-Scale Monte Carlo Study of a Realistic Lattice Model for Ga1−xMnxAs. Phys. Rev. Lett. 2007, 99, 057207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şen, C. Weak Ferromagnetism in a One-Orbital Double-Exchange Model with Ising Spins for Cerium Oxides. Condens. Matter 2021, 6, 53. https://doi.org/10.3390/condmat6040053
Şen C. Weak Ferromagnetism in a One-Orbital Double-Exchange Model with Ising Spins for Cerium Oxides. Condensed Matter. 2021; 6(4):53. https://doi.org/10.3390/condmat6040053
Chicago/Turabian StyleŞen, Cengiz. 2021. "Weak Ferromagnetism in a One-Orbital Double-Exchange Model with Ising Spins for Cerium Oxides" Condensed Matter 6, no. 4: 53. https://doi.org/10.3390/condmat6040053
APA StyleŞen, C. (2021). Weak Ferromagnetism in a One-Orbital Double-Exchange Model with Ising Spins for Cerium Oxides. Condensed Matter, 6(4), 53. https://doi.org/10.3390/condmat6040053





