Protocol Article: A Cross-Sectional Evaluation of Children’s Feet and Lower Extremities
Abstract
1. Introduction
- To make reference material of the demography of the foot, the rotational status, and the range of motion in joints in the lower extremities of children in Denmark to evaluate if these anthropometric parameters in children influence pain status.
- Examining the prevalence of pain in children as well as exploring the prevalence of pathologies in children’s feet to evaluate if these conditions in children influence pain status.
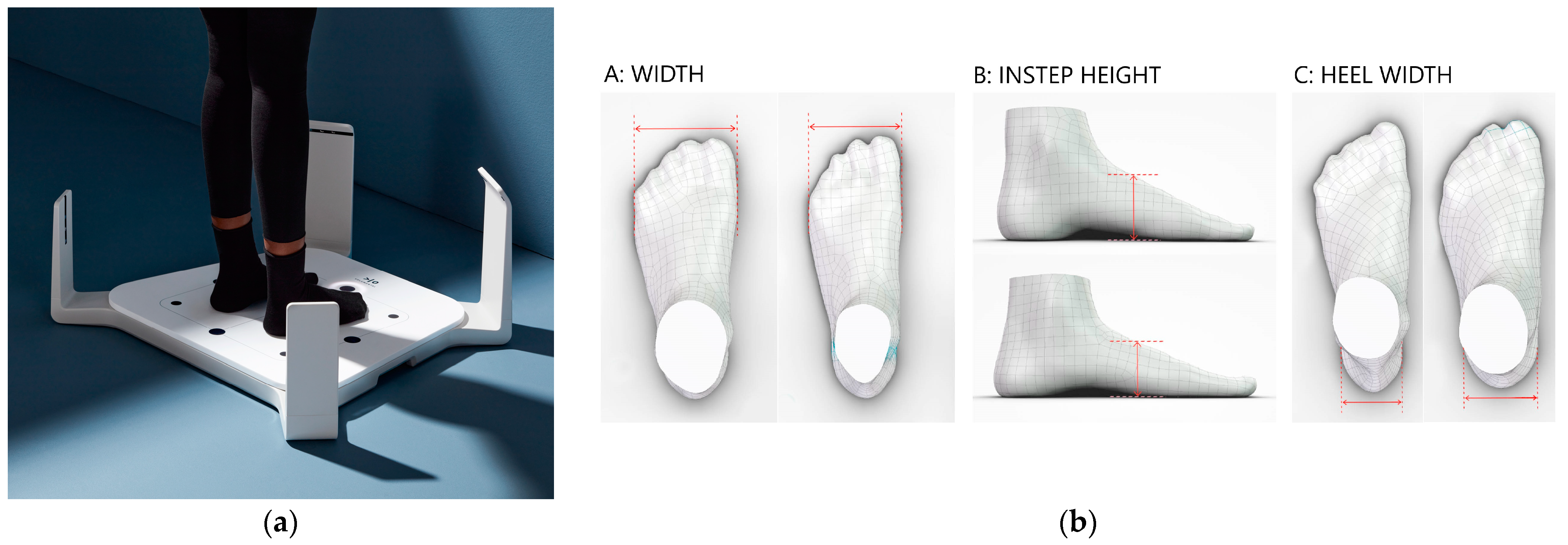
- Evaluating the appropriateness of shoe fitting using commercial 3D technology and foot pressure mapping to evaluate if shoe fitting in children influences foot pain status.
2. Method and Materials
2.1. Study Design
2.1.1. Study Plan
2.1.2. Study Organization
3. Participants
3.1. Inclusion Process
- The subject and caregiver log on to Aula with NemID.
- A link to RedCap is sent via Aula to the subject and caregiver (who has now been verified via the NemID login).
- The subject and caregiver enter RedCap to be able to assess written patient information and preliminary oral information via video, with the possibility to orally inquire about additional information on the project (see below).
- After this, they will be able to confirm his/her consent by signing into the digital system.
3.2. Inclusion Criteria
- Children aged 6–18, and the children must attend 1st, 5th, or 9th grade at a Danish primary school.
- Children from selected schools in Region Hovedstaden and Region Sjælland.
3.3. Exclusion Criteria
- Not accepting to participate in this study.
- Malignancies or infections discovered throughout this study that warrant acute medical treatment.
3.4. Treatment during and after This Study Has Ended/Exclusion from This Study and Discontinuation of This Study
3.5. Insurance
4. Ethical Consideration
Ethics Approval and Consent to Participate
5. Procedure and Method
5.1. Assessments
5.2. Clinical Examinations
- -
- Mapping the foot anatomy and pathologies of warts, foot deformities, ingrown toenails, callosities, hallux valgus, metatarsus varus, and the ROM of the subtalar joint and midfoot, hindfoot valgus, navicular height, static foot pressure mapping, and foot type (square, Roman, or Greek) [26].
- -
- Mapping the anthropometric data on children’s height and weight, hip range of motion (ROM) (flexion, internal and external ROM [27], the ROM of knee flexion, and the extension ROM of the ankle joint on a flexed and extended knee [27], as well as the femoral anteversion and torsion of the tibia (foot–thigh ankle)). This is evaluated when the child is either standing, prone, or supine in a standardized manner and using a goniometer (see Supplementary).
5.3. Patient-Related Outcome Measures (PROMs) and Pain Status
5.4. Equipment
5.4.1. Measurement Procedure for the Foot
5.4.2. Data Collection Footwear
Shoe Length
Shoe Width
5.4.3. Foot Pressure Mapping
6. Analyses and Statistics
6.1. Availability of Data and Materials
6.2. Safety Endpoints and Evaluations
6.3. Statistical Analysis and Randomization
6.4. Test and Re-Test Analysis and Sample Size
7. Expected Results
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ward, M.G.K.; Baird, B. Medical Neglect: Working with Children, Youth, and Families. Paediatr. Child. Health 2022, 27, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.C.; Tait, M.; Bong, E.; Kane, K.J.; Nester, C. Symptomatic Pes Planus in Children: A Synthesis of Allied Health Professional Practices. J. Foot Ankle Res. 2020, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Brønnum-Hansen, H.; Baadsgaard, M. Widening Social Inequality in Life Expectancy in Denmark. A Register-Based Study on Social Composition and Mortality Trends for the Danish Population. BMC Public. Health 2012, 12, 994. [Google Scholar] [CrossRef]
- Bharmal, N.; Derose, K.P.; Felician, M.F.; Weden, M.M. Understanding the Upstream Social Determinants of Health; RAND Corporation: Santa Monica, CA, USA, 2015. [Google Scholar]
- Fuglkjær, S.; Dissing, K.B.; Hestbæk, L. Prevalence and Incidence of Musculoskeletal Extremity Complaints in Children and Adolescents. A Systematic Review. BMC Musculoskelet. Disord. 2017, 18, 418. [Google Scholar] [CrossRef] [PubMed]
- Fuglkjær, S.; Hartvigsen, J.; Wedderkopp, N.; Boyle, E.; Jespersen, E.; Junge, T.; Larsen, L.R.; Hestbæk, L. Musculoskeletal Extremity Pain in Danish School Children—How Often and for How Long? The CHAMPS Study-DK. BMC Musculoskelet. Disord. 2017, 18, 492. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, M.K.; Williams, D.R.; Menestrel, S.; Le Flaubert, J.L. (Eds.) The Future of Nursing 2020–2030; National Academies Press: Washington, DC, USA, 2021; ISBN 978-0-309-68506-1. [Google Scholar]
- Gold, J.I.; Mahrer, N.E.; Yee, J.; Palermo, T.M. Pain, Fatigue, and Health-Related Quality of Life in Children and Adolescents with Chronic Pain. Clin. J. Pain. 2009, 25, 407–412. [Google Scholar] [CrossRef]
- Haraldstad, K.; Christophersen, K.A.; Helseth, S. Health-Related Quality of Life and Pain in Children and Adolescents: A School Survey. BMC Pediatr. 2017, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Stebbins, J.; Zavatsky, A.B.; Theologis, T. Health-Related Quality of Life in Children with Flexible Flatfeet: A Cross-Sectional Study. J. Child. Orthop. 2014, 8, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.L.; Cheng, J.C.Y.; Mak, A.F.T. A Cross-Sectional Study on the Development of Foot Arch Function of 2715 Chinese Children. Prosthet Orthot Int 2005, 29, 241–253. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, H.; Zhuang, G.; Liang, X.; Hu, X.; Zhu, Y.; Zhang, R.; Fan, X.; Cao, Y. Flexible Flatfoot of 6–13-Year-Old Children: A Cross-Sectional Study. J. Orthop. Sci. 2018, 23, 552–556. [Google Scholar] [CrossRef]
- Pourghasem, M.; Kamali, N.; Farsi, M.; Soltanpour, N. Prevalence of Flatfoot among School Students and Its Relationship with BMI. Acta Orthop. Traumatol. Turc. 2016, 50, 554–557. [Google Scholar] [CrossRef]
- Stavlas, P.; Grivas, T.B.; Michas, C.; Vasiliadis, E.; Polyzois, V. The Evolution of Foot Morphology in Children between 6 and 17 Years of Age: A Cross-Sectional Study Based on Footprints in a Mediterranean Population. J. Foot Ankle Surg. 2005, 44, 424–428. [Google Scholar] [CrossRef]
- Uden, H.; Scharfbillig, R.; Causby, R. The Typically Developing Paediatric Foot: How Flat Should It Be? A Systematic Review. J. Foot Ankle Res. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Müller, S.; Carlsohn, A.; Müller, J.; Baur, H.; Mayer, F. Static and Dynamic Foot Characteristics in Children Aged 1-13 Years: A Cross-Sectional Study. Gait Posture 2012, 35, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.W.K.; Kong, P.W.; Tong, J.W.K.; Kong, P.W. Medial Longitudinal Arch Development of Children Aged 7 to 9 Years: Longitudinal Investigation. Phys. Ther. 2016, 96, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, D.J.; Kim, S.J.; Suh, J.S. Does the Long-Term Use of Medial Arch Support Insole Induce the Radiographic Structural Changes for Pediatric Flexible Flat Foot?—A Prospective Comparative Study. Foot Ankle Surg. 2020, 26, 449–456. [Google Scholar] [CrossRef]
- Bouchard, M.; Mosca, V.S. Flatfoot Deformity in Children and Adolescents: Surgical Indications and Management. J. Am. Acad. Orthop. Surg. 2014, 22, 623–632. [Google Scholar] [CrossRef] [PubMed]
- González-Elena, M.L.; Castro-Méndez, A.; Córdoba-Fernández, A.; Coheña-Jiménez, M. Relationship of the Use of Short Footwear with the Development of Hallux Valgus in a Sample of Andalusian Schoolchildren. Int. J. Environ. Res. Public. Health 2021, 18, 11244. [Google Scholar] [CrossRef] [PubMed]
- Puszczałowska-Lizis, E.; Zarzyczna, P.; Mikuľáková, W. Impact of Footwear Fitting on Foot Shape in Primary Schoolgirls. Acta Bioeng. Biomech. 2020, 22, 1. [Google Scholar] [CrossRef]
- Morrison, S.C.; Price, C.; McClymont, J.; Nester, C. Big Issues for Small Feet: Developmental, Biomechanical and Clinical Narratives on Children’s Footwear. J. Foot Ankle Res. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Yurt, Y.; Sener, G.; Yakut, Y. Footwear Suitability in Turkish Preschool-Aged Children. Prosthet. Orthot. Int. 2014, 38, 224–231. [Google Scholar] [CrossRef] [PubMed]
- González Elena, M.L.; Córdoba-Fernández, A. Footwear Fit in Schoolchildren of Southern Spain: A Population Study. BMC Musculoskelet. Disord. 2019, 20, 208. [Google Scholar] [CrossRef]
- Haley, M.R.; Pavey, M.; Price, C.; Nester, C. Children’s Foot Size versus Children’s Shoe Size When They Return to Store. Footwear Sci. 2019, 11, S130–S132. [Google Scholar] [CrossRef]
- Haight, H.J.; Dahm, D.L.; Smith, J.; Krause, D.A. Measuring Standing Hindfoot Alignment: Reliability of Goniometric and Visual Measurements. Arch. Phys. Med. Rehabil. 2005, 86, 571–575. [Google Scholar] [CrossRef]
- Staheli, L.T. Torsion-Treatment Indications. Clin. Orthop. Relat. Res. 1989, 247, 61–66. [Google Scholar] [CrossRef]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of Four Pain Intensity Rating Scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef]
- Martinkevich, P.; Møller-Madsen, B.; Gottliebsen, M.; Kjeldgaard Pedersen, L.; Rahbek, O. Validation of the Translated Oxford Ankle Foot Questionnaire in 82 Danish Children Aged between Five and 16 Years. Bone Jt. J. 2015, 97-B, 420–426. [Google Scholar] [CrossRef]
- Wenger, D.R.; Mauldin, D.; Speck, G.; Morgan, D.; Lieber, R.L. Corrective Shoes and Inserts as Treatment for Flexible Flatfoot in Infants and Children. J. Bone Jt. Surg. Ser. A 1989, 71, 800–810. [Google Scholar] [CrossRef]
- Stacoff, A.; Reinschmidt, C.; Nigg, B.M.; van den Bogert, A.J.; Lundberg, A.; Denoth, J.; Stüssi, E. Ects of Foot Orthoses on Skeletal Motion during Running. Clin. Biomech. 2000, 15, 54–64. [Google Scholar] [CrossRef]
- Puszczalowska-Lizis, E.; Zarzyczna, P.; Mikulakova, W.; Migala, M.; Jandzis, S. Influence of Footwear Fitting on Feet Morphology in 9 Year Old Girls. BMC Pediatr. 2020, 20, 349. [Google Scholar] [CrossRef]
- Yalçin, N.; Esen, E.; Kanatli, U.; Yetkin, H. Evaluation of the Medial Longitudinal Arch: A Comparison between the Dynamic Plantar Pressure Measurement System and Radiographic Analysis. Acta Orthop. Traumatol. Turc. 2010, 44, 241–245. [Google Scholar] [CrossRef]
- Chen, K.C.; Chen, Y.C.; Yeh, C.J.; Hsieh, C.L.; Wang, C.H. The Effect of Insoles on Symptomatic Flatfoot in Preschool-Aged Children: A Prospective 1-Year Follow-up Study. Medicine 2019, 98, e17074. [Google Scholar] [CrossRef]
- Klein, C.; Groll-Knapp, E.; Kundi, M.; Kinz, W. Increased Hallux Angle in Children and Its Association with Insufficient Length of Footwear: A Community Based Cross-Sectional Study. BMC Musculoskelet. Disord. 2009, 10, 159. [Google Scholar] [CrossRef]
- Saini, U.C.; Bali, K.; Sheth, B.; Gahlot, N.; Gahlot, A. Normal Development of the Knee Angle in Healthy Indian Children: A Clinical Study of 215 Children. J. Child. Orthop. 2010, 4, 579–586. [Google Scholar] [CrossRef]
- Jae, H.Y.; In, H.C.; Cho, T.J.; Chin, Y.C.; Won, J.Y. Development of Tibiofemoral Angle in Korean Children. J. Korean Med. Sci. 2008, 23, 714–717. [Google Scholar] [CrossRef]
- Arazi, M.; Memik, R. Normal Development of the Tibiofemoral Angle in Children: A Clinical Study of 590 Normal Subjects from 3 to 17 Years of Age. J. Pediatr. Orthop. 2001, 21, 264–267. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing. 2023. Available online: https://www.R-project.org/ (accessed on 7 October 2023).
- Gäverth, J.; Sandgren, M.; Lindberg, P.G.; Forssberg, H.; Eliasson, A.-C. Test-Retest and Inter-Rater Reliability of a Method to Measure Wrist and Finger Spasticity. J. Rehabil. Med. 2013, 45, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Gisev, N.; Bell, J.S.; Chen, T.F. Interrater Agreement and Interrater Reliability: Key Concepts, Approaches, and Applications. Res. Soc. Adm. Pharm. 2013, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- De Vet, H.; Terwee, C.; Mokkink, L.; Knol, D. Measurement in Medicine: A Practical Guide. Measurement in Medicine: A Practical Guide; Cambridge University Press: Cambridge, UK, 2011; pp. 1–338. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Measuring Agreement in Method Comparison Studies. Available online: https://pubmed.ncbi.nlm.nih.gov/10501650/ (accessed on 7 October 2023).
- Chéron, C.; Le Scanff, C.; Leboeuf-Yde, C. Association between Sports Type and Overuse Injuries of Extremities in Children and Adolescents: A Systematic Review. Chiropr. Man. Therap 2016, 24, 41. [Google Scholar] [CrossRef]
- Chéron, C.; Leboeuf-Yde, C.; Le Scanff, C.; Jespersen, E.; Rexen, C.T.; Franz, C.; Wedderkopp, N. Leisure-Time Sport and Overuse Injuries of Extremities in Children Age 6-13, a 2.5 Years Prospective Cohort Study: The CHAMPS-Study DK. BMJ Open 2017, 7, e012606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wedderkopp, N.; Jespersen, E.; Franz, C.; Klakk, H.; Heidemann, M.; Christiansen, C.; Møller, N.C.; Leboeuf-Yde, C. Study Protocol. The Childhood Health, Activity, and Motor Performance School Study Denmark (The CHAMPS-Study DK). BMC Pediatr. 2012, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.R.; Kristensen, P.L.; Junge, T.; Rexen, C.T.; Wedderkopp, N. Motor Performance as Predictor of Physical Activity in Children: The CHAMPS Study-DK. Med. Sci. Sports Exerc. 2015, 47, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Rønne, M.S.; Heidemann, M.; Lylloff, L.; Schou, A.J.; Tarp, J.; Laursen, J.O.; Jørgensen, N.R.; Husby, S.; Wedderkopp, N.; Mølgaard, C. Bone Mass Development in Childhood and Its Association with Physical Activity and Vitamin D Levels. CHAMPS-Study DK. Calcif. Tissue Int. 2019, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.; Bjerge, C.Y.; Jurca, A.; Petersen, M.M.; Boedtker, S.; Balslev-Clausen, A.; Harsted, S. Protocol Article: A Cross-Sectional Evaluation of Children’s Feet and Lower Extremities. Methods Protoc. 2023, 6, 115. https://doi.org/10.3390/mps6060115
Wong C, Bjerge CY, Jurca A, Petersen MM, Boedtker S, Balslev-Clausen A, Harsted S. Protocol Article: A Cross-Sectional Evaluation of Children’s Feet and Lower Extremities. Methods and Protocols. 2023; 6(6):115. https://doi.org/10.3390/mps6060115
Chicago/Turabian StyleWong, Christian, Christina Ystrøm Bjerge, Ales Jurca, Michael Mørk Petersen, Soren Boedtker, Andreas Balslev-Clausen, and Steen Harsted. 2023. "Protocol Article: A Cross-Sectional Evaluation of Children’s Feet and Lower Extremities" Methods and Protocols 6, no. 6: 115. https://doi.org/10.3390/mps6060115
APA StyleWong, C., Bjerge, C. Y., Jurca, A., Petersen, M. M., Boedtker, S., Balslev-Clausen, A., & Harsted, S. (2023). Protocol Article: A Cross-Sectional Evaluation of Children’s Feet and Lower Extremities. Methods and Protocols, 6(6), 115. https://doi.org/10.3390/mps6060115






