Synthesis of PTh/PEDOT Films into FTO Substrate by Electrodeposition, for Energy Storage Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrosynthesis
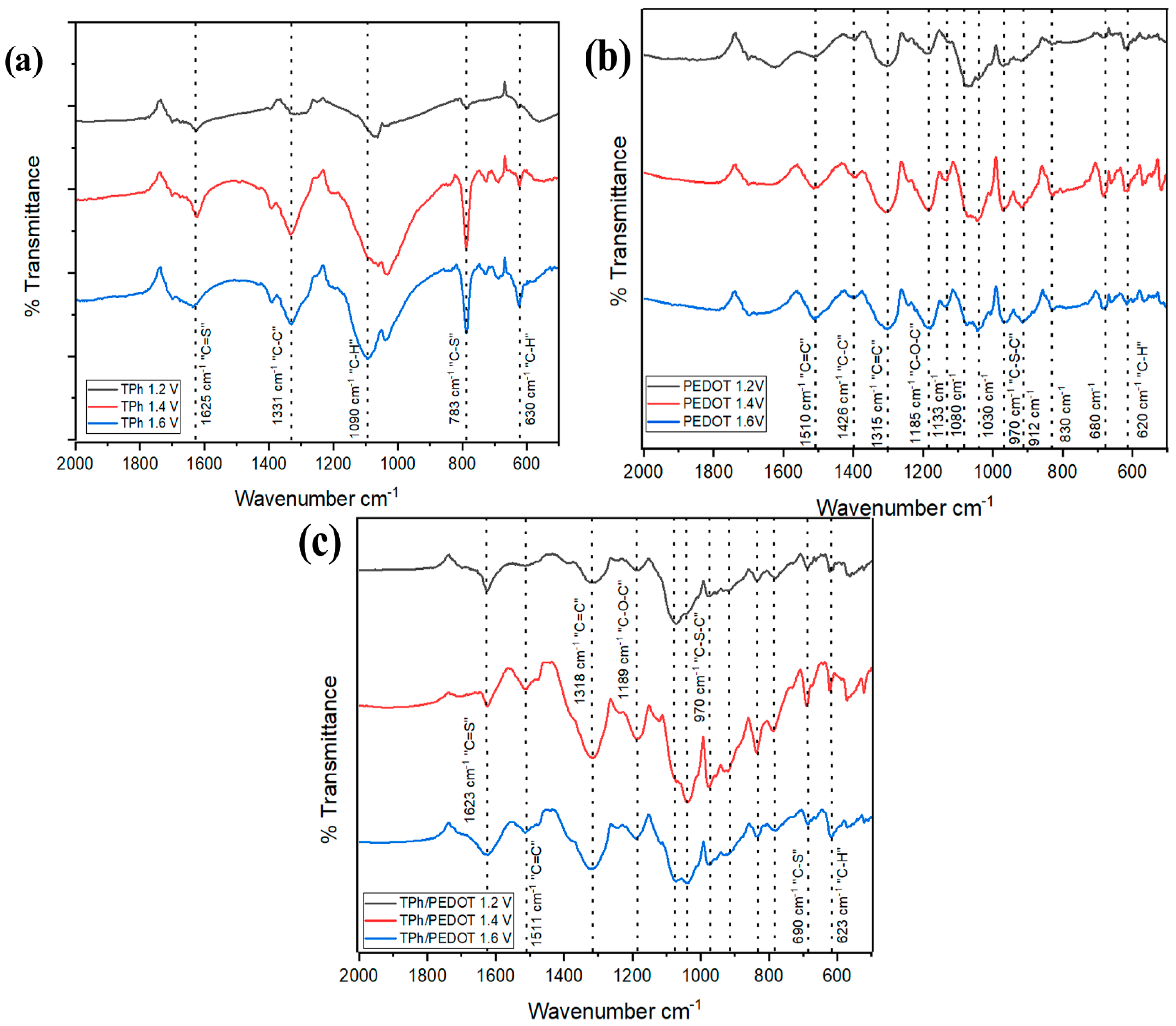
2.2. FTIR Characterization
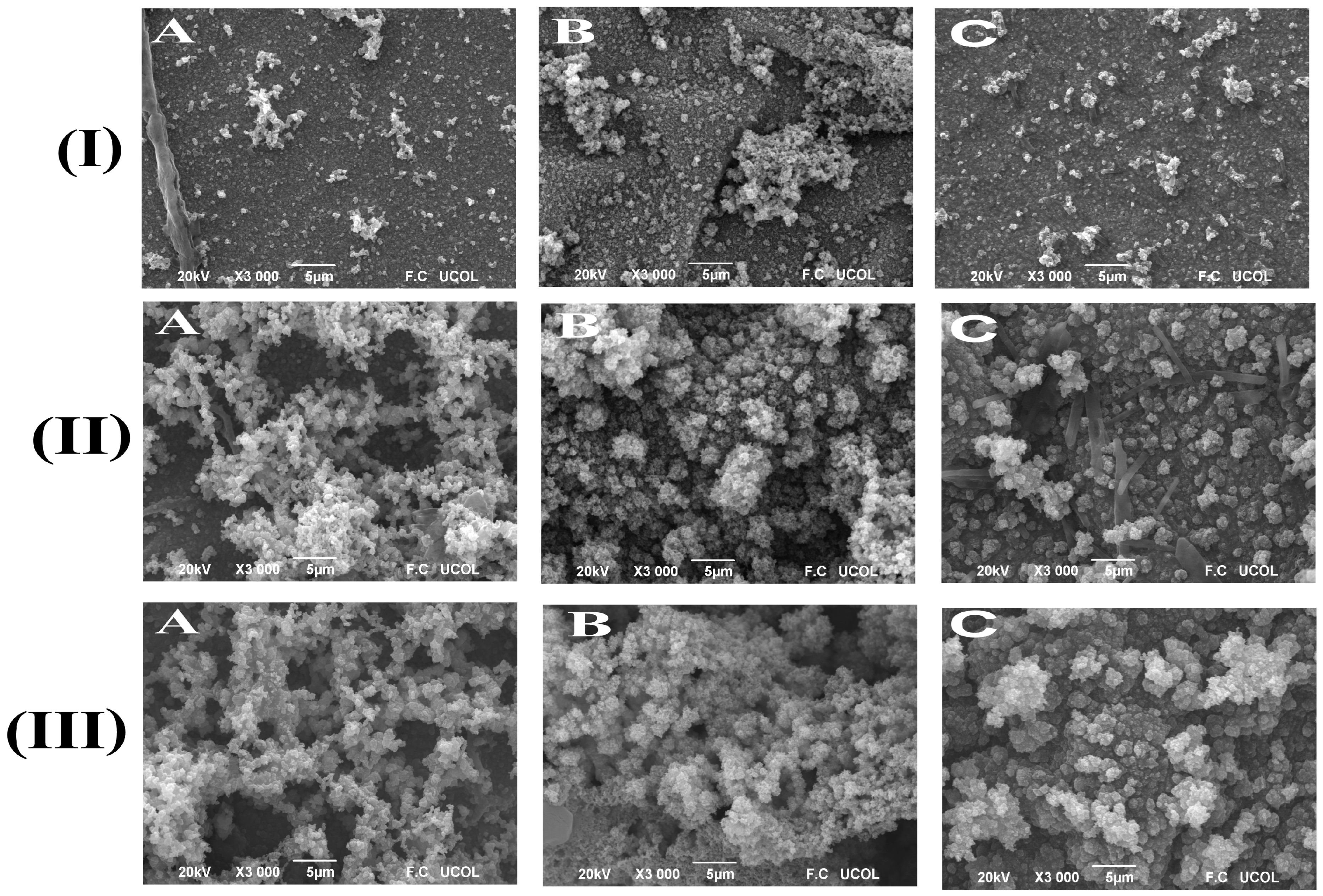
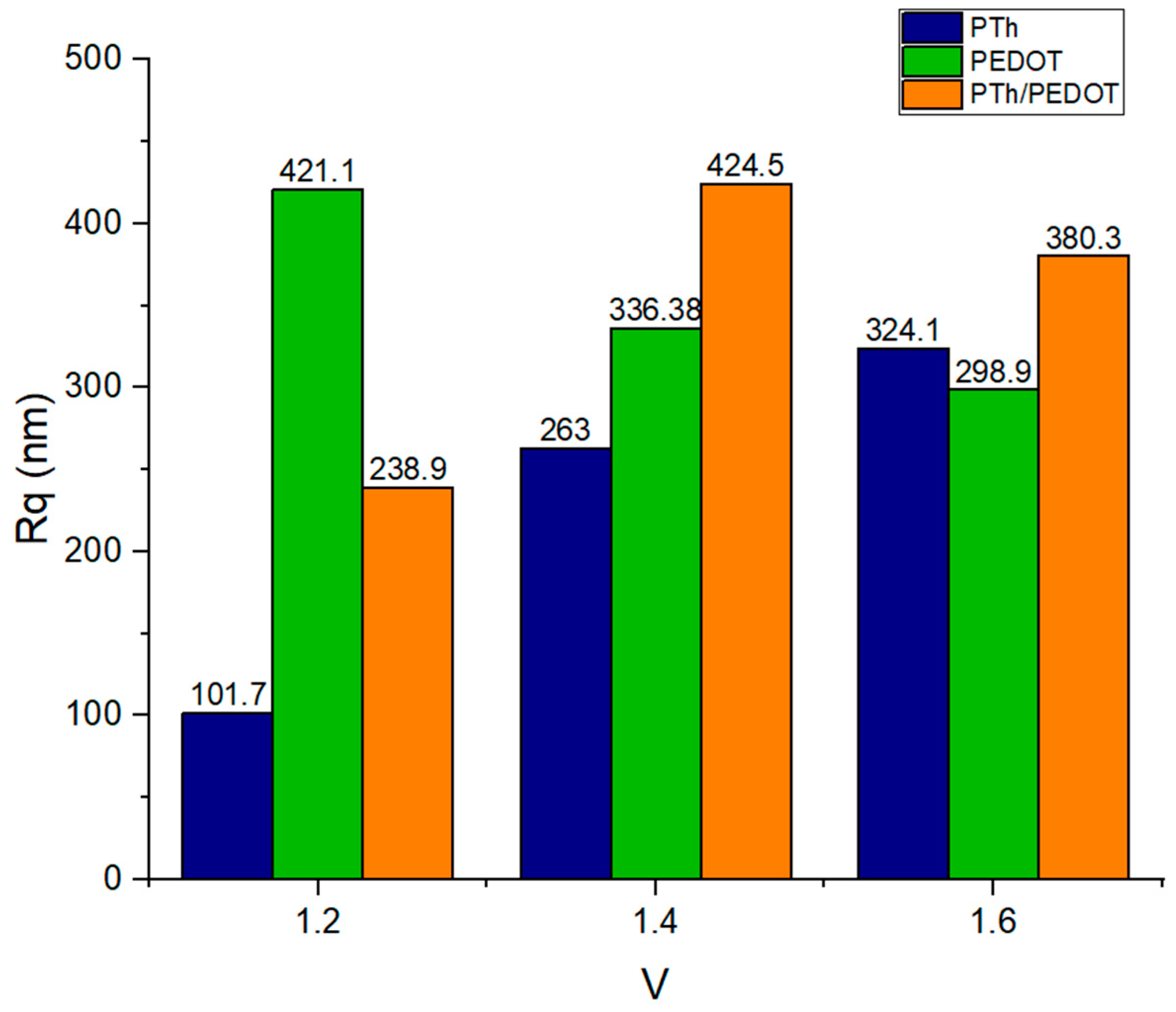
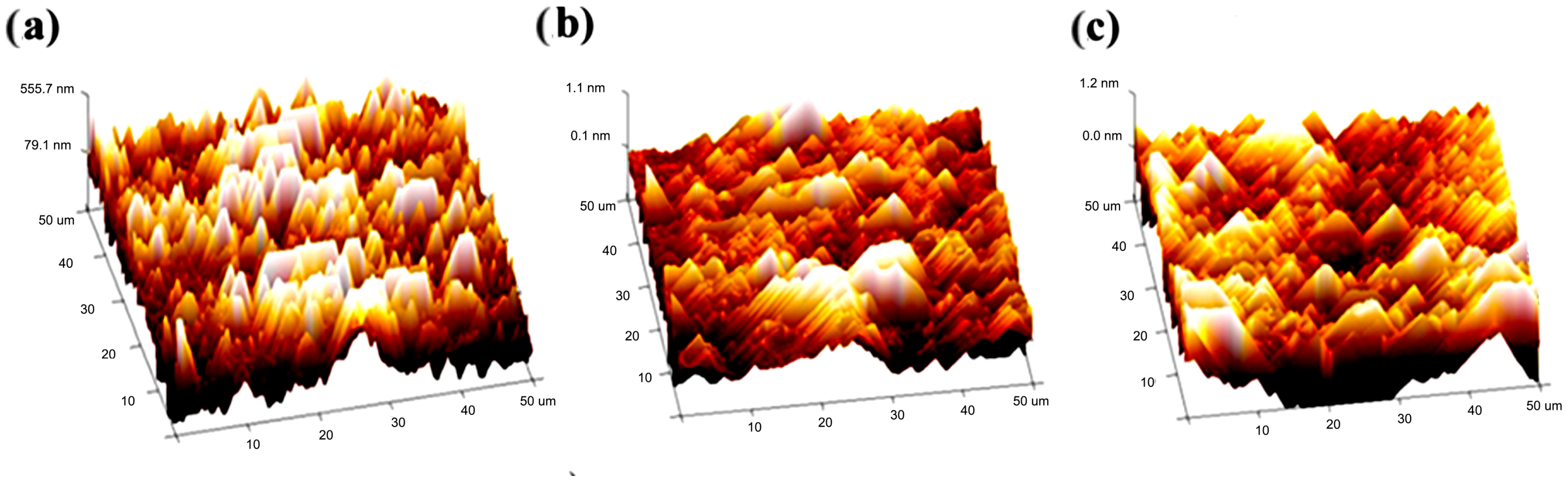
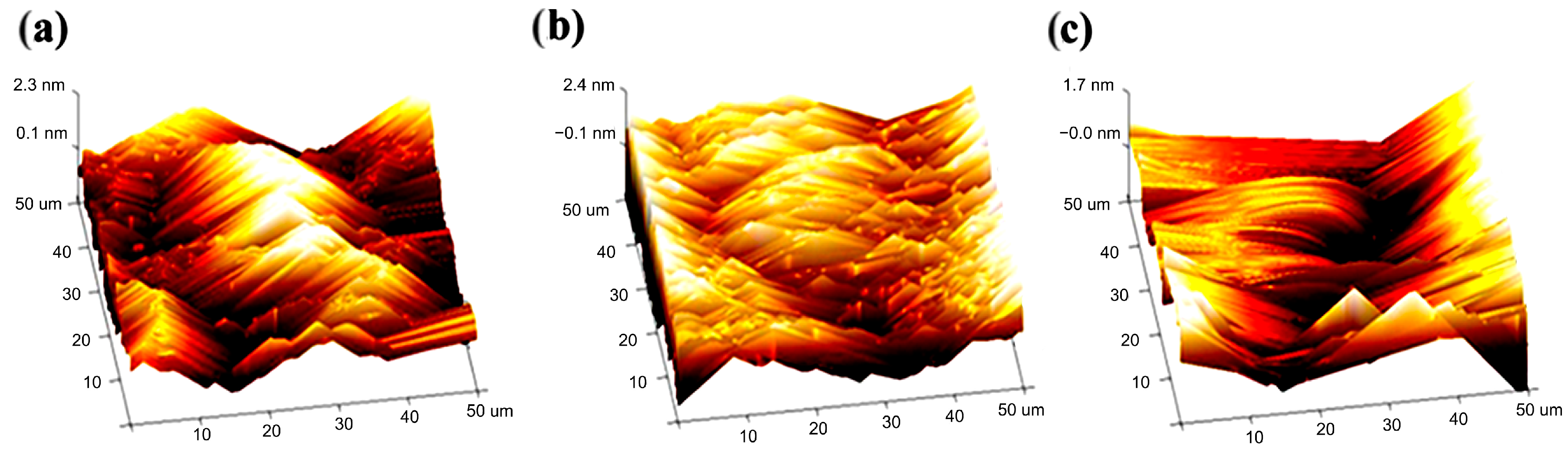
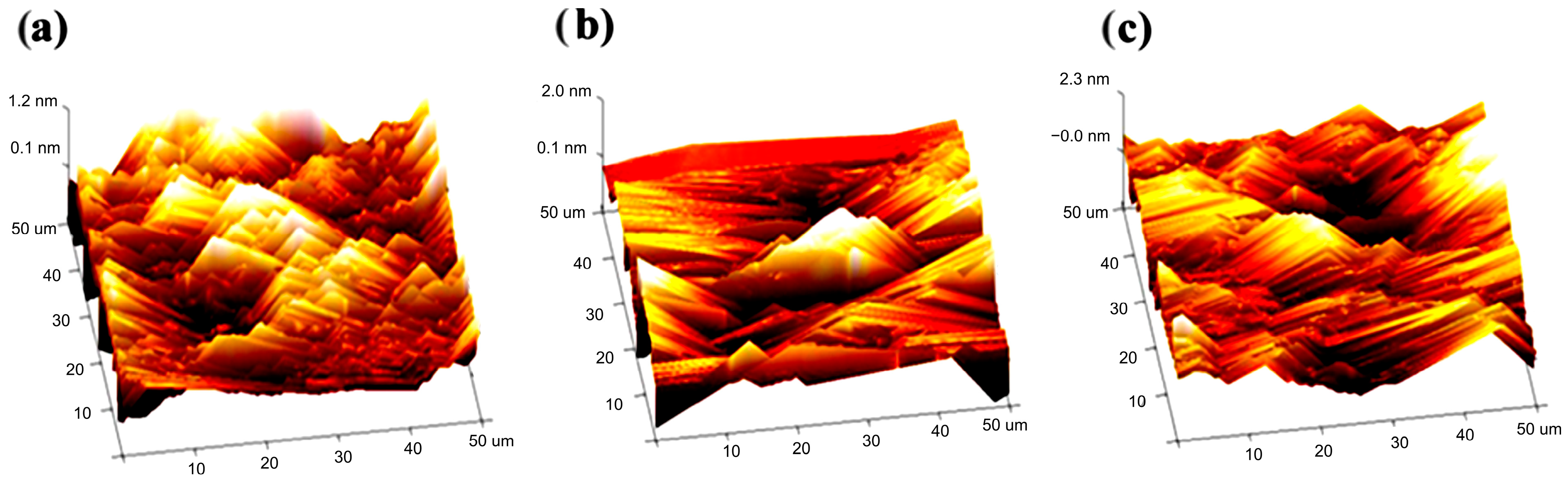
2.3. Morphological Characterization
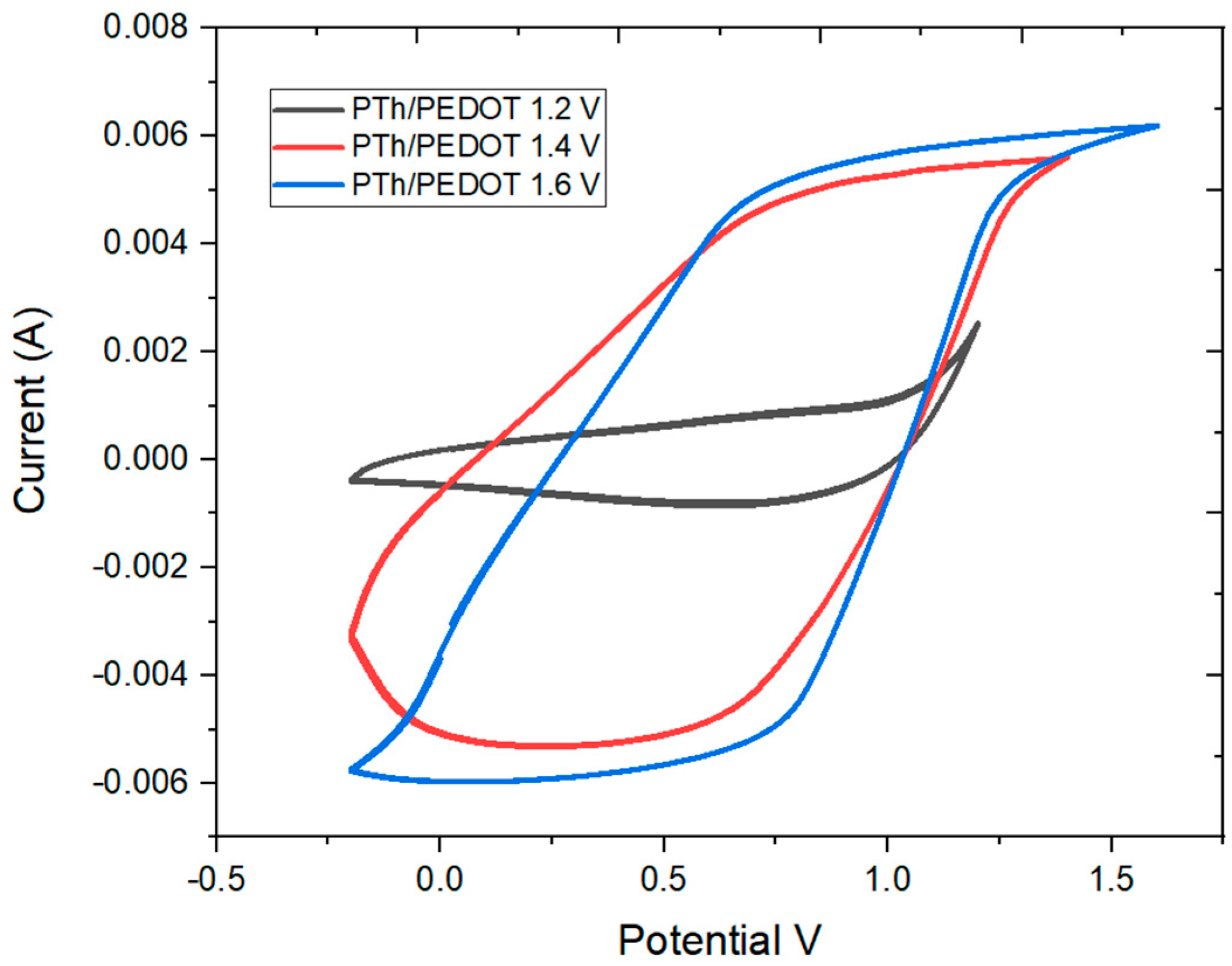
2.4. Electrochemical Characterization
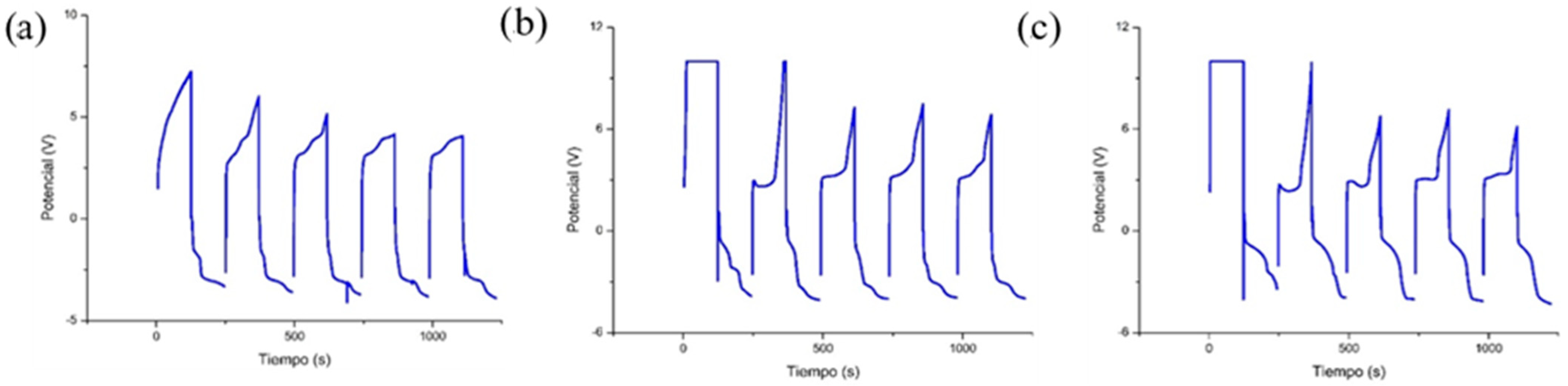
2.4.1. Pulsed Chronoamperometry (Charge Capacity)
2.4.2. Scanning Electron Microscope (SEM) After Charge Capacity Test
3. Materials and Methods
3.1. Reagents
3.2. Electrochemical Synthesis of Conductive Polymers
3.3. Materials Characterization
3.4. Chemical Characterization
3.5. Morphological Characterization
3.6. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cumurcu, A.; Duvigneau, J.; Lindsay, I.D.; Schön, P.M.; Vancso, G.J. Multimodal imaging of heterogeneous polymers at the nanoscale by AFM and scanning near-field ellipsometric microscopy. Eur. Pol. J. 2013, 49, 1935–1942. [Google Scholar]
- Rotering, N.; Lic, M. Optimal Charge Control of Plug-In Hybrid Electric Vehicles in Deregulated Electricity Markets. IEEE Trans. Power Syst. 2011, 26, 1021–1029. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Xu, J.; Jiang, Q.; Shi, H.; Liu, E. Improving the electrical conductivity of PEDOT:PSS films by binary secondary doping. Electron. Mater. Lett. 2016, 12, 54–58. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.-Y.; Ho, K.-C.; Chu, C.-W. Highly conductive PEDOT:PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Zeng, L.C.; Li, W.H.; Jiang, Y.; Yu, Y. Recent progres in Li-S and Li Se batteries. Rare Met. 2017, 36, 339–364. [Google Scholar] [CrossRef]
- He, G.; Ji, X.L.; Nazar, L. High “C” rate Li-S cathodes: Sulfuer imbibed bimodal porous carbons. Energy Environ. Sci. 2011, 4, 2878–2883. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Aloni, S.; Wang, L.; Cairns, E.J.; Zhang, Y. Porous carbon nanofibers-sulfur composites electrodes for lithium/sulfur cells. Energy Environ. Sci. 2011, 4, 5053–5059. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Hu, J.; Zhang, X.; Li, X.; Zhang, G.; Li, Y.; Zheng, C.; Hong, X.; Duan, H. Tailoring polysulfide trapping and kinetics by engineering hollow carbon bubble nanocreactors for high-energy Li-S pouch cells. Nano Res. 2021, 14, 1355–1363. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Liao, J.; Sun, Q.; Zhao, F.; Luo, J.; Lin, X.; Niu, X.; Wu, M.; Li, R. Efficient trapping and catalytic conversion of polysulfides by VS4 nanosites for Li-S batteries. ACS Energy Lett. 2019, 4, 755–763. [Google Scholar] [CrossRef]
- Islam, M.S.; Driscoll, D.J.; Fisher, C.A.J.; Slater, P.R. Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olive-type battery material. Chem. Mat. 2005, 17, 5085–5092. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z. Carbon material for high volumetric performances supercapacitors: Design progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Jadhav, N.; Kasisomayajula, S.; Gelling, V. Polypyrrole/metal Oxides-Based Composites/Nanocomposites for Corrosion Protection. Front. Mater. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Mohammad, M.; Farina, A. Polythiophene (PTh)-TiO2-Reduced Graphene Oxide (rGO) Nanocomposites Coating: Synthesis, Characterization, and Corrosion Protection Performance on Low-Carbon Stell in 3.5 wt% NaCl. ACS Omega 2022, 7, 46717–46730. [Google Scholar] [CrossRef]
- Ansari, M.O.; Mohammad, F. Thermal stability, electrical conductivity and ammonia sensing studies on p-toluenesulfonic acid doped polyaniline:titanium dioxide (pTSA/Pani:TiO2) nanocomposites. Sens. Act. B 2011, 157, 122–129. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Raju, K.; Lee, J.; Cho, M.H. Enhanced thermal stability under DC electrical conductivity retention and visible light activity of Ag/TiO2@Polyaniline nanocomposite film. ACS Appl. Mater. Interfaces 2014, 6, 8124–8133. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.C.; Balogh, D.T. Optical chemical sensors using polythiophene derivates as active layer for detection of volatile organic compounds. Sens. Act. B 2012, 162, 307–312. [Google Scholar] [CrossRef]
- McCullough, R.D. The chemistry of conducting polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- Murugavel, S.; Malathi, M. Synthesis and characterization of polythiophene nanofibers. Int. J. Chem. Sci. 2016, 14, 363–371. [Google Scholar]
- Yamamoto, T.; Maruyama, T.; Zhou, Z.-H.; Miyazaki, Y.; Kanbara, T.; Sanechika, K. New method using nickel (0) complex for preparation of poly(p-phenylene), poly(2,5-thiwnylwnw) and related π-conjugated polymers. Synth. Met. 1991, 41, 345–348. [Google Scholar] [CrossRef]
- Chandraprabha, G.; Sankarappa, T.; Lokhande, B.J.; Sujatha, T. Studies on conduction mechanism, magnetization and electrochemical propierties of polythiophene-cobalt nanocomposites. J. Nanosci. Technol. 2018, 4, 304–307. [Google Scholar] [CrossRef]
- Ko, H.C.; Kang, M.; Moon, B.; Lee, H. Enhancement of electrochomic contrast of poly(3,4-ethylenedioxythiophene) by incorporating a pendent viologen. Adv. Mat. 2004, 16, 1712–1716. [Google Scholar] [CrossRef]
- Gadgil, B.; Damlin, P.; Aaritalo, T.; Kankare, J.; Kvarnstrom, C. Eletrosynthesis and characterization of viologen cross linked thiophene copolymer. Electrochim. Acta 2013, 97, 378–385. [Google Scholar] [CrossRef]
- Ustamehmetoǧlu, B.; Sezer, E.; Kizilcan, N.; Yazic, P.; Tayyar, S.; Saraç, A.A.S. Inhibition of pyritr corrosion and photocorrosion by MEKFER modified carbazoles. Prog. Org. Coat. 2013, 76, 533–540. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present and future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.S.; Kim, J.H. Synthesis of polythiopehene/poly(3,4-ethylenedioxythiophene) Nanocomposites and their Application in thermoelectric devices. J. Electr. Mat. 2014, 43, 3276–3282. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Funt, B.L.; Lowen, S.V. Mechanistic studies of the electropolymerization of 2,2’-bithiophene and pyrrole to form conductiong polymers. Synth. Met. 1985, 11, 129–137. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Xu, J.; Song, H.; Lu, B.; Jiang, F.; Jiang, Q. Facile fabrication of PEDOT: PSS/polythiophenes bilayered nanofilms on pure organic electrodes and their thermoelectric performance. ACS Appl. Mat. Interfaces 2013, 5, 12811–12819. [Google Scholar] [CrossRef]
- Yang, L.; Huang, X.; Gogoll, A.; Strømme, M.; Sjödin, M. Conducting redox polymer based anode materials for high power electrical energy storage. Electrochim. Acta 2016, 204, 270–275. [Google Scholar] [CrossRef]
- Lacerda, G.R.B.S.; Calado, C.R.; Calado, H.D.R. Electrochromic and electrochemical properties of copolymer films based on EDOT and phenylthiophene derivatives. J. Solid State Electrochem. 2019, 23, 823–835. [Google Scholar] [CrossRef]
- Liu, F.-H.; Bai, J.; Yu, G.; Ma, F.-H.; Hou, Y.-J.; Niu, H.-J. Synthesis, electrochromic properties and flash memory behaviors of novel DAD polyazomethines containing EDOT and thiophene units. Org. Electron. 2020, 77, 105538. [Google Scholar] [CrossRef]
- Topal, S.; Topal, S.; Sezer, E.; Ustamehmetoglu, B.; Ozturk, T. Flexible energy storage device fabricated by electrochromic smart copolymer based on thienothiophene-triphenyamine and EDOT. J. Energy Storage 2024, 99, 113272. [Google Scholar] [CrossRef]
- Yılmaz, K.; Karaman, M. Highly conductive and uniform PEDOT on poly(acrylic acid-vinylbenzyl chloride) functionalized surfaces. Surf. Interfaces 2024, 55, 105320. [Google Scholar] [CrossRef]
- Gök, A.; Omastova, M.; Yavuz, A. Synthesis and characterization of polythiophenes prepared in the presence of surfactants. Synth. Met. 2007, 157, 23–29. [Google Scholar] [CrossRef]
- Karim, M.R.; Lee, C.J.; Lee, M.S. Synthesis and characterization of conducting polythiophene/carbon nanotubes composites. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5283–5290. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative study of polyaniline (PANI), poly (3, 4-ethylenedioxythiophene) (PEDOT) and PANI-PEDOT films electrochemically deposited on transparent indium thin oxide-based electrodes. Polymer 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Zhu, M.; Eyraud, M.; Judikael, L.-R.; Ahmed, N.A.; Boulc’h, F.; Alfonso, C.; Knauth, P.; Flory, F. Simple approach for the fabrication of PEDOT-coated Si nanowires. Beilstein J. Nanotech. 2015, 6, 640–650. [Google Scholar] [CrossRef]
- Patil, B.H.; Jagadale, A.D.; Lokhande, C.D. Synthesis of polythiophene thin films by simple successive ionic layer adsorption and reaction (SILAR) method for supercapacitor application. Synth. Met. 2012, 162, 1400–1405. [Google Scholar] [CrossRef]
- Kumarraja, M.; Pitchumani, K. Simple and efficient reduction of nitroarenes by hydrazine in faujasite zeolites. Appl. Cat. A Gen. 2004, 265, 135–139. [Google Scholar] [CrossRef]
- Baderstscher, M.; Bühlmann, P.; Pretsch, E. Structure Determination of Organic Compounds: Tables of Spectra Data, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Schuhmann, W.; Kranz, C.; Wohlschläger, H.; Strohmeier, J. Pulse technique for the electrochemical deposition of polymer films on electrodes surfaces. Biosens. Bioelectron. 1997, 12, 1157–1167. [Google Scholar] [CrossRef]
- Kaynak, A. Effect of synthesis parameters on the surface morphology of conducting polypyrrole films. Mater. Res. Bull. 1997, 32, 271–285. [Google Scholar] [CrossRef]
- Kiss, L.; Bôsz, D.; Koväcs, F.; Li, H.; Nagy, G.; Kunsagi-Máté, S. Investigation of pheno electrooxidation in aprotic non-aqueous solvents by using cyclic and normal pulse voltammetry. Polym. Bull. 2019, 76, 5849–5864. [Google Scholar] [CrossRef]
- Band, L.E. Spatial aggregation of complex terrain. Geograph. Anal. 1989, 21, 279–293. [Google Scholar] [CrossRef]
- Froeck, C.; Bartl, A.; Dunsch, L. STM-and AFM-investigations of one-and two-dimensional polypyrrole structures on electrodes. Electrochim. Acta 1995, 40, 1421–1425. [Google Scholar] [CrossRef]
- Mardia, K.V. Statistics of Directional Data. J. Royal Stat. Soc. Series B (Method) 1975, 37, 349–371. [Google Scholar] [CrossRef]
- Fingleton, B.; Upton, G.J. Spatial Data Analysis by Example. In Vol. 1: Point Pattern and Quantitative Data; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1985. [Google Scholar]
- Jonda, C.; Mayer, A.B.R.; Stolz, U.; Elschner, A.; Karbach, A. Surface roughness effects and their influence on the degradation of organic light emitting divices. J. Mat. Sci. 2000, 35, 5645–5651. [Google Scholar] [CrossRef]
- Carneiro, K.; Jesen, C.P.; Jørgensen, J.F.; Garnoes, J.; McKeown, P.A. Roughness parameters of surfaces by atomic force microscopy. CIRP Ann. 1995, 44, 517–522. [Google Scholar] [CrossRef]
- Yu, J.; Namba, Y. Atomic Surface roughness. Appl. Phys. Lett. 1998, 73, 3607–3609. [Google Scholar] [CrossRef]
- Suárez, M.F.; Compton, R.G. In situ atomic force microscopy study of polypyrrole synthesis and the volume changes induced by oxidation and reduction of the polymer. J. Electroanal. Chem. 1999, 462, 211–221. [Google Scholar] [CrossRef]
- Yang, Y.; Mu, S.; Chen, H. Electrochemical synthesis of polypyrrole for the immobilization of galactose oxidase. Synth. Met. 1998, 92, 173–178. [Google Scholar] [CrossRef]
- Castagnola, V.; Bayon, C.; Descamps, E.; Bergaud, C. Morphology and conductivity of PEDOT layers produced by different electrochemical routes. Synth. Met. 2014, 189, 7–16. [Google Scholar] [CrossRef]
- Silk, T.; Hong, Q.; Tamm, J.; Compton, R.G. AFM studies of polypyrrole film surface morphology I. The influence of film thickness and dopant nature. Synth. Met. 1998, 93, 59–64. [Google Scholar] [CrossRef]
- Schönherr, H. Imaging Polymer Morphology using Atomic Force Microscopy. In Polymer Morphology: Principles, Characterization, and Processing; Guo, Q., Ed.; Wiley: Hoboken, NJ, USA, 2016; Chapter 6; pp. 100–117. [Google Scholar] [CrossRef]
- Tüken, T.; Yazıcı, B.; Erbil, M. Electrochemical synthesis of polythiophene on nickel coated mild steel and corrosion performance. Appl. Surf. Sci. 2005, 239, 398–409. [Google Scholar] [CrossRef]
- Li, X.; Lu, M.; Li, H. Electrochemical copolymerization of pyrrole and thiophene nanofibrils using template-synthesis method. J. Appl. Pol. Sci. 2002, 86, 2403–2407. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Loredo, D.A.; Páramo-García, U.; Pino-Pérez, L.A.M.D.; Gallardo-Rivas, N.V.; García-Alamilla, R.; Campa-Guevara, D.L. Synthesis of PTh/PEDOT Films into FTO Substrate by Electrodeposition, for Energy Storage Systems. Condens. Matter 2025, 10, 26. https://doi.org/10.3390/condmat10020026
Vázquez-Loredo DA, Páramo-García U, Pino-Pérez LAMD, Gallardo-Rivas NV, García-Alamilla R, Campa-Guevara DL. Synthesis of PTh/PEDOT Films into FTO Substrate by Electrodeposition, for Energy Storage Systems. Condensed Matter. 2025; 10(2):26. https://doi.org/10.3390/condmat10020026
Chicago/Turabian StyleVázquez-Loredo, Daniel Alejandro, Ulises Páramo-García, Luis Alejandro Macclesh Del Pino-Pérez, Nohra Violeta Gallardo-Rivas, Ricardo García-Alamilla, and Diana Lucia Campa-Guevara. 2025. "Synthesis of PTh/PEDOT Films into FTO Substrate by Electrodeposition, for Energy Storage Systems" Condensed Matter 10, no. 2: 26. https://doi.org/10.3390/condmat10020026
APA StyleVázquez-Loredo, D. A., Páramo-García, U., Pino-Pérez, L. A. M. D., Gallardo-Rivas, N. V., García-Alamilla, R., & Campa-Guevara, D. L. (2025). Synthesis of PTh/PEDOT Films into FTO Substrate by Electrodeposition, for Energy Storage Systems. Condensed Matter, 10(2), 26. https://doi.org/10.3390/condmat10020026





