Features of Metal Hydrogenation during Electron Irradiation
Abstract
:1. Introduction
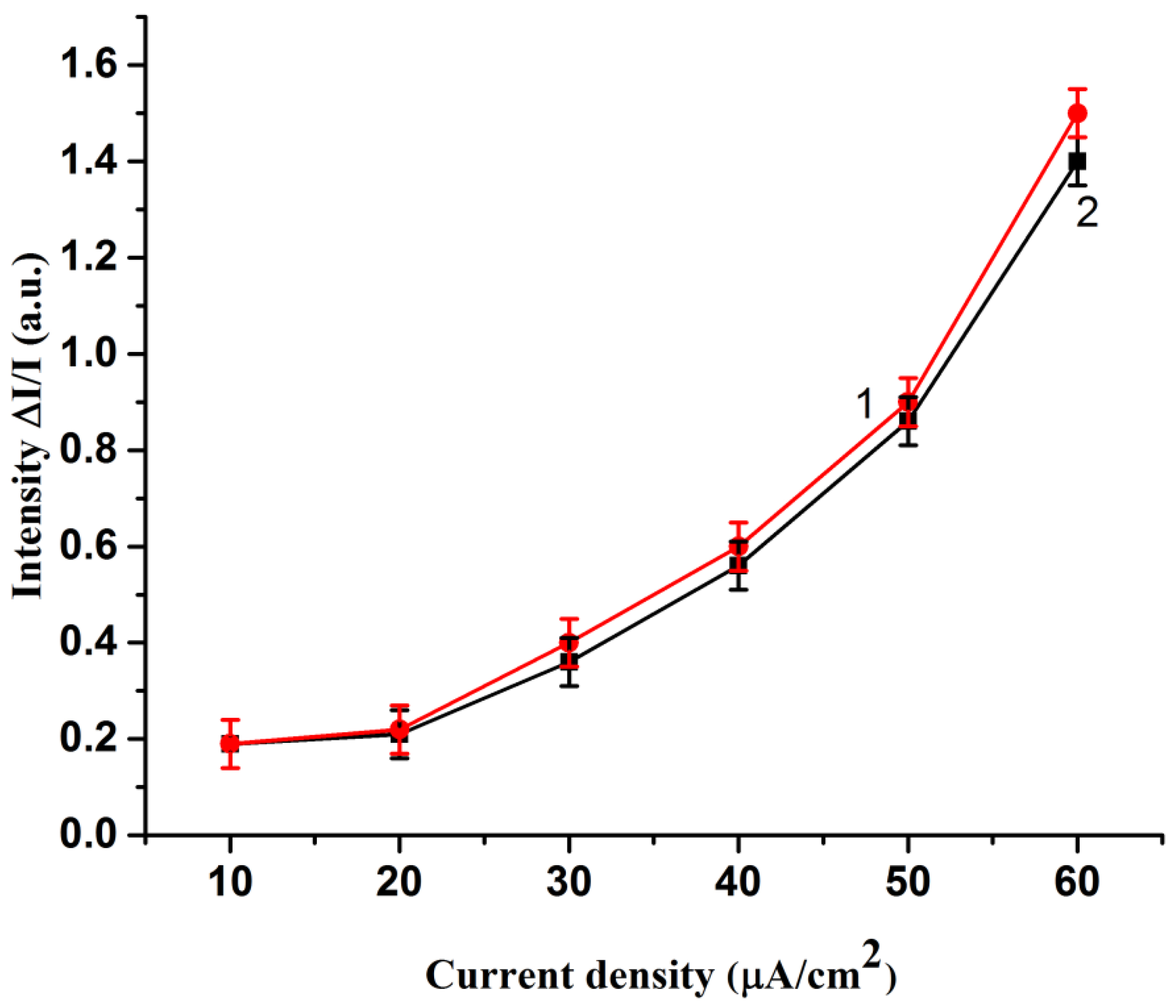
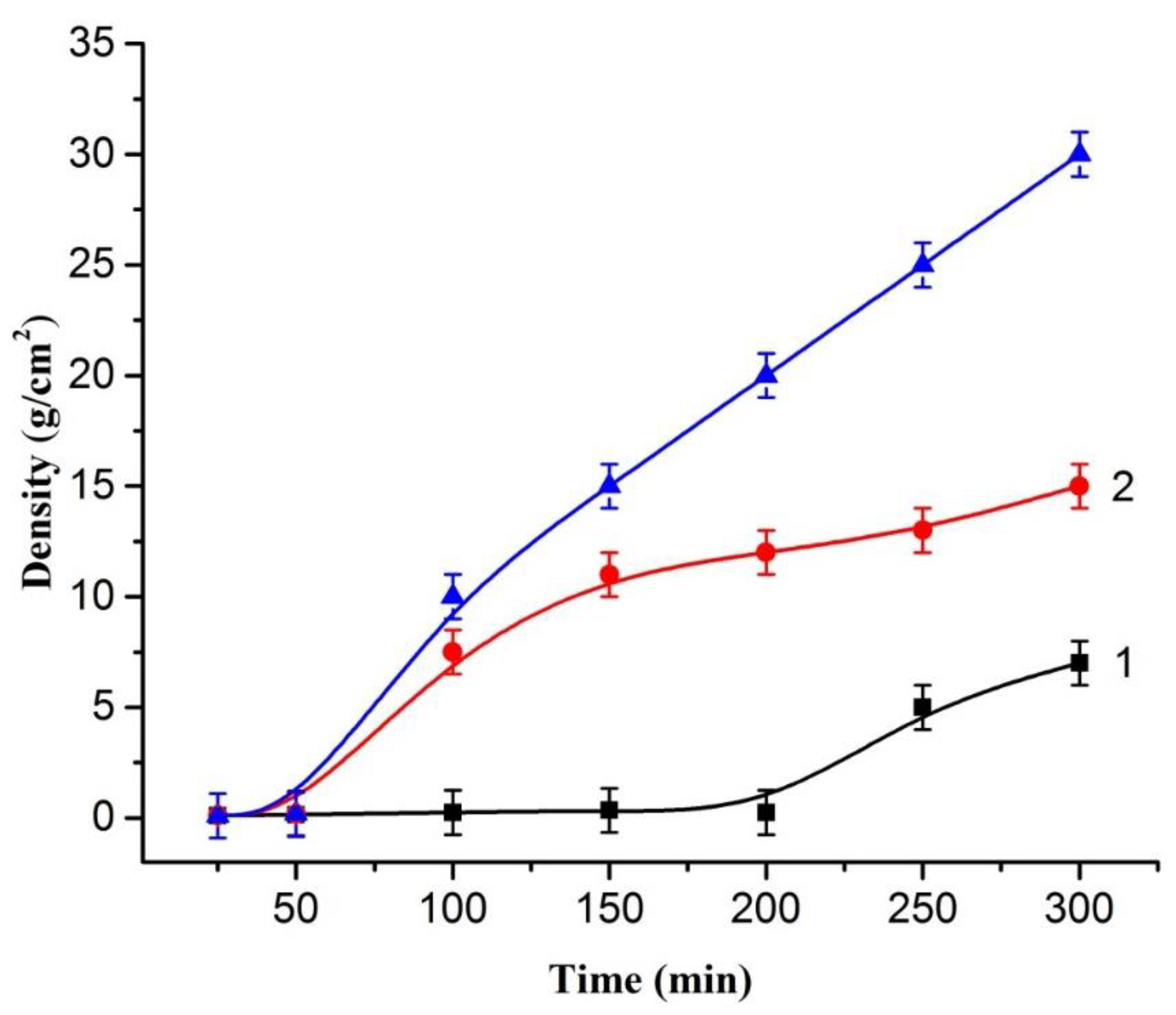
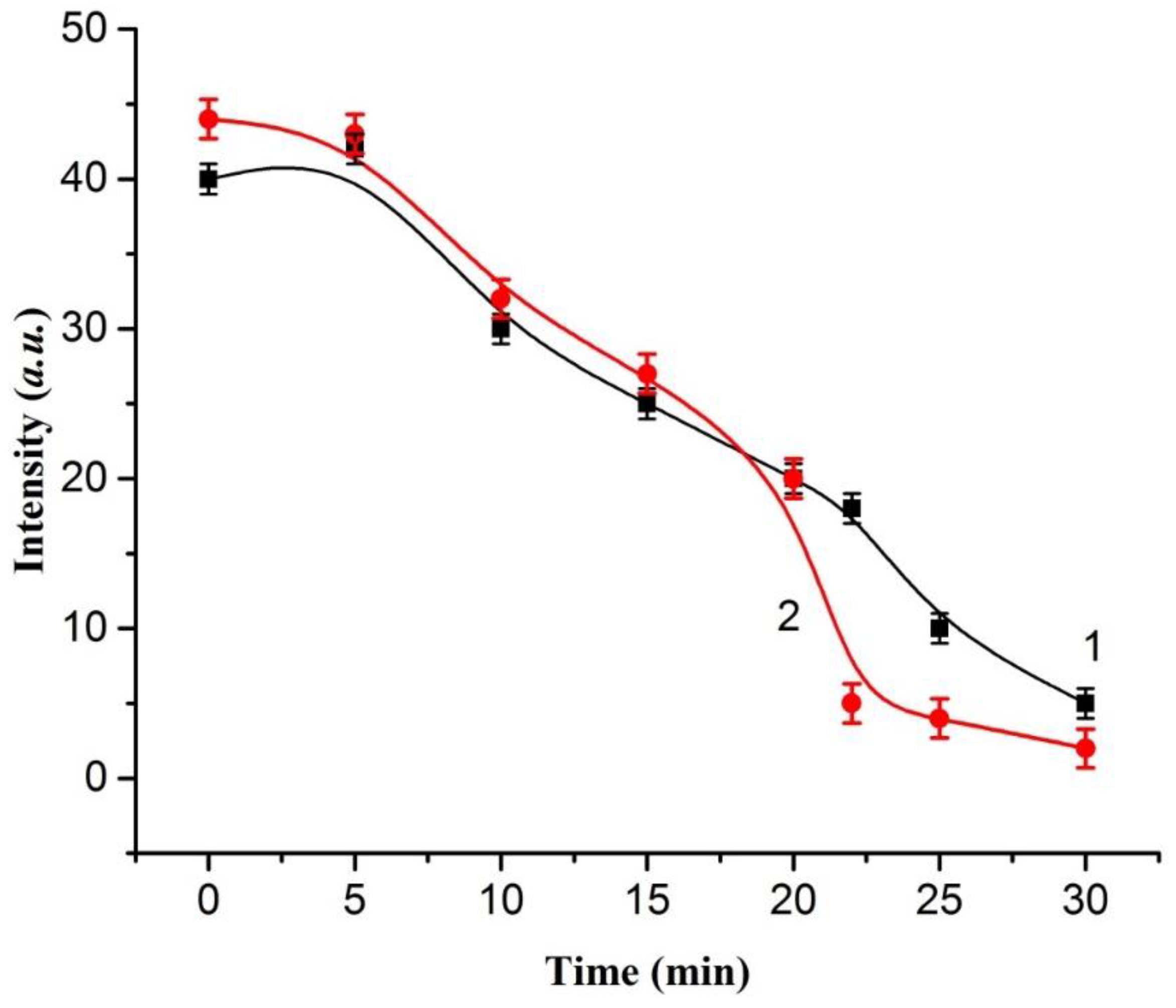
2. Results and Discussion
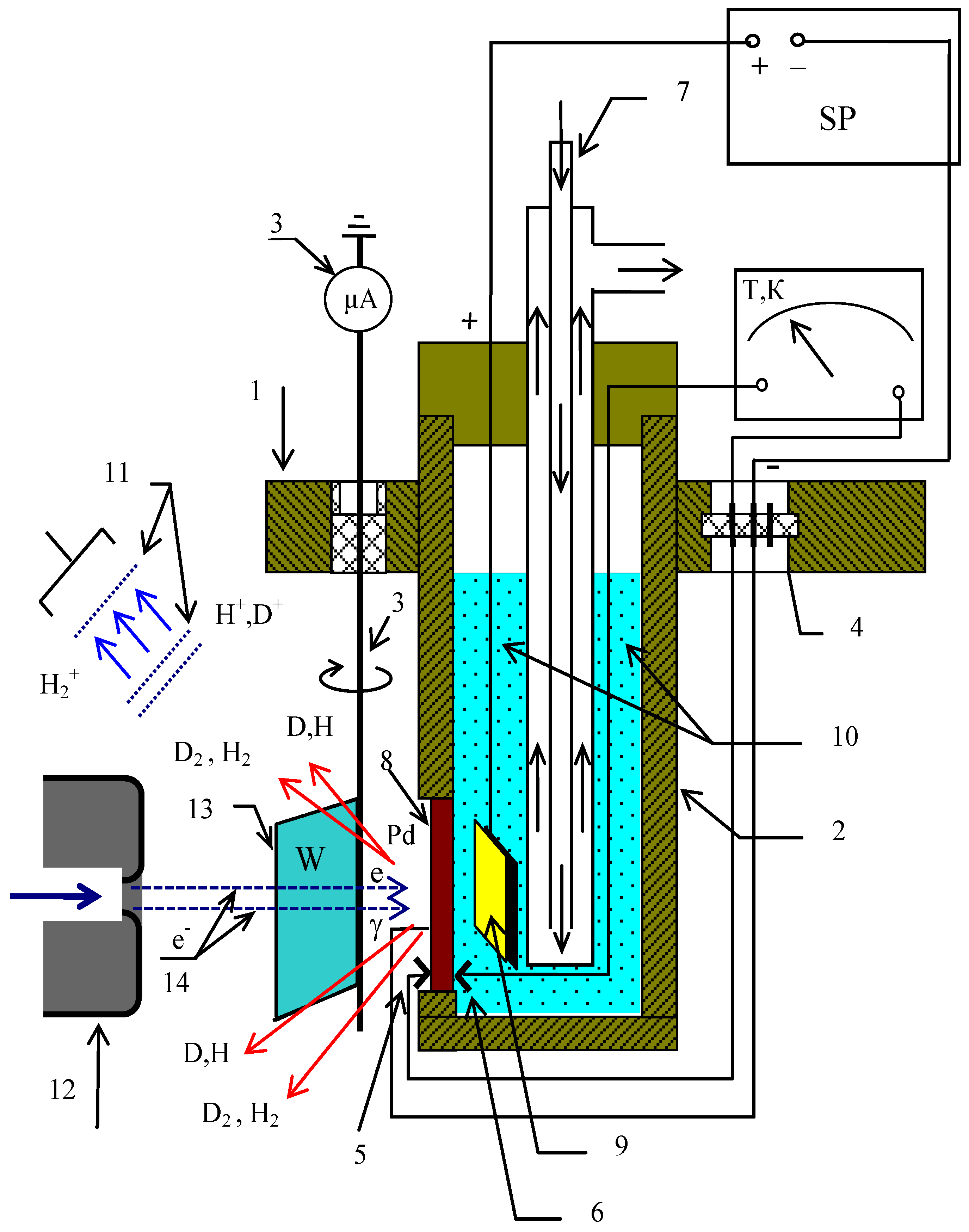
3. Materials and Methods
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alefeld, G.; Völkl, J. Hydrogen in Metals I-Basic Properties; Springer-Verlag: Berlin, Germany, 1978. [Google Scholar]
- Elias, R.J.; Corso, H.L.; Gervasoni, J.L. Fundamental aspects of the Ti–H system: Theoretical and experimental behaviour. Int. J. Hydrog. Energy 2002, 27, 91–97. [Google Scholar] [CrossRef]
- Evard, E.A.; Gabis, I.E.; Voyt, A.P. Study of the kinetics of hydrogen sorption and desorption from titanium. J. Alloys Compd. 2005, 404, 335–338. [Google Scholar] [CrossRef]
- Scholz, J.; Züchner, H.; Paulus, H.; Müller, K.H. Ion bombardment induced segregation effects in VDx studied by SIMS and SNMS. J. Alloys Compd. 1997, 253, 459–462. [Google Scholar] [CrossRef]
- Ignat’ev, A.I.; Nashchekin, A.V.; Nevedomskii, V.M.; Podsvirov, O.A.; Sidorov, A.I.; Solov’ev, A.P.; Usov, O.A. Formation of silver nanoparticles in photothermorefractive glasses during electron irradiation. Tech. Phys. 2011, 56, 662–667. [Google Scholar] [CrossRef]
- Popov, V.V.; Basteev, A.V.; Solovey, V.V.; Prognimak, A.M. The effect of metal-hydride activation of hydrogen and investigation of its influence on the characteristics of gas-discharge hydrogen-using energy conversion devices. Int. J. Hydrog. Energy 1996, 21, 259–265. [Google Scholar] [CrossRef]
- Prognimak, A.M. Mass-spectrometry investigation on the effect of metal-hydride activation of molecular hydrogen. Int. J. Hydrog. Energy 1995, 20, 449–453. [Google Scholar] [CrossRef]
- Aliotta, M.; Raiola, F.; Gyürky, G.; Formicola, A.; Bonetti, R.; Broggini, C.; Campajola, L.; Corvisiero, P.; Costantini, H.; D’Onofrio, A.; et al. Electron screening effect in the reactions 3He(d,p)4He and d(3He,p)4He. Nucl. Phys. A 2001, 690, 790–800. [Google Scholar] [CrossRef]
- Lipson, A.G.; Roussetski, A.S.; Karabut, A.B.; Miley, G. Strong Enhancement of DD-reaction and Soft X-ray Generation in a High-current Deuterium Glow Discharge at Voltages 0.8-2.5 keV. J. Exp. Theor. Phys. 2005, 100, 1334–1349. [Google Scholar] [CrossRef]
- Chan, C.T.; Louie, S.G. Self-consistent pseudopotential calculation of the electronic structure of PdH and Pd4H. Phys. Rev. B 1983, 27, 3325–3337. [Google Scholar] [CrossRef]
- Nechaev, I.A.; Zhukov, V.P.; Chulkov, E.V. Ab initio calculation of the lifetime of quasiparticle excitations in transition metals within the framework of the GW approximation. Phys. Solid State 2007, 49, 1811–1819. [Google Scholar] [CrossRef]
- Chernov, I.P.; Larionov, V.V.; Lider, A.M.; Maximova, N.G. Energy storage using palladium and titanium targets. Indian J. Sci. Technol. 2015, 8, 1–5. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.M. Hydrogen trapping phenomena in metals with BCC and FCC crystals structures by the desorption thermal analysis technique. Surf. Coat. Technol. 1986, 28, 301–314. [Google Scholar] [CrossRef]
- Podsiadły-Paszkowska, A.; Krawiec, M. Tuning the Electronic Structure of Hydrogen-Decorated Silicene. Condens. Matter 2017, 2, 1. [Google Scholar] [CrossRef]
- Chernov, I.P.; Larionov, V.V.; Lipson, A.G.E.; Tyurin, Y.I. Production of high-energy deuterons upon electron-beam excitation of deuterium-saturated palladium. High Temp. 2010, 48, 599–602. [Google Scholar] [CrossRef]
- Larionov, V.V.; Nikitenkov, N.N.; Tyurin, Y.I. Hydrogen diffusion in steels under electron bombardment. Tech. Phys. 2016, 61, 793–797. [Google Scholar] [CrossRef]
- Bordulev, Y.S.; Laptev, R.S.; Kudiiarov, V.N.; Lider, A.M. Investigation of commercially pure titanium structure during accumulation and release of hydrogen by means of positron lifetime and electrical resistivity measurements. Adv. Mater. Res. 2014, 880, 93–100. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larionov, V.; Tyurin, Y.; Murashkina, T.; Sigfusson, T. Features of Metal Hydrogenation during Electron Irradiation. Condens. Matter 2018, 3, 17. https://doi.org/10.3390/condmat3020017
Larionov V, Tyurin Y, Murashkina T, Sigfusson T. Features of Metal Hydrogenation during Electron Irradiation. Condensed Matter. 2018; 3(2):17. https://doi.org/10.3390/condmat3020017
Chicago/Turabian StyleLarionov, Vitaliy, Yuriy Tyurin, Tatyana Murashkina, and Thorsteinn Sigfusson. 2018. "Features of Metal Hydrogenation during Electron Irradiation" Condensed Matter 3, no. 2: 17. https://doi.org/10.3390/condmat3020017
APA StyleLarionov, V., Tyurin, Y., Murashkina, T., & Sigfusson, T. (2018). Features of Metal Hydrogenation during Electron Irradiation. Condensed Matter, 3(2), 17. https://doi.org/10.3390/condmat3020017




