Finite Element Analysis of Functionally Loaded Subperiosteal Implants Evaluated on a Realistic Model Reproducing Severe Atrophic Jaws
Abstract
:1. Introduction
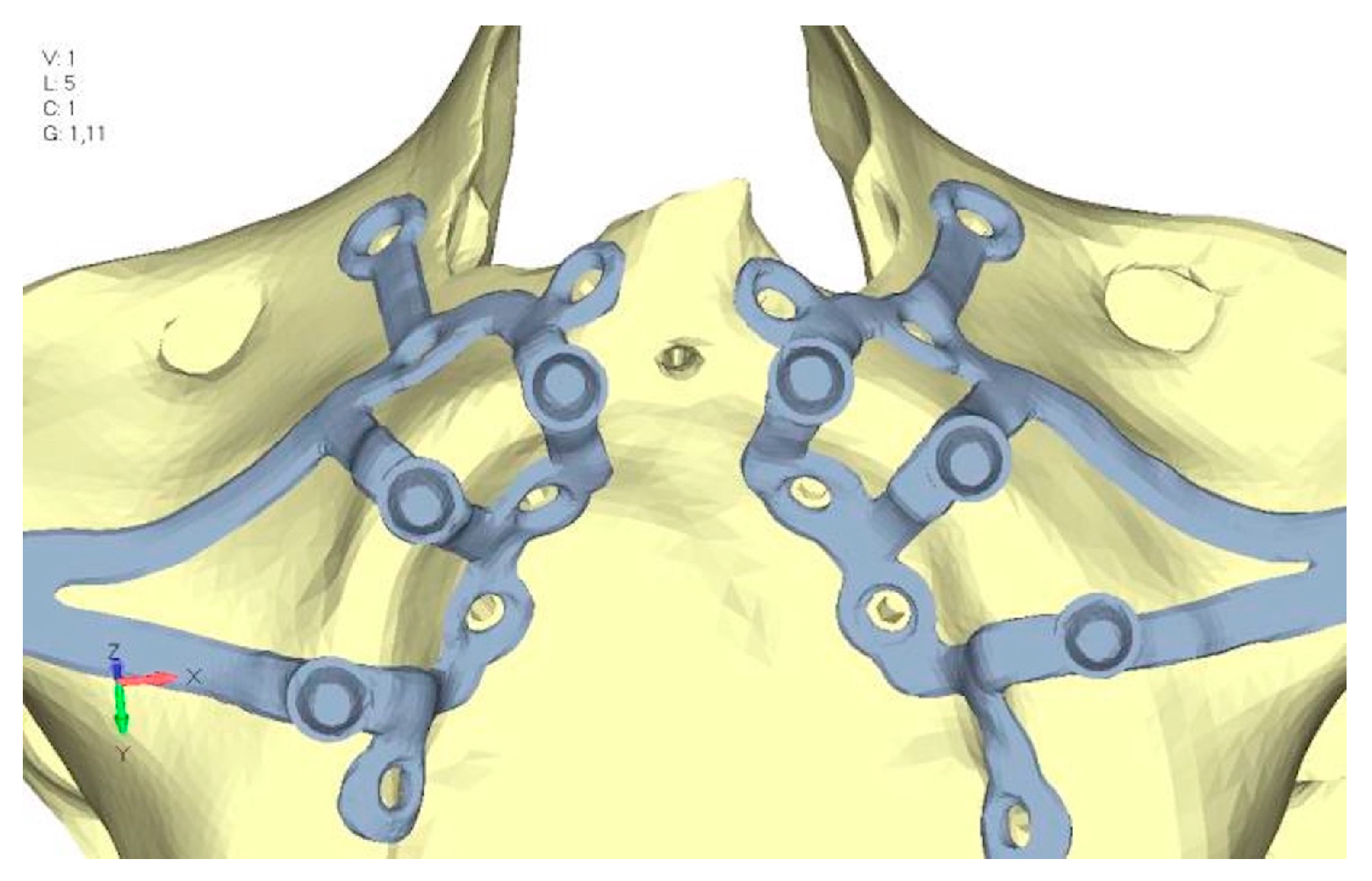
2. Materials and Methods
- Glued contact: this type permanently bonds surfaces, preventing detachment or sliding.
- Frictional contact: allows surfaces to slide and detach from each other.
- Between bone support and juxta support surfaces: frictional contact simulates realistic interaction.
- Between cortical bone and spongy bone surfaces: glued contact simulates their natural bond.
- Between screw threads and bone surfaces: simplified with glued contact due to screw geometry and assumed osseointegration.
- Between screw underhead and juxta seats: contact type property allows small displacements.
- Between juxta abutments and prosthesis: glued contact solidifies the implant-to-prosthesis connection.
3. Results
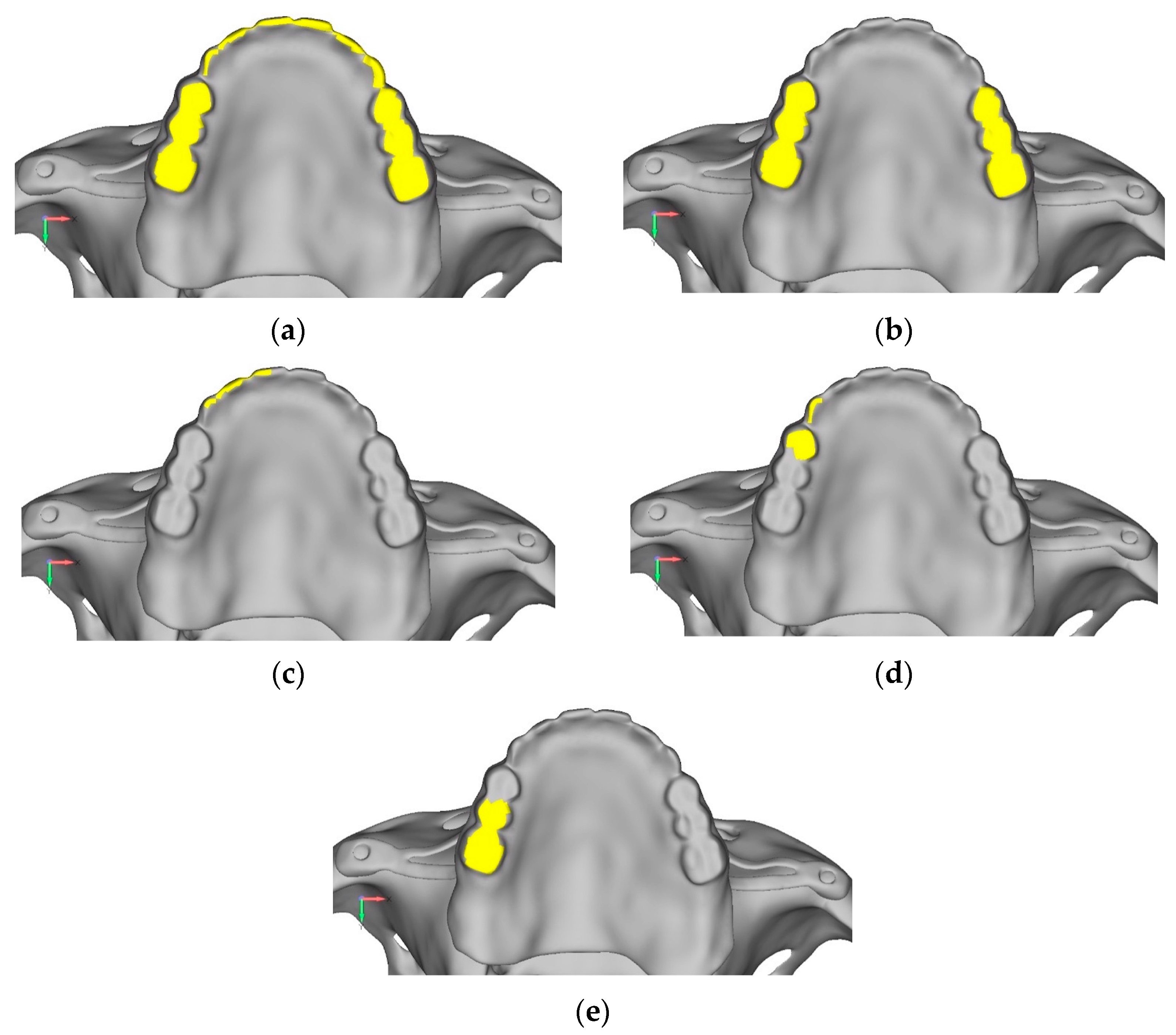
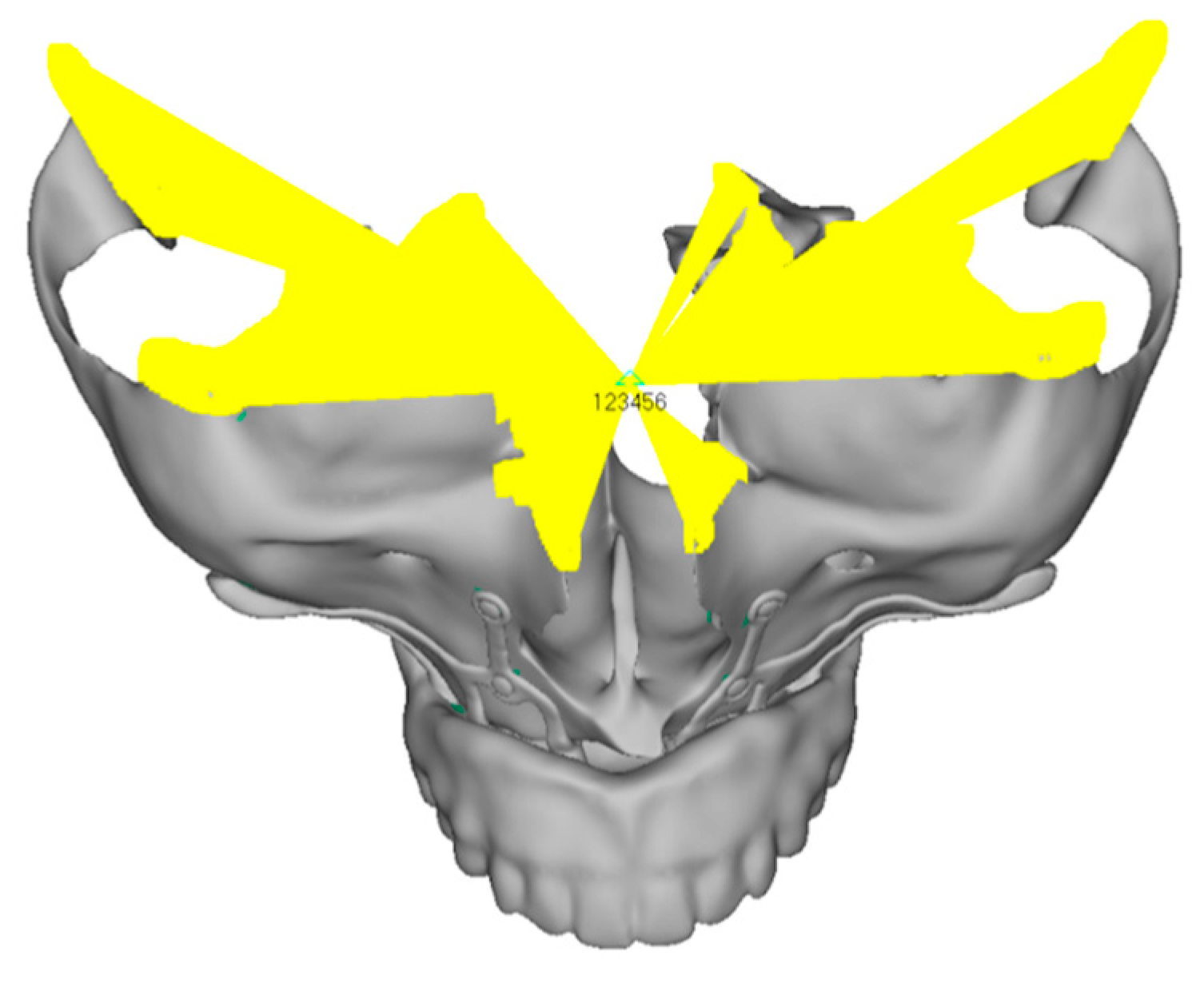
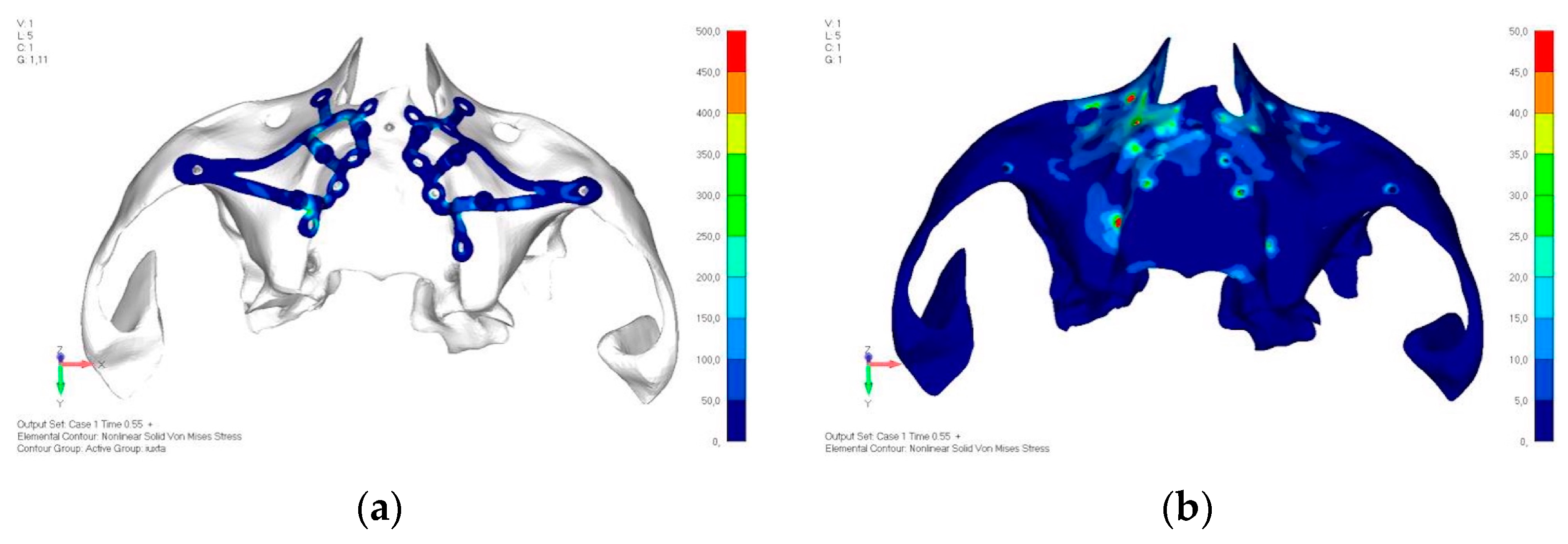
- Model V0. From the analysis of Model V0, it emerged that the most critical situation is related to load configuration 3 (Figure 5a,b), which represents a load applied to the anterior right side. The least critical situations are load configurations 1 and 2 (Figure 6 and Figure 7), corresponding to a load distributed across the entire dentition and a load distributed only on the posterior teeth, respectively. Regarding the stress values observed, no critical issues were identified with the juxta-osseous implant. In load configuration 3, the stresses are below the breaking limits of titanium laser melting: peak stresses of 500 MPa are reached only in very localized areas of the implant.
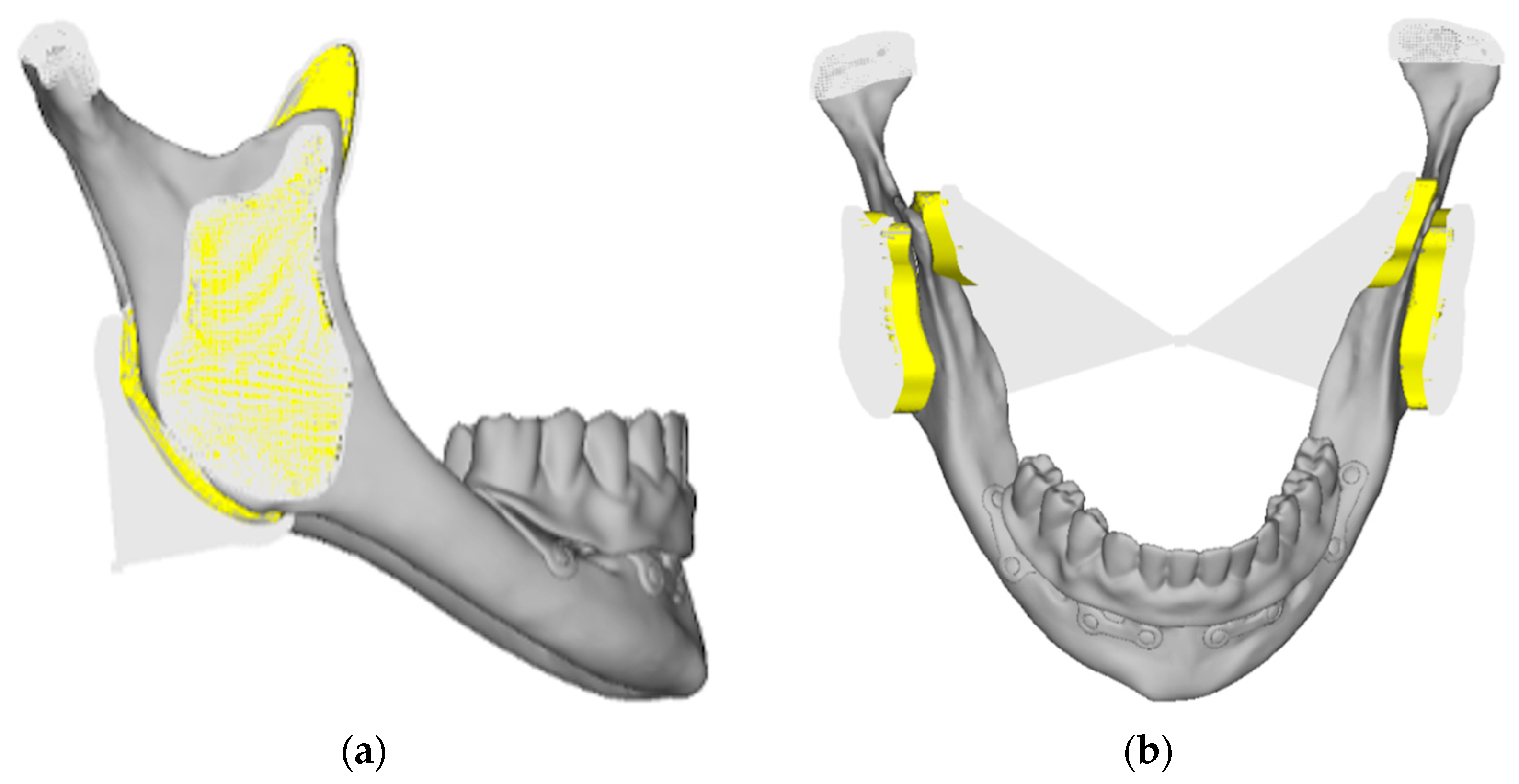
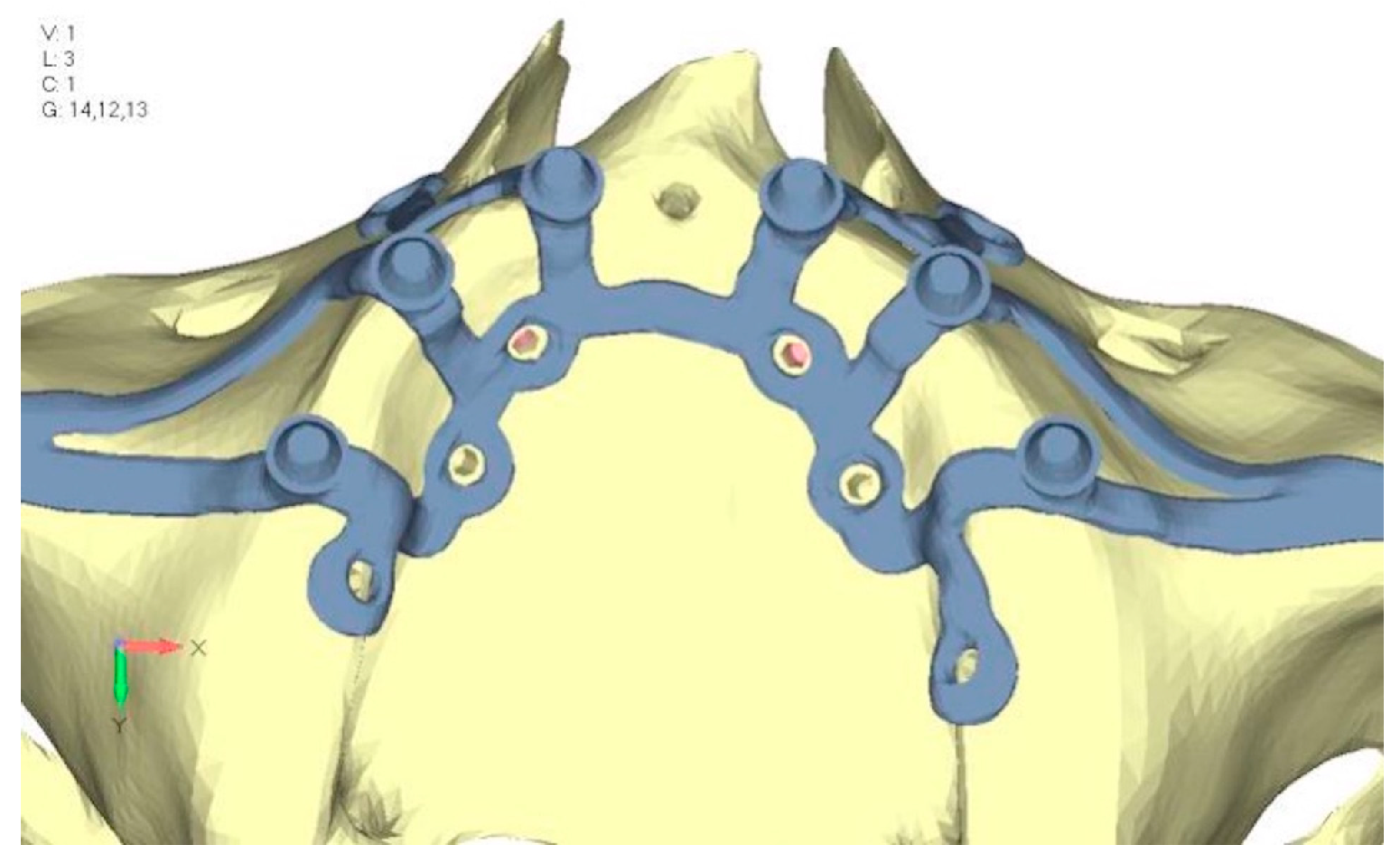
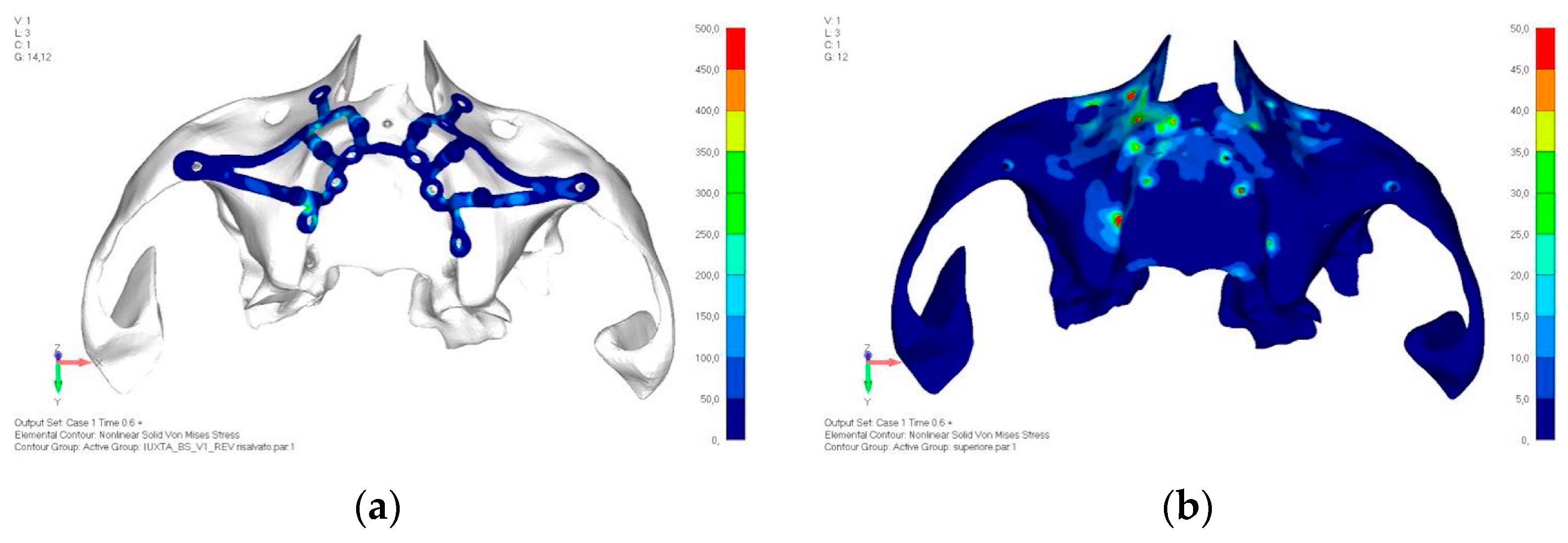
- Model V1. Added posterior screws, reducing stress on anterior parts and achieving more balanced distribution. The addition of the posterior screw has certainly alleviated the load on the palatal screw, which was excessively stressed in the previous model (Figure 8). The screw now experiencing the most stress is the posterior screw: compared to the previous case, only part of the hole shows a stress exceeding 50 MPa, and the area affected by this stress is therefore much more contained (Figure 9a,b).
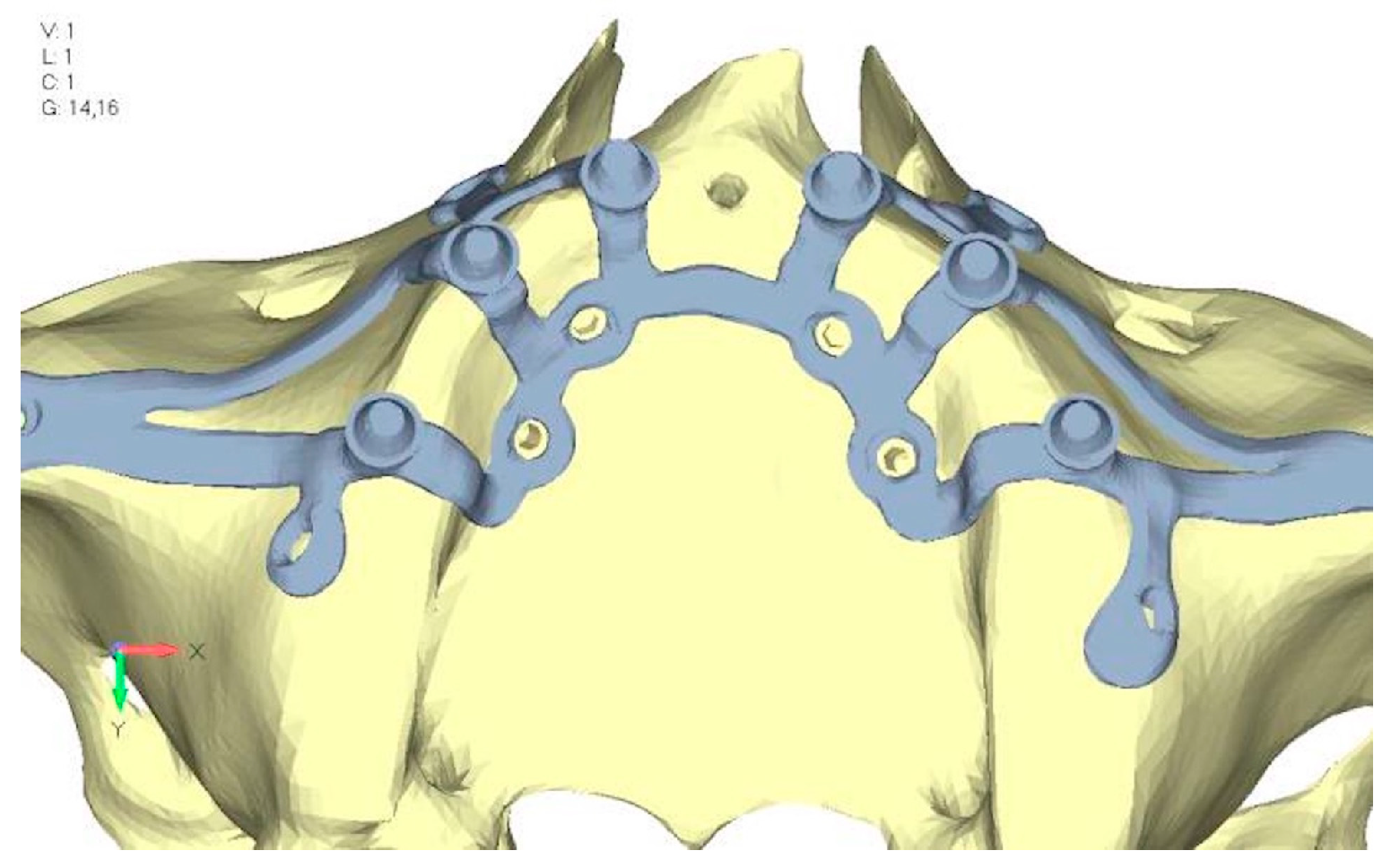
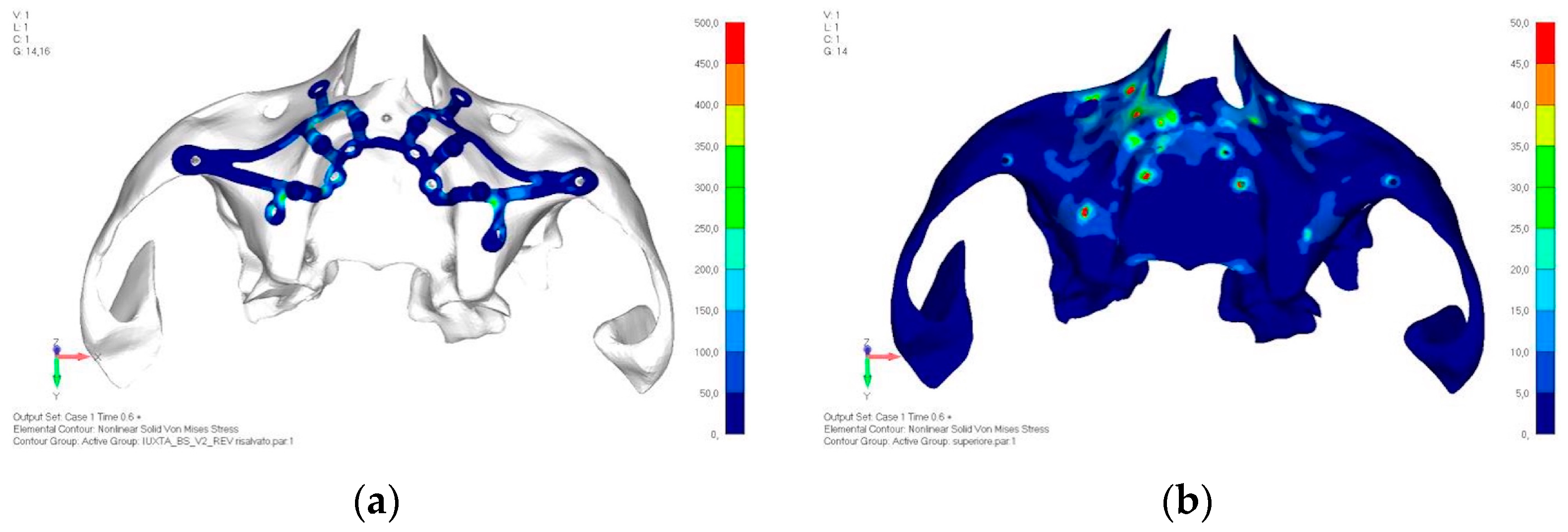
- Model V2. This model serves as an alternative to model V1, as it aims to stabilize the structure posteriorly using screws placed in the vestibular direction rather than the palatal direction (Figure 10). The model displayed similar behavior to V1, leading to the decision to proceed with V1 for further development (Figure 11a,b).
- Model V3. Based on the findings from model 1, attention was shifted to the anterior section to optimize the anchors in that area. Two additional screws were placed anterior to the nasal spine to reduce the load on the frontal screws (Figure 12). The analysis revealed minimal changes; the stress on the frontal screws remains the same, while the pressure on the anterior crestal support has decreased to below 35–40 Mpa (Figure 13a,b).
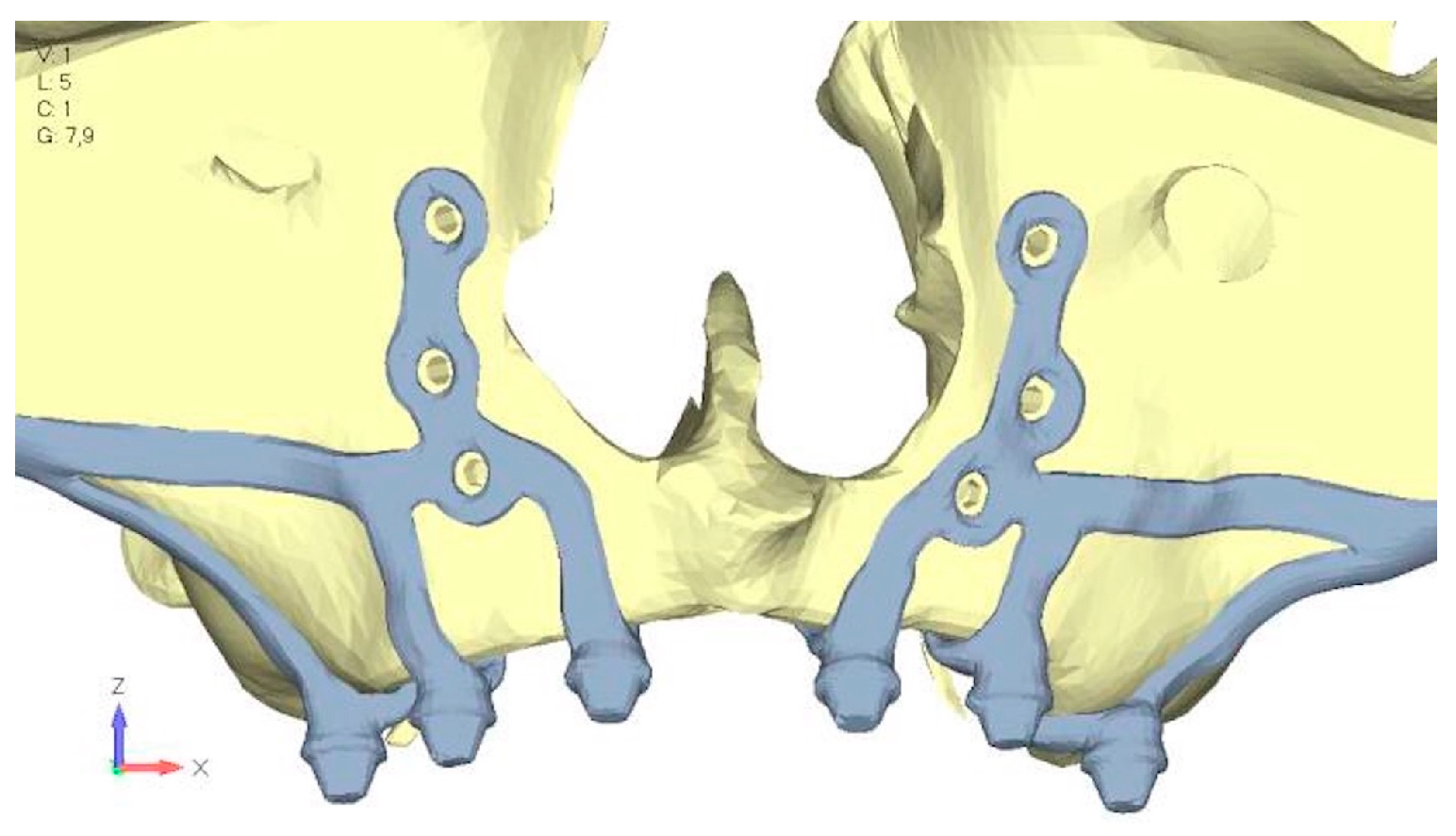
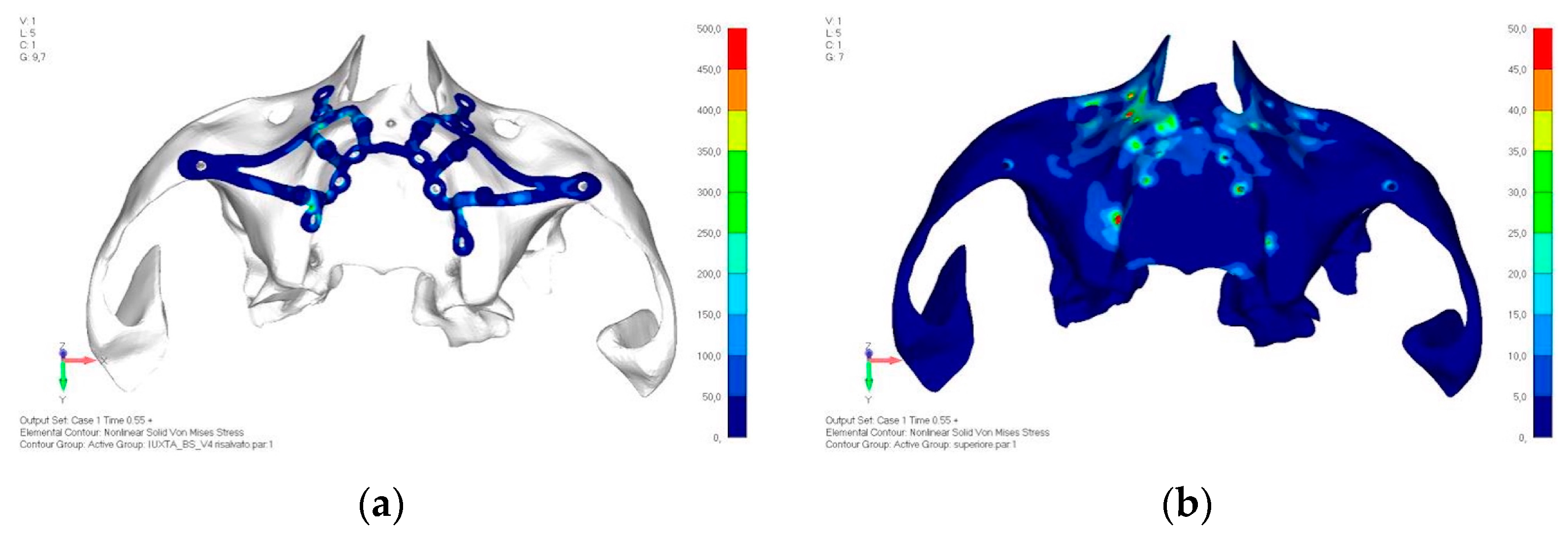
- Model V4. The previously added screw was relocated towards the frontal process, aligning it vertically with the other screws and ensuring that both arms of the first and second abutments connect to this screw (Figure 14). This solution proved to be more effective than V3; the addition of the screw reduces the stress on the other screws and on the support. The area where stress exceeds 50 MPa in the vicinity of the screws is now more contained, and the crestal support shows stresses between 30 and 35 MPa, which are absolutely acceptable (Figure 15a,b).
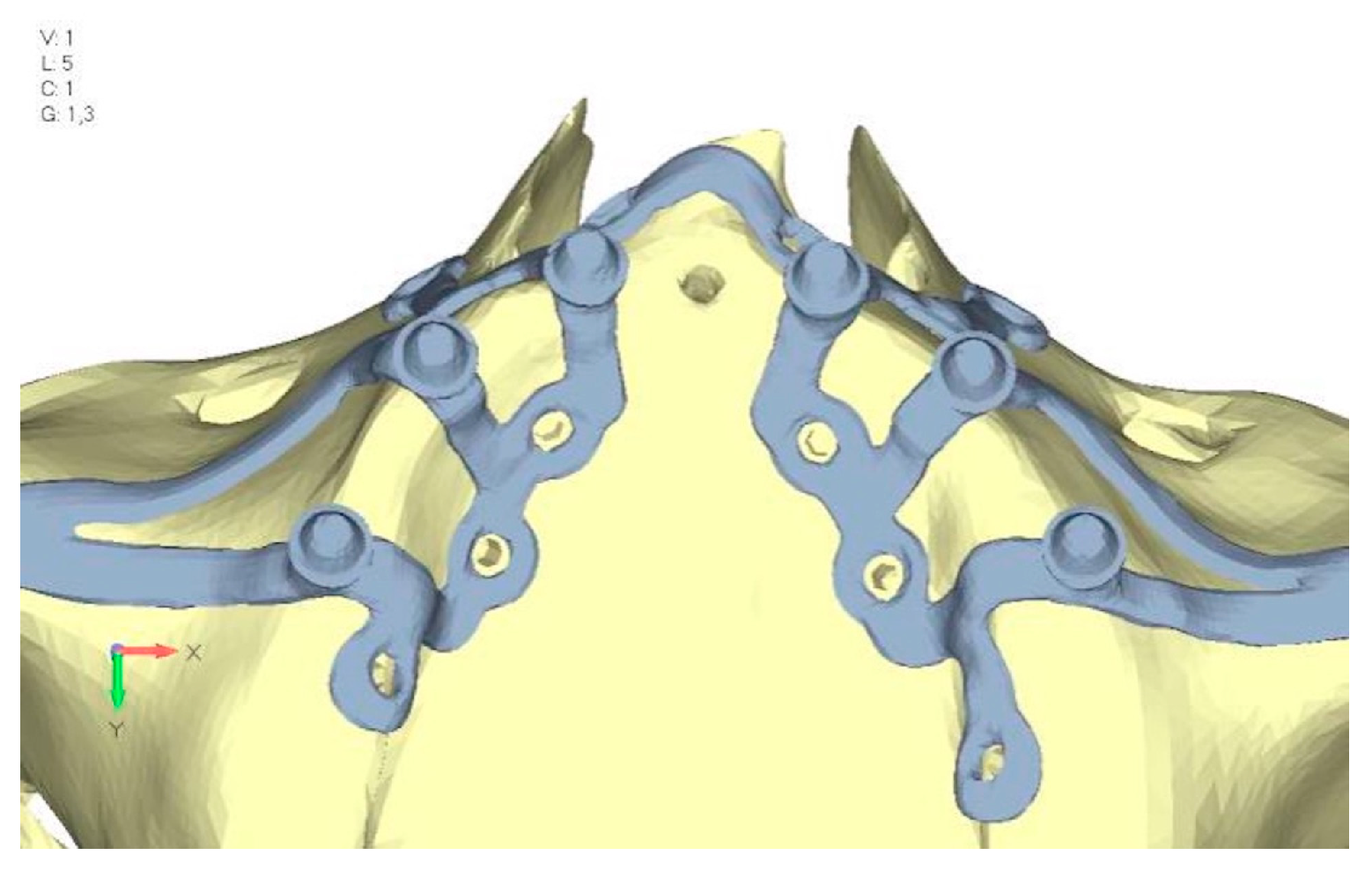
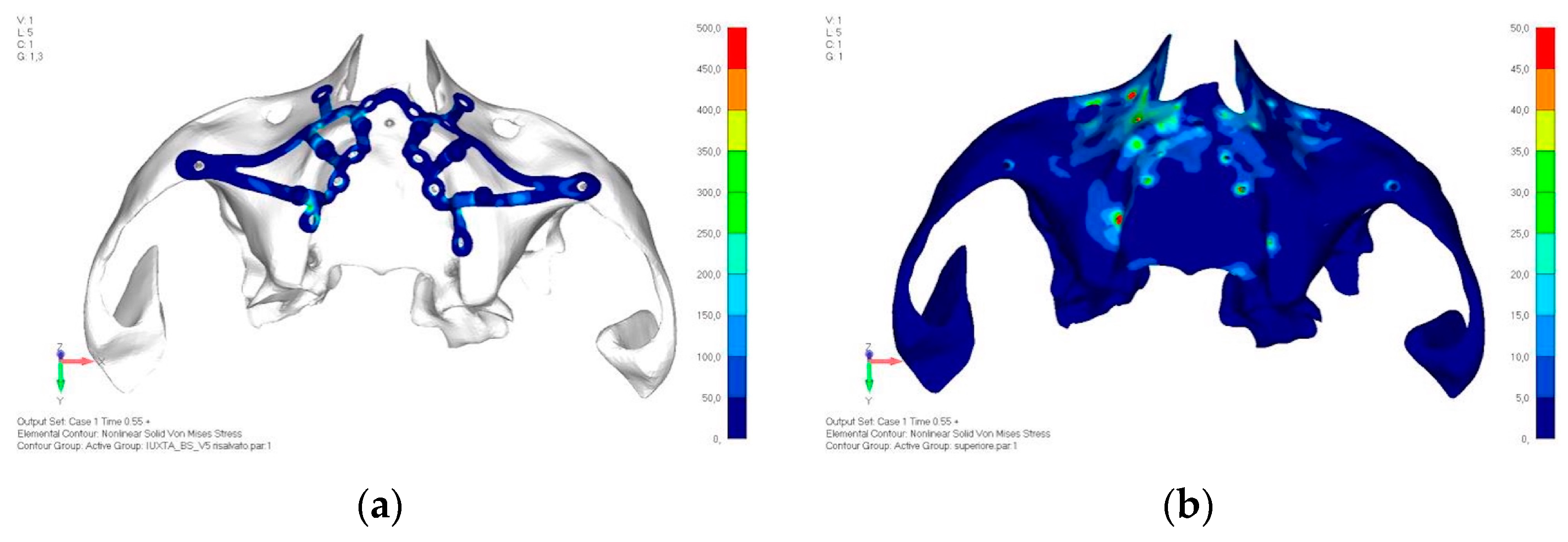
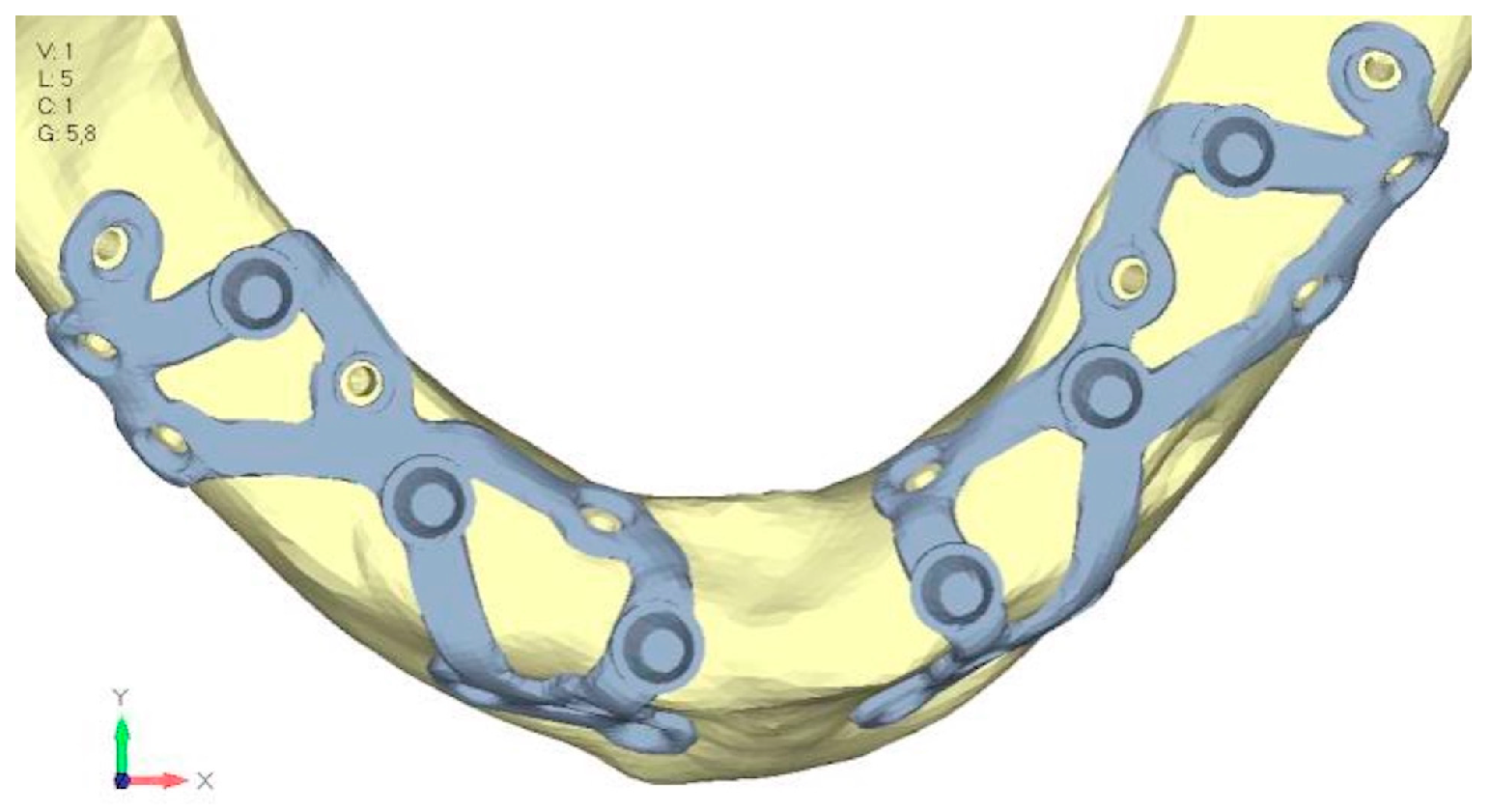
- Model V6. This model analyzes an implant divided into two hemi-arches without any connecting element. As can be easily observed, the presence or absence of an element joining the two halves of the implant has no effect on the stress state of the model. In all previously analyzed models, the bar connecting the two hemi-arches of the implant shows no stress (Figure 18). Removing this bar in model 6 does not alter the results in any way; the stress state of the bone and implant remains the same as in cases with the connection (Figure 19a,b).
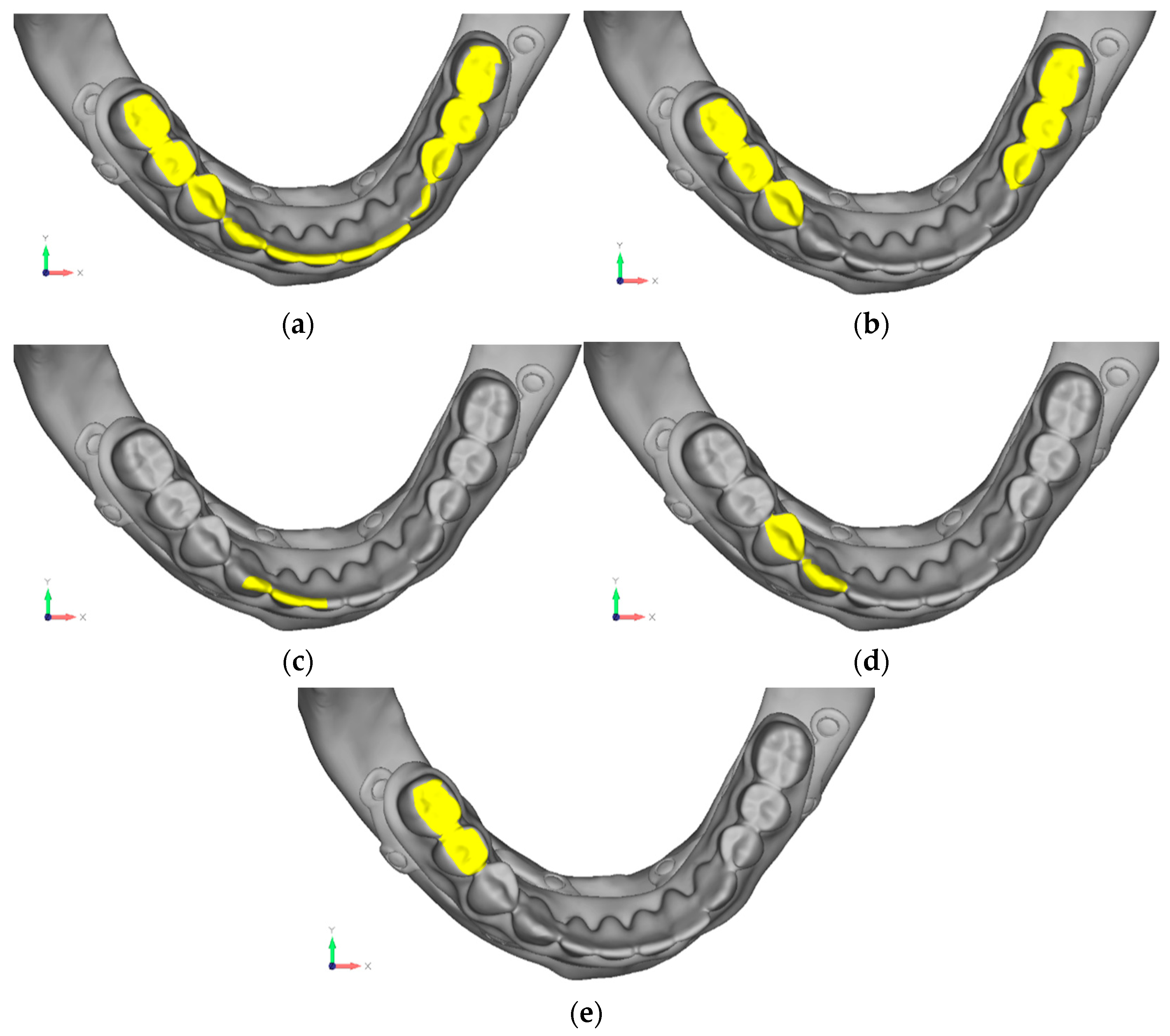
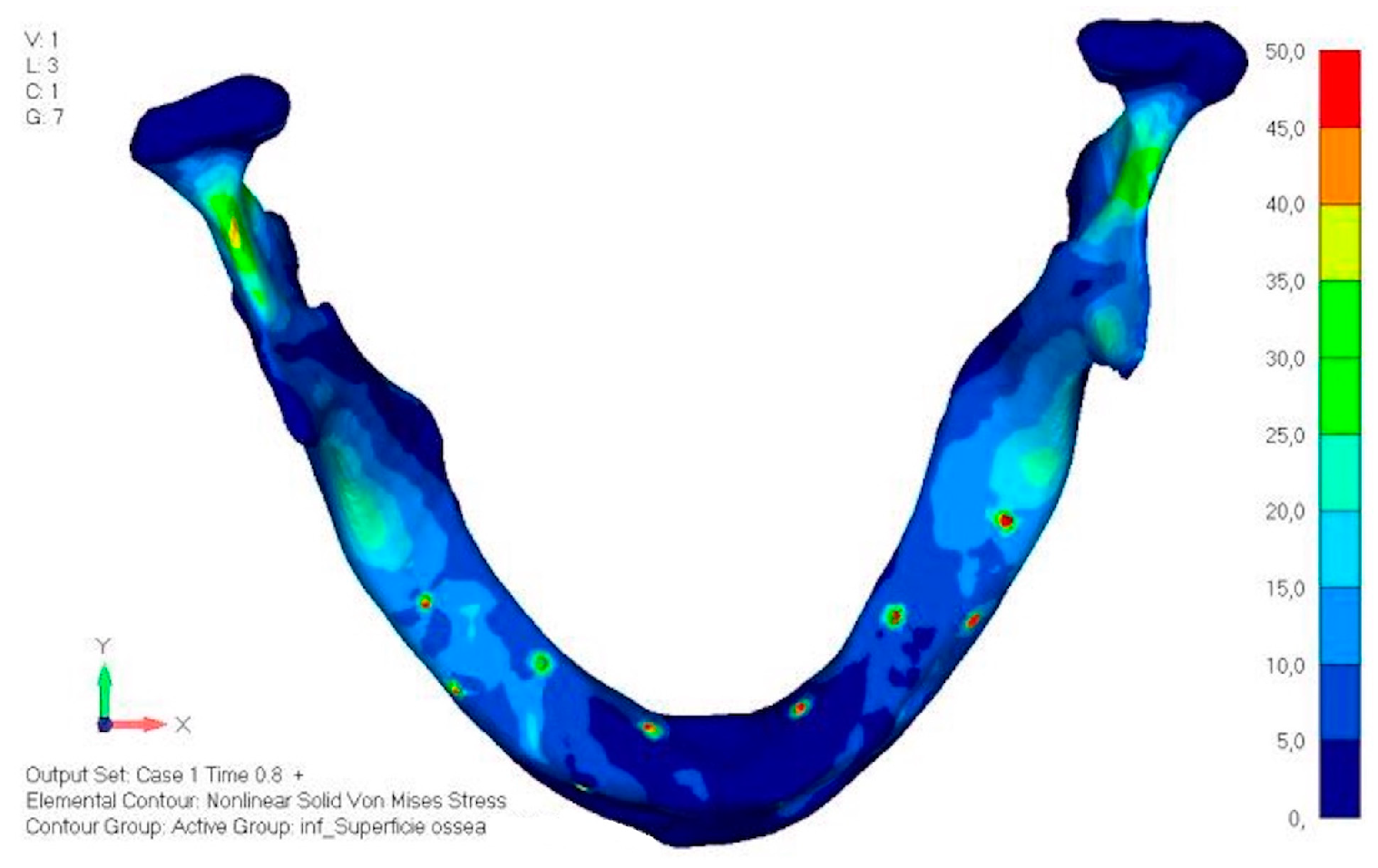
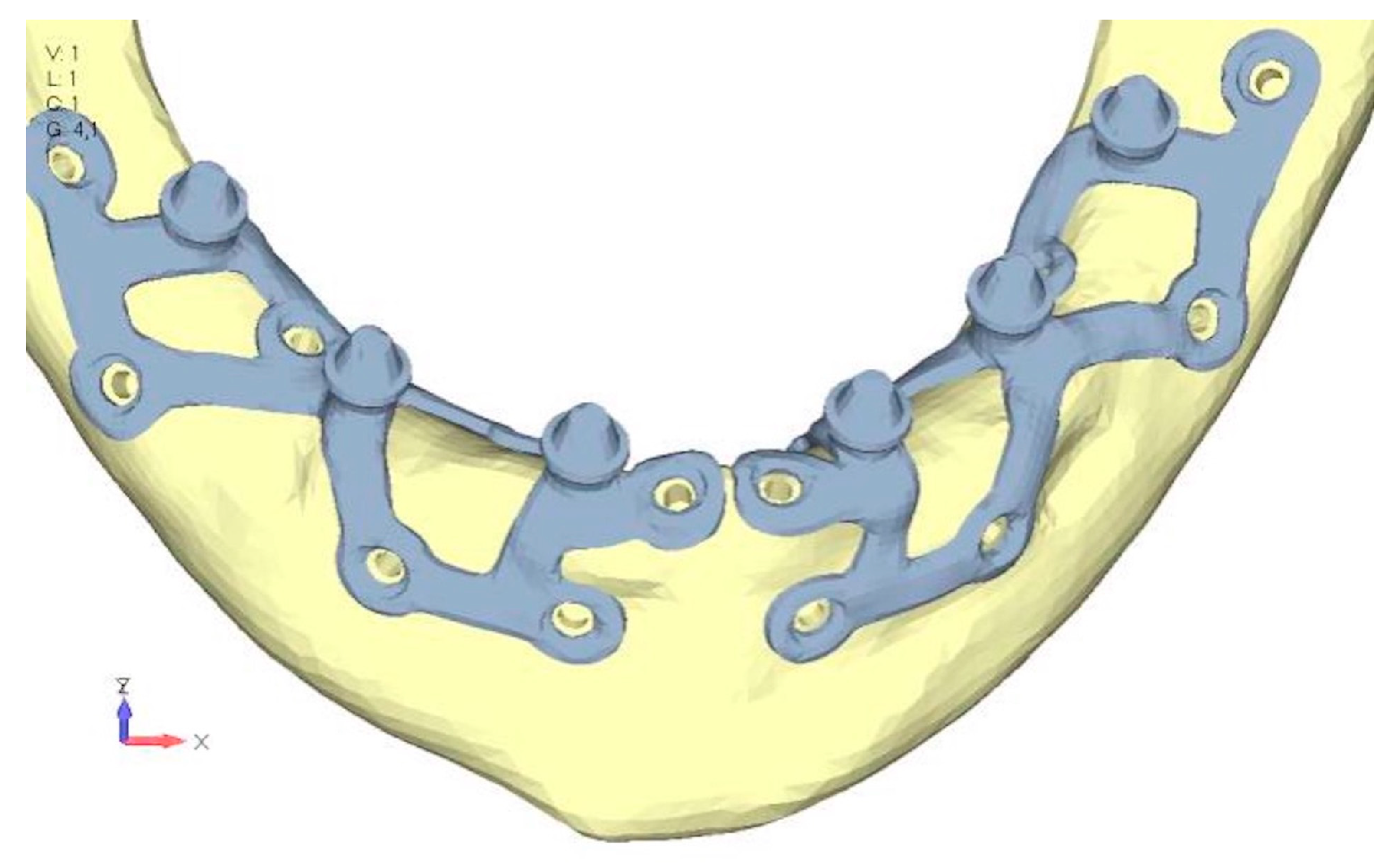
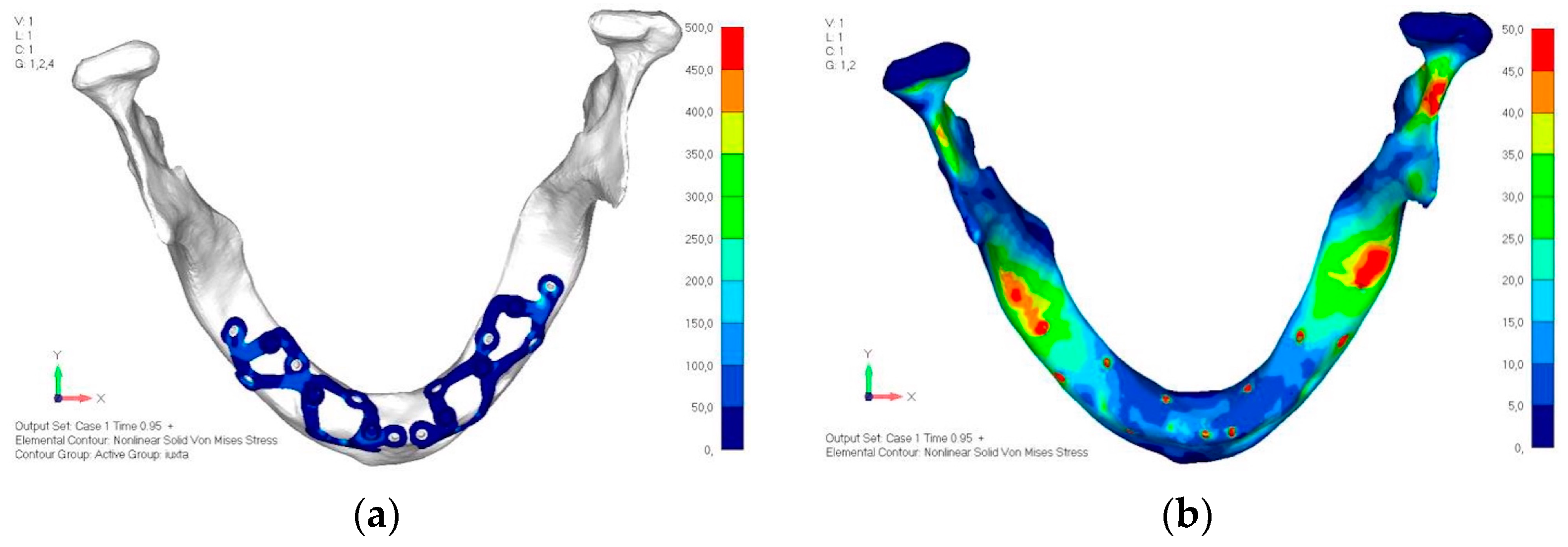
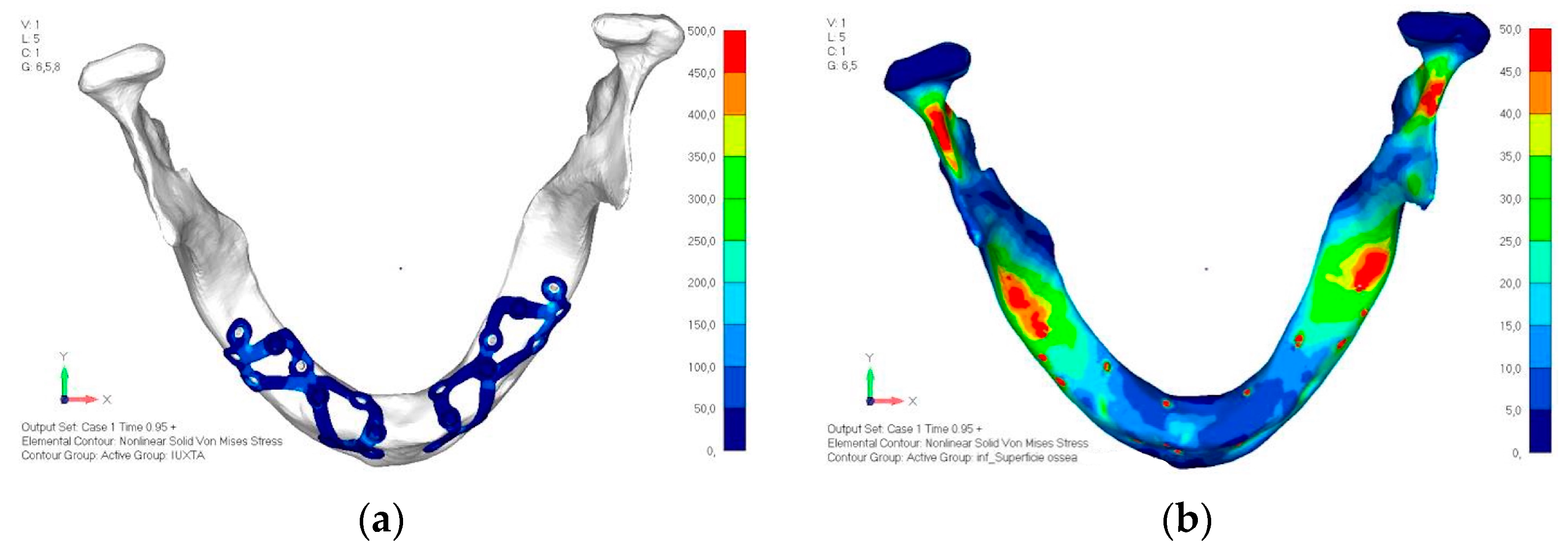
- Model V0. This model represents the initial analysis performed on the lower arch. The implant consists of two completely separate hemi-arches. The situation observed in the lower model is very similar to that found in the upper model. The most significant load is load 3 (Figure 20a,b), corresponding to chewing in the anterior right sector. Loads distributed over larger areas, such as configurations 1 and 2, result in less stress on both the implant and the bone (Figure 21 and Figure 22).
- Even in the lower model, the stresses observed in the peri-implant bone are always acceptable and significantly lower compared to those found in the upper implant. In the worst case, peak stresses reached 250 MPa, which ensures an adequate safety margin. From the bone perspective, in load configuration 3, it is noted that stresses exceed 50 MPa even in areas distant from the implant, such as near the condyles and in the posterior alveolar process.
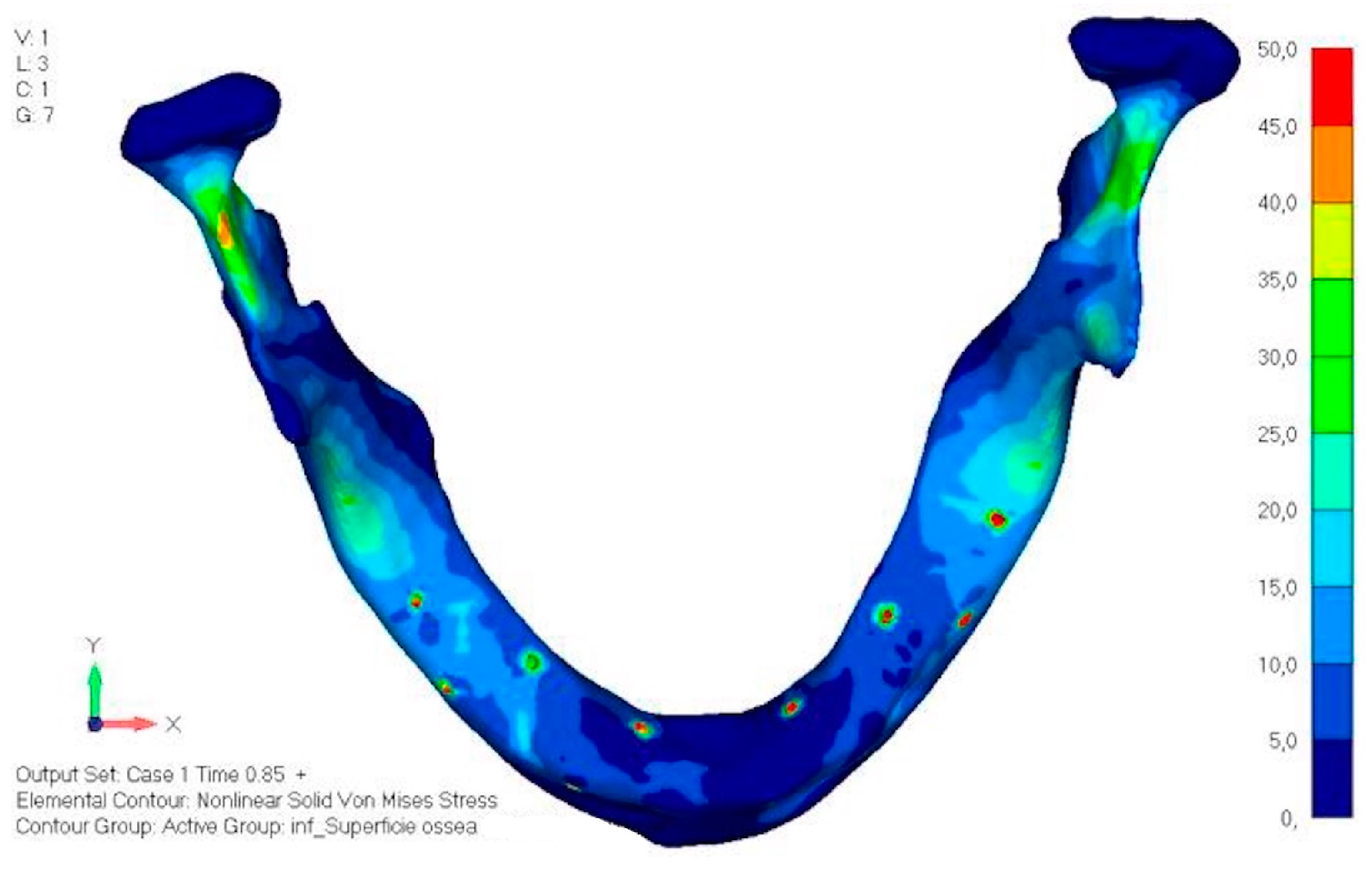
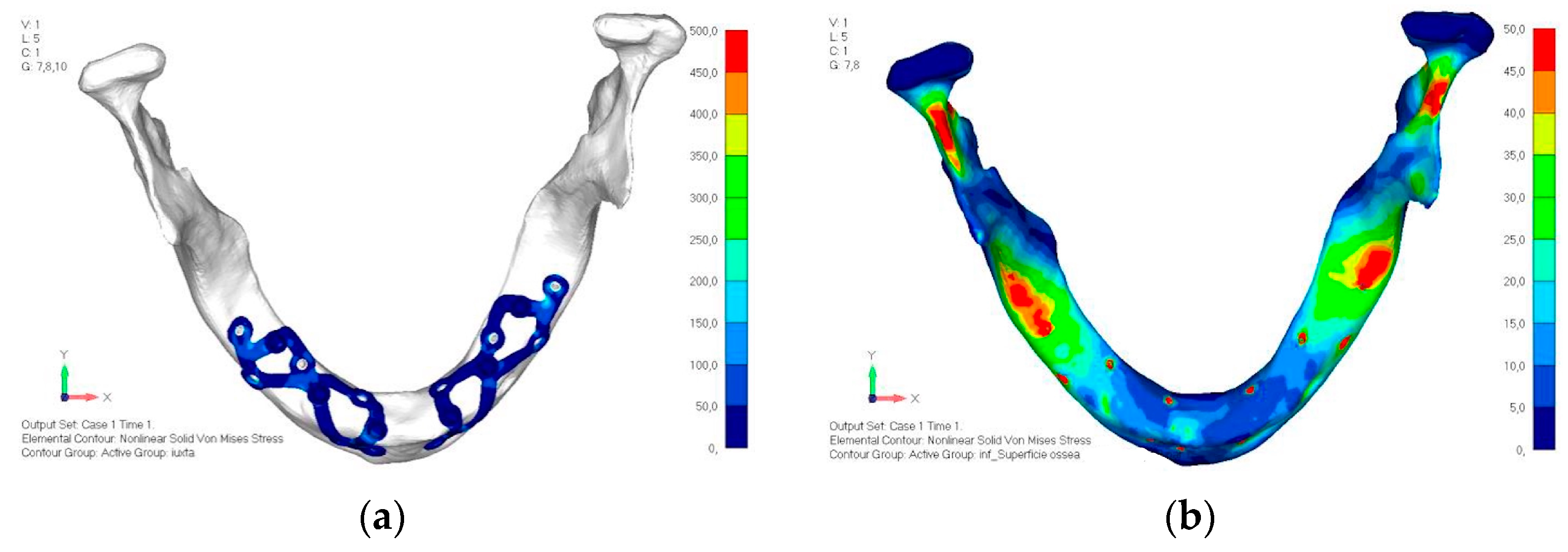
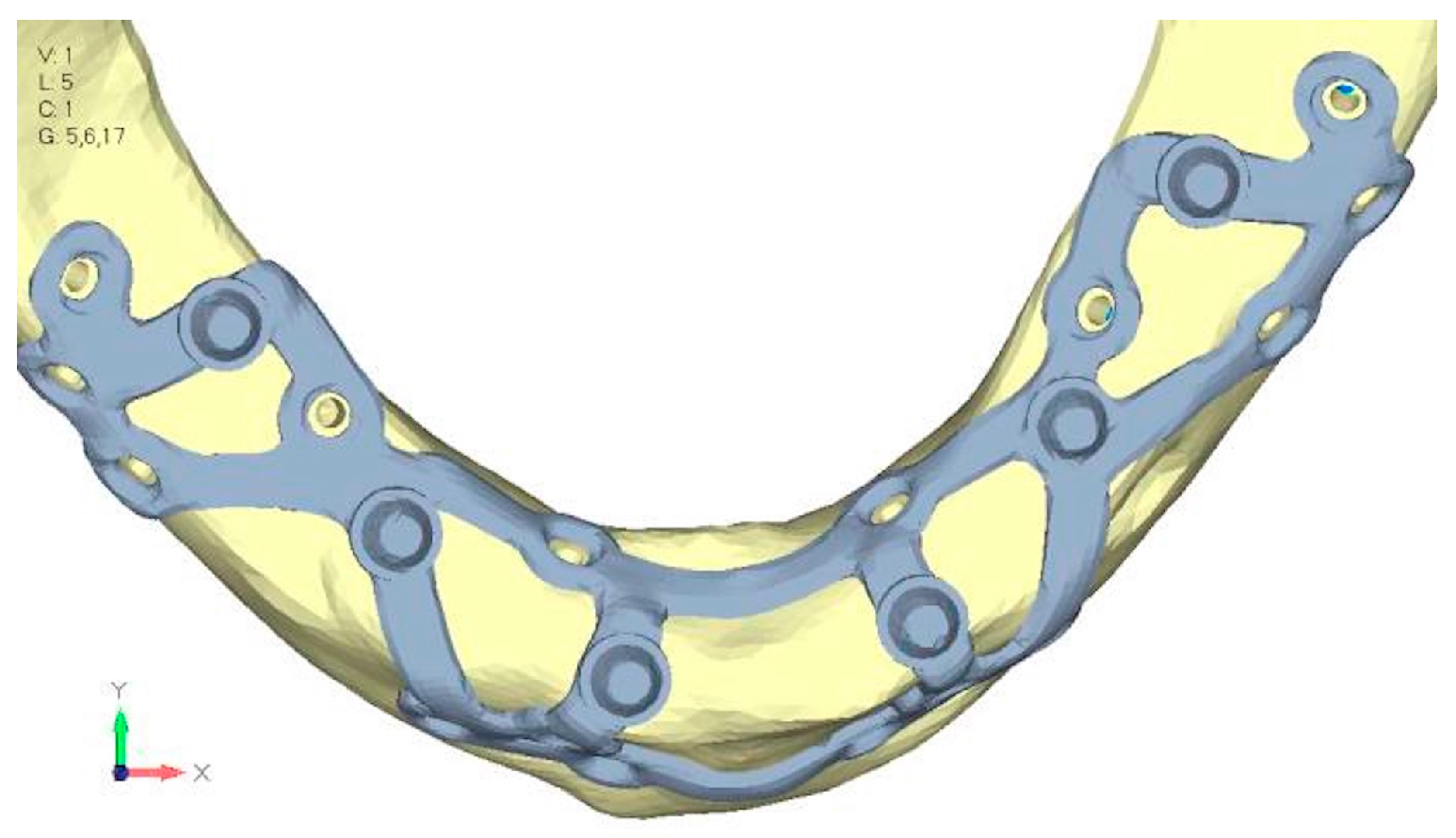
- Model V1. In this version of the implant, two anterior appendages have been added in a crestal position with the aim of better distributing the load in that area (Figure 23). The examined configuration does not result in improvements. Additionally, from a practical standpoint, it is unfeasible because the presence of the crestal screws would create an obstacle in managing the soft tissues, increasing the risk of dehiscence and exposure of the implant (Figure 24a,b).
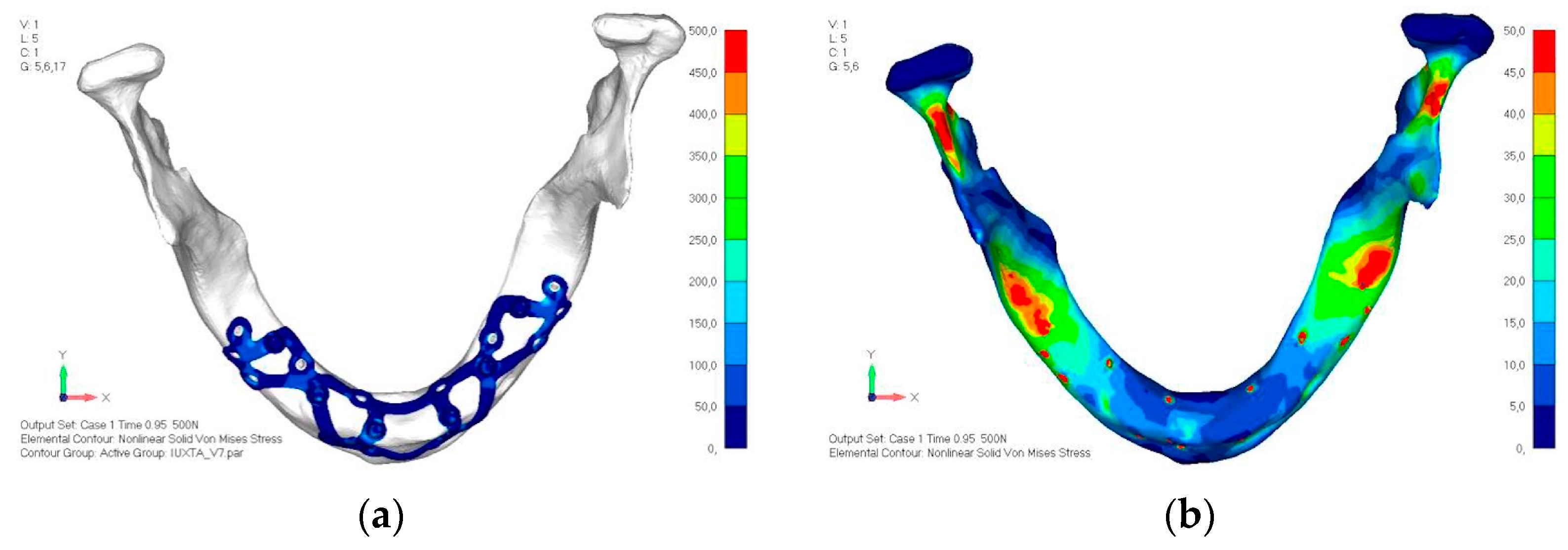
- Model V2. The implant has been modified anteriorly by extending the anterior vestibular arms that connect to the first abutment (Figure 25). This change aims to achieve greater flexibility of the implant in that area, promoting the transmission of masticatory load to the bone through support rather than through the screws. The modification did not reveal significant changes in the stress state. The stresses near the holes are similar to those observed in model 1 (Figure 26a,b).
- Model V3. To reduce the load on the front screws, it was decided to add an additional screw, distributing the load of the anterior abutment across three screws instead of two (Figure 27). The addition of the anterior screw has certainly improved the distribution of stresses, as the volume of material experiencing stresses greater than 50 MPa near the screws has decreased (Figure 28a,b).
- Model V4. This version was derived from version 3 by adding a screw in the posterior sector, positioned in the vestibular direction (Figure 29). Again, the addition of an anchoring screw has allowed for more effective distribution of the stresses. The posterior alveolar area, particularly around the more posterior screws, remains notably stressed (Figure 30a,b). However, this phenomenon is attributed to the geometry and configuration of the bone rather than the presence of a cortical screw.
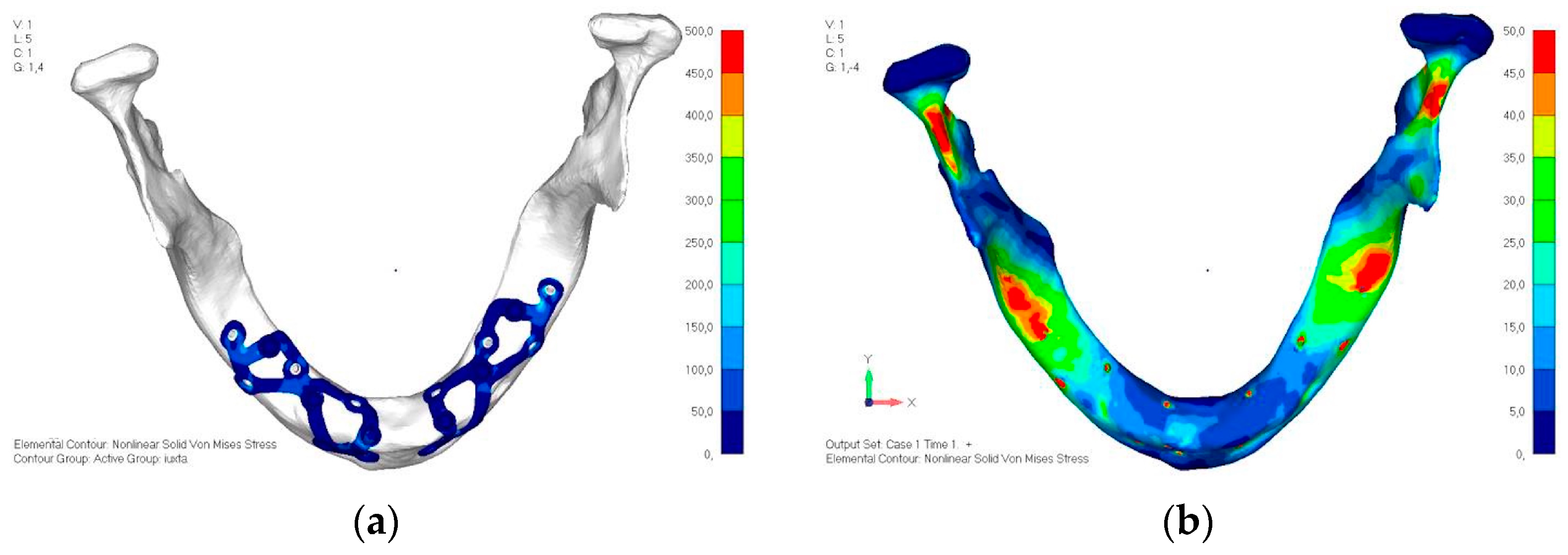
- Model V5. This version of the implant retains the same geometry as version 4, with the addition of two connecting bars, one on the lingual side and one on the vestibular side (Figure 31). The purpose of this analysis is to identify the differences between a monolithic implant and an implant divided into two hemi-arches. The results are quite similar to those observed in the upper model: the presence of a connection between the two halves of the implant does not contribute to its stability. It is immediately noticeable that the two connecting bars exhibit stresses close to 0, indicating that no force is transmitted through them (Figure 32a,b). Once again, a significant contribution is provided by the prosthesis, which stiffens the structure through the abutments.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alotaibi, F.F.; Rocchietta, I.; Buti, J.; D’Aiuto, F. Comparative evidence of different surgical techniques for the management of vertical alveolar ridge defects in terms of complications and efficacy: A systematic review and network meta-analysis. J. Clin. Periodontol. 2023, 50, 1487–1519. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Barausse, C.; Barone, A.; Zucchelli, G.; Piattelli, M.; Pistilli, R.; Ippolito, D.R.; Simion, M. Interpositional augmentation technique in the treatment of posterior mandibular atrophies: A retrospective study comparing 129 autogenous and heterologous bone blocks with 2 to 7 years follow-up. Int. J. Periodontics Restor. Dent. 2017, 37, 469–480. [Google Scholar] [CrossRef]
- Esposito, M.; Cannizzaro, G.; Barausse, C.; Cosci, F.; Soardi, E.; Felice, P. Cosci versus Summers technique for crestal sinus lift: 3-year results from a randomized controlled trial. Eur. J. Oral Implantol. 2014, 7, 129–137. [Google Scholar]
- Stacchi, C.; Andolsek, F.; Berton, F.; Perinetti, G.; Navarra, C.O.; Di Lenarda, R. Intraoperative Complications During Sinus Floor Elevation with Lateral Approach: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2017, 32, e107–e118. [Google Scholar] [CrossRef]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sanchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 319–339. [Google Scholar] [CrossRef] [PubMed]
- Lizio, G.; Pellegrino, G.; Corinaldesi, G.; Ferri, A.; Marchetti, C.; Felice, P. Guided bone regeneration using titanium mesh to augment 3-dimensional alveolar defects prior to implant placement: A pilot study. Clin. Oral Implants Res. 2022, 33, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Barausse, C.; Ravidà, A.; Bonifazi, L.; Pistilli, R.; Saleh, M.H.A.; Gasparro, R.; Sammartino, G.; Wang, H.-L.; Felice, P. Extra-short (4-mm) implants placed after regenerative failures in the posterior atrophic mandible: A retrospective study. Int. J. Oral Implantol. 2023, 16, 31–38. [Google Scholar]
- Stacchi, C.; Rapani, A.; Lombardi, T.; Bernardello, F.; Nicolin, V.; Berton, F. Does new bone formation vary in different sites within the same maxillary sinus after lateral augmentation? A prospective histomorphometric study. Clin. Oral Implants Res. 2022, 33, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Pistilli, R.; Zucchelli, G.; Simion, M.; Karaban, M.; Bonifazi, L.; Barausse, C. Decision criteria proposed for the treatment of vertical bone atrophies in the posterior mandible. Int. J. Periodont. Rest. Dent. 2021, 41, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, M.; Bellini, C.M.; Romeo, D.; Francetti, L. Tilted implants for the rehabilitation of edentulous jaws: A systematic review. Clin. Implant Dent. Relat. Res. 2012, 14, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Pozzan, M.C.; Grande, F.; Mochi Zamperoli, E.; Tesini, F.; Carossa, M.; Catapano, S. Assessment of preload loss after cyclic loading in the OT Bridge System in an “All-on-Four” rehabilitation model in the absence of one and two prosthesis screws. Materials 2022, 15, 1582. [Google Scholar] [CrossRef]
- Gabriele, G.; Chisci, G.; Cascino, F.; Ricci, N.M.; Marruganti, C.; Ferrari, M. Technique-related survival rate and complications of zygomatic implant placement: A systematic review and meta-analysis. Int. J. Maxillofac. Implants 2023, 38, 855–875. [Google Scholar] [CrossRef]
- Mello, C.C.; Lemos, C.A.A.; Gomes, J.M.D.L.; Verri, F.R.; Pellizzer, E.P. CAD/CAM vs conventional technique for fabrication of implant-supported frameworks: A systematic review and meta-analysis of in vitro studies. Int. J. Prosthodont. 2019, 32, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.S.; Ibad, H.; Suchal, Z.A.; Gosain, A.K. Implementation of 3D printing and computer-aided design and manufacturing (CAD/CAM) in craniofacial reconstruction. J. Craniofacial Surg. 2022, 33, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Basile, F.; Relics, D.; Ferri, A.; Grande, F.; Tarsitano, A.; Marchetti, C. Computer-aided rehabilitation supported by zygomatic implants: A cohort study comparing atrophic with oncologic patients after five years of follow-up. J. Clin. Med. 2020, 9, 3254. [Google Scholar] [CrossRef]
- Stefanelli, L.V.; Franchina, A.; Pranno, A.; Pellegrino, G.; Ferri, A.; Pranno, N.; Di Carlo, S.; De Angelis, F. Use of intraoral scanners for full dental arches: Could different strategies or overlapping software affect accuracy? Int. J. Environ. Res. Public Health 2021, 18, 9946. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, M.A.; Hegazy, S.A. Subperiosteal implants constructed with digital technology: A systematic review. Oral Maxillofac. Surg. 2024, 28, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Strappa, E.M.; Memè, L.; Cerea, M.; Roy, M.; Bambini, F. Custom-made additively manufactured subperiosteal implant. Minerva Dent. Oral Sci. 2022, 71, 353–360. [Google Scholar] [CrossRef]
- Alemayehu, D.-B.; Jeng, Y.-R. Three-dimensional finite element investigation into effects of implant thread design and loading rate on stress distribution in dental implants and anisotropic bone. Materials 2021, 14, 6974. [Google Scholar] [CrossRef]
- Anitua, E.; Eguia, A.; Stradigl, C.; Alkhraisat, M.H. Clinical performance of additively manufactured subperiosteal implants: A systematic review. Int. J. Implant Dent. 2024, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Brizuela Velasco, A.; Ríos-Santos, J.-V.; Sánchez Lasheras, F.; Ferreira Lemos, B.; Gil, F.J.; Carvalho, A.; Herrero-Climent, M. Effect of Different Implant Designs on Strain and Stress Distribution under Non-Axial Loading: A Three-Dimensional Finite Element Analysis. Int. J. Environ. Res. Public Health 2020, 17, 4738. [Google Scholar] [CrossRef] [PubMed]
- Pellizzer, E.P.; Lemos, C.A.A.; Almeida, D.A.F.; de Souza Batista, V.E.; Santiago Júnior, J.F.; Verri, F.R. Biomechanical Analysis of Different Implant-Abutment Interfaces in Different Bone Types: An In Silico Analysis. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhang, L.; Wang, Y.; Chu, Y.; Lu, Y. Finite element analysis in the Dental Sciences: A Bibliometric and a Visual Study. Int. Dent. J. 2024. S0020-6539(24)01416-3. [Google Scholar] [CrossRef]
- Hamada, T.; Gonda, T.; Murase, K.; Maeda, Y.; Ikebe, K. Mechanical Influence of Edentulous Mandible Morphology on Peri-implant Bone in Implant Prosthetics: 3D Finite Element Analysis. Int. J. Oral Maxillofac. Implants 2024, 39, 931–937. [Google Scholar]
- Tribst, J.P.M.; Dal Piva, A.M.O.; Lo Giudice, R.; Borges, A.L.S.; Bottino, M.A.; Epifania, E.; Ausiello, P. The Influence of Custom-Milled Framework Design for an Implant-Supported Full-Arch Fixed Dental Prosthesis: 3D-FEA Study. Int. J. Environ. Res. Public Health 2020, 17, 4040. [Google Scholar] [CrossRef]
- Elleuch, S.; Jrad, H.; Wali, M.; Dammak, F. Mandibular bone remodeling around osseointegrated functionally graded biomaterial implant using three-dimensional finite element model. Clin. Num. Mater. 2023, 39, e3750. [Google Scholar] [CrossRef] [PubMed]
- Anniwaer, A.; Yin, Z.; Zhu, J.; Jin, C.; Muhetaer, A.; Huang, C. Comparison of three implant systems under preload loss: A finite element analysis validated by digital image correlation methods. J. Prosthodont. Res. 2024. [Google Scholar] [CrossRef]
- Elleuch, S.; Jrad, H.; Kessentini, A.; Wali, M.; Dammak, F. Design optimization of implant geometrical characteristics enhancing primary stability using FEA of stress distribution around dental prosthesis. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 1035–1051. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Saneja, R.; Singh, A.; Dubey, P.K.; Bhatnagar, A. Peri-implant Stress Distribution Assessment of Various Attachment Systems for Implant-Supported Overdenture Prosthesis by Finite Element Analysis—A Systematic Review. J. Oral Biol. Craniofacial Res. 2022, 12, 802–808. [Google Scholar] [CrossRef]
- Kundakcioglu, A.; Ayhan, M. Evaluation of different subperiosteal implant thicknesses on mechanical strength and stress on bone by finite element analysis. Int. J. Med. Sci. 2024, 21, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Sundram, R.; Abdemagyd, H.A.E. Application of Finite Element Model in Implant Dentistry: A Systematic Review. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. 2), S85–S91. [Google Scholar] [CrossRef] [PubMed]
- Marcián, P.; Wolff, J.; Horáčková, L.; Kaiser, J.; Zikmund, T.; Borák, L. Micro Finite Element Analysis of Dental Implants under Different Loading Conditions. Comput. Biol. Med. 2018, 96, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Nimmawitt, P.; Abdu Aliyu, A.A.; Lohwongwatana, B.; Arunjaroensuk, S.; Puncreobutr, C.; Mattheos, N.; Pimkhaokham, A. Understanding the Stress Distribution on Anatomic Customized Root-Analog Dental Implant at Bone-Implant Interface for Different Bone Densities. Materials 2022, 15, 6379. [Google Scholar] [CrossRef]
- Anitua, E.; Larrazabal Saez de Ibarra, N.; Saracho Rotaeche, L. Implant-supported prostheses in the edentulous mandible: Biomechanical analysis of different implant configurations via finite element analysis. Dent. J. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Keleş, H.G.; Karaca, Ç. Comparison of stress distribution among standard dental implants placed in grafted bone, zygomatic implants, and subperiosteal implants in the atrophic edentulous maxilla: 3D finite element analysis. Int. J. Oral Maxillofac. Implants 2023, 38, 347–356. [Google Scholar] [CrossRef]
- Saranya, V.; Harris, M.; Abraham, S.; Venkitachalam, R.; Nair, S.S.; Mathew, A. Three-dimensional finite element analysis of stress distribution on different complex macro designs in commercially available implants: An in-vitro study. J. Oral Biol. Craniofacial Res. 2024, 14, 761–766. [Google Scholar]
- Mommaerts, M.Y. Management of adverse effects following additively manufactured subperiosteal jaw implantation in the maxilla. J. Stomatol. Oral Maxillofac. Surg. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.B.; Jain, S.; Jain, V.; Grover, R.K.; Kale, A.A.; Talreja, L. Evaluation and Comparison of Stresses Between All-on-4 and All-on-6 Treatment Concepts With Three Different Prosthetic Materials in the Maxilla: A Finite Element Analysis Study. Cureus 2024, 16, e71362. [Google Scholar] [CrossRef]
- Mousa, M.A.; Abdullah, J.Y.; Jamayet, N.B.; El-Anwar, M.I.; Ganji, K.K.; Alam, M.K.; Husein, A. Biomechanics in Removable Partial Dentures: A Literature Review of FEA-Based Studies. Biomed. Res. Int. 2021, 2021, 5699962. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mao, Z.-H.; Peng, W.; Wen, S. Biomechanical Influence of Thread Form on Stress Distribution Over Short Implants (≤6 mm) Using Finite Element Analysis. Biomed. Tech. 2022, 67, 53–60. [Google Scholar] [CrossRef]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef] [PubMed]
- De Moore, E.; Huys, S.E.F.; Vn Lenthe, D.H.; Mommaerts, M.Y.; Sloten, J.V. Mechanical evaluation of a patient-specific additively manufactured subperiosteal jaw implant (AMSJI) using finite-element analysis. Int. J. Oral Maxillofac. Surg. 2022, 51, 405–411. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ujigawa, K.; Kizu, Y.; Tonogi, M.; Yamane, G.Y. Biomechanical three-dimensional finite-element analysis of maxillary prostheses with implants: Design of number and position of implants for maxillary prostheses after hemimaxillectomy. Int. J. Oral Maxillofac. 2010, 39, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, M.; Cankaya, A.B. Custom-made subperiosteal implants: A finite element analysis on monoblock and dual implant systems in atrophic maxilla. Int. J. Med. Sci. 2023, 20, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Antiparmak, N.; Polat, S.; Onal, S. Finite element analysis of the biomechanical effects of titanium and Cfr-peek additively manufactured subperiosteal jaw implant (AMSJI) on maxilla. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101290. [Google Scholar] [CrossRef]
- Zielinski, R.; Sowinski, J.; Piechaczek, M.; Okulski, J.; Kozakiewicz, M. Finite element analysis of subperiosteal implants in edentulism—On the basis of the MaI Implant® by Integra Implants®. Materials 2023, 16, 7466. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Z.; Chen, J.; Yuan, X.; Sadowsky, S.J.; Coyac, B.R.; Brunski, J.B.; Helms, J.A. Mechano-adaptive Responses of Alveolar Bone to Implant Hyper-loading in a Pre-clinical In Vivo Model. Clin. Oral Implants Res. 2020, 31, 1159–1172. [Google Scholar] [CrossRef]
- Jafarian, M.; Mirhashemi, F.S.; Emadi, N. Finite Element Analysis of Stress Distribution Around a Dental Implant with Different Amounts of Bone Loss: An In Vitro Study. Dent. Med. Probl. 2019, 56, 27–32. [Google Scholar] [CrossRef]
- Cantó-Navés, O.; Medina-Galvez, R.; Marimon, X.; Ferrer, M.; Figueras-Álvarez, Ó.; Cabratosa-Termes, J. A 3D Finite Element Analysis Model of Single Implant-Supported Prosthesis under Dynamic Impact Loading for Evaluation of Stress in the Crown, Abutment, and Cortical Bone Using Different Rehabilitation Materials. Materials 2021, 14, 3519. [Google Scholar] [CrossRef]
- Liao, X.; Cao, R.; Zhong, J.; Chen, C.; Pan, S. Influence of implant distribution on the biomechanical behaviors of mandibular implant-retained overdentures: A three-dimensional finite element analysis. Int. J. Med. Sci. 2024, 24, 405. [Google Scholar] [CrossRef] [PubMed]








| Type of Material | Elastic Module E (MPa) | Poisson Coefficient | σmax Maximum Allowable (Mpa) |
|---|---|---|---|
| Cortical bone | 13,700 | 0.3 | 50 |
| Trabecular bone | 1370 | 0.3 | - |
| Titanium Gr5 (load model) | 101,000 | 0.34 | 950 |
| Titanium Gr5 (bar) | 101,000 | 0.34 | 970 |
| Resin for prosthetics | 3000 | 0.3 | - |
| Muscle simulators | 25 | 0.4 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, G.; Karaban, M.; Scalchi, V.; Urbani, M.; Giudice, A.; Barausse, C.; Felice, P. Finite Element Analysis of Functionally Loaded Subperiosteal Implants Evaluated on a Realistic Model Reproducing Severe Atrophic Jaws. Methods Protoc. 2025, 8, 8. https://doi.org/10.3390/mps8010008
Pellegrino G, Karaban M, Scalchi V, Urbani M, Giudice A, Barausse C, Felice P. Finite Element Analysis of Functionally Loaded Subperiosteal Implants Evaluated on a Realistic Model Reproducing Severe Atrophic Jaws. Methods and Protocols. 2025; 8(1):8. https://doi.org/10.3390/mps8010008
Chicago/Turabian StylePellegrino, Gerardo, Maryia Karaban, Veronica Scalchi, Marco Urbani, Amerigo Giudice, Carlo Barausse, and Pietro Felice. 2025. "Finite Element Analysis of Functionally Loaded Subperiosteal Implants Evaluated on a Realistic Model Reproducing Severe Atrophic Jaws" Methods and Protocols 8, no. 1: 8. https://doi.org/10.3390/mps8010008
APA StylePellegrino, G., Karaban, M., Scalchi, V., Urbani, M., Giudice, A., Barausse, C., & Felice, P. (2025). Finite Element Analysis of Functionally Loaded Subperiosteal Implants Evaluated on a Realistic Model Reproducing Severe Atrophic Jaws. Methods and Protocols, 8(1), 8. https://doi.org/10.3390/mps8010008









