Development of a Quantitative PCR Method for Detecting Enterococcus faecalis Cytolysin in Human Stool Samples
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials for Bacterial DNA Isolation from Stool
- 0.5 mm Zirconium Oxide Beads (Next Advance, Raymer town, NY, USA, Cat. No.: ZROB05)
- Absolute ethanol
- Sterile 2 mL Screw-Cap Tubes (Stellar scientific, Baltimore, MD, USA, Cat. No.: T20-C3220-SG)
- QIAmp Fast DNA stool Mini Kit (QIAGEN, Hilden, Germany; Cat. No.: 51604)
- Pipettes and sterile tips
- Eppendorf tubes 1.5 and 2 mL
2.2. Isolation of Enterococcus Strains
- Enterococcosel Broth (BD Biosciences, La Jolla, CA, USA, Cat. No.: 212207)
- Agar (BD Biosciences, La Jolla, CA, USA, Cat. No.: 214010)
- Blood agar plates (BD Biosciences, La Jolla, CA, USA, Cat. No.: B21739X)
- 4 mm glass sterile beads (Fischer Scientific, Waltham, MA, USA, Cat. No.: 11-312B)
2.3. Materials for qPCR
- Primer mix (forward and reverse) for bacteria (see Primers at Procedure 3.2)
- Genomic DNA (10 ng/μL)
- MicroAmp Fast 96-Well Reaction Plate (0.1 mL) (Applied Biosystems, Waltham, MA, USA, Cat. No.: 4346907)
2.4. Materials for Gel Electrophoresis
- Agarose gel (Invitrogen, Waltham, MA, USA, Cat. No:.16500500)
- DNA ladder 100 bp (Biopioneer Inc. San Diego, CA, USA, Cat. No.: MDL-100)
- SYBR® Safe (Invitrogen, Waltham, MA, USA, Cat. No.: S33102)
- TAE buffer (Quality Biological Inc, Gaithersburg, MD, USA, Cat. No.: 10128-398)
2.5. Equipment
- Centrifuge (capacity to speed at 20,000× g)
- Nanodrop
- Vortex
- Heat blocks
- Mini-Beadbeater 96, BioSpec Products (capacity to speed at 2000 rpm)
- Applied BiosystemsTM StepOnePlusTM real-time PCR system
- Gel Electrophoresis Equipment
2.6. Methods for Human Studies, Mouse Studies, and Statistics
2.6.1. Patient Cohorts
2.6.2. Statistics
3. Procedure
3.1. DNA Isolation from Human Stool
- Before starting:
- Heat the heat block to 95 °C for use in step 4 and 70 °C for use in step 11.
- Read the instructions from the QIAamp Fast DNA Stool Mini Kit to add absolute ethanol to the Buffer AW1 and Buffer AW2 concentrates. Mix all buffers before use, and redissolve any precipitates in Buffer AL and InhibitEX Buffer by incubating at 37–70 °C. Prepare screw-cap tubes with 2 scoops (0.25 mL) of 0.5 mm beads, and leave them on ice.
- Weigh 200 mg of stool using a scalpel to scrape bits of the frozen stool sample, and place it in 2 mL screw-cap tubes.
CRITICAL STEP: It is important to maintain the sample frozen at all times; keep the sample on ice at all times.
CRITICAL STEP: The protocol is optimized for use with 200 mg of stool, but it can also be used with lower or higher amounts. For higher amounts, you need to increase the amount of buffers. For example, weigh the stool sample, and add 10 volumes of Buffer ASL (e.g., add 10 mL InhibitEX to 1 g stool).
- Add 500 μL of InhibitEX Buffer to each stool sample while keeping it on ice.
- Use the bead beater at 2000 rpm for 2 cycles of 30 s to homogenize the samples.
- Using the heat block, heat samples at 95 °C for 5 min.
- Centrifuge the samples at room temperature (15–25 °C) at 20,000× g for 3 min.
CRITICAL STEP: If you see particles in the supernatant, centrifuge the samples again. It is important not to transfer debris.
- In a new 1.5 mL Eppendorf tube, pipette 20 μL of proteinase K.
- Transfer 400 μL of the supernatant from step 7 into the 1.5 mL Eppendorf tube containing proteinase K.
- Pipette 400 μL of AL buffer.
- Vortex for 15 s.
- Using the heat block, heat the samples at 70 °C for 10 min.
- Add 400 μL of absolute ethanol to each tube to the lysate, and vortex for 15 s.
- Carefully pipette 600 μL of the lysate to the spin column.
- Centrifuge the samples at room temperature (15–25 °C) at 20,000× g for 1 min.
- Place the spin column in a new 2 mL collection tube, and discard the collection tube that contains the filtrate.
- Pipette the remaining 600 μL of the lysate to the spin column.
- Centrifuge the samples at room temperature (15–25 °C) at 20,000× g for 1 min.
- Place the spin column in a new 2 mL collection tube, and discard the collection tube that contains the filtrate.
- Carefully open the spin column, and add 500 μL of buffer AW1.
- Centrifuge the samples at room temperature (15–25 °C) at 20,000× g for 1 min.
- Place the spin column in a new 2 mL collection tube and discard.
- Pipette 500 μL of buffer AW2 in the spin column.
- Centrifuge the samples at room temperature (15–25 °C) at 20,000× g for 6 min.
- Transfer the spin column into a new labeled 1.5 mL Eppendorf tube, and pipette 50 μL of sterile double-distilled water into the middle of the membrane of the spin column.
- Incubate 1 min at room temperature.
- Centrifuge the samples at room temperature (15–25 °C) at 20,000× g for 3 min.
- Discard the spin column, and keep the samples on ice.
PAUSE STEP After collecting all the samples from the centrifuge, they can be stored at −20 °C.
- Quantification of DNA is accomplished by measuring the absorbance at 260 nm using the Nanodrop.
CRITICAL STEP The assessment of DNA purity involves calculating the ratio between the absorbance values at 230 nm, 260 nm, and 280 nm. A desirable A260/A280 ratio for pure DNA falls within the range of 1.8 to 2.0, indicating pure DNA. The A260/A230 ratio assesses contaminants, like phenol, salts, and carbs. A ratio above 2.0 suggests minimal contamination.
CRITICAL STEP To ensure accurate measurements, the absorbance readings at 260 nm should ideally range between 0.1 and 1.0. Maintaining absorbance values within this range is crucial for obtaining reliable and valid quantification results.
3.2. Real-Time Quantitative PCR
CRITICAL STEP Ensure all pipetting and handling procedures are conducted with appropriate sterile techniques to prevent contamination. Proper controls (negative and positive) should be included in each qPCR run for result validation. Additionally, it is important to verify the specificity of the primers and optimize the primer concentrations, if necessary, to achieve reliable qPCR results.
- Prepare the qPCR reaction mix for each sample according to the following composition:
- Sybr Green: 10 μL
- Primer mix (forward and reverse, 10 μM): 1 μL
- Extracted DNA (10 ng/μL): 9 μL
- Mix the components gently by pipetting up and down a few times and vortex 10 s.
- Distribute the reaction mix into the wells of a qPCR 96-well plate, ensuring proper allocation for samples and controls.
- Seal the qPCR plate with an optical adhesive cover to prevent contamination during the amplification process.
- Spin the qPCR plate 10 s at 20,000× g.
- Load the sealed qPCR plate into the ABI StepOnePlus real-time PCR system.
- Set up the qPCR program on the ABI StepOnePlus system as follows:
- Initial denaturation: 95 °C for 3 min
- Amplification (40 cycles)
- Denaturation: 95 °C for 15 s
- Annealing and extension: 60 °C for 1 min
During the amplification cycles, the ABI StepOnePlus system will collect real-time fluorescence data. - After the amplification is complete, the system will automatically generate Ct (cycle threshold) values for each reaction, representing the cycle at which the fluorescence signal crosses a predetermined threshold.
3.3. Verification of qPCR Product
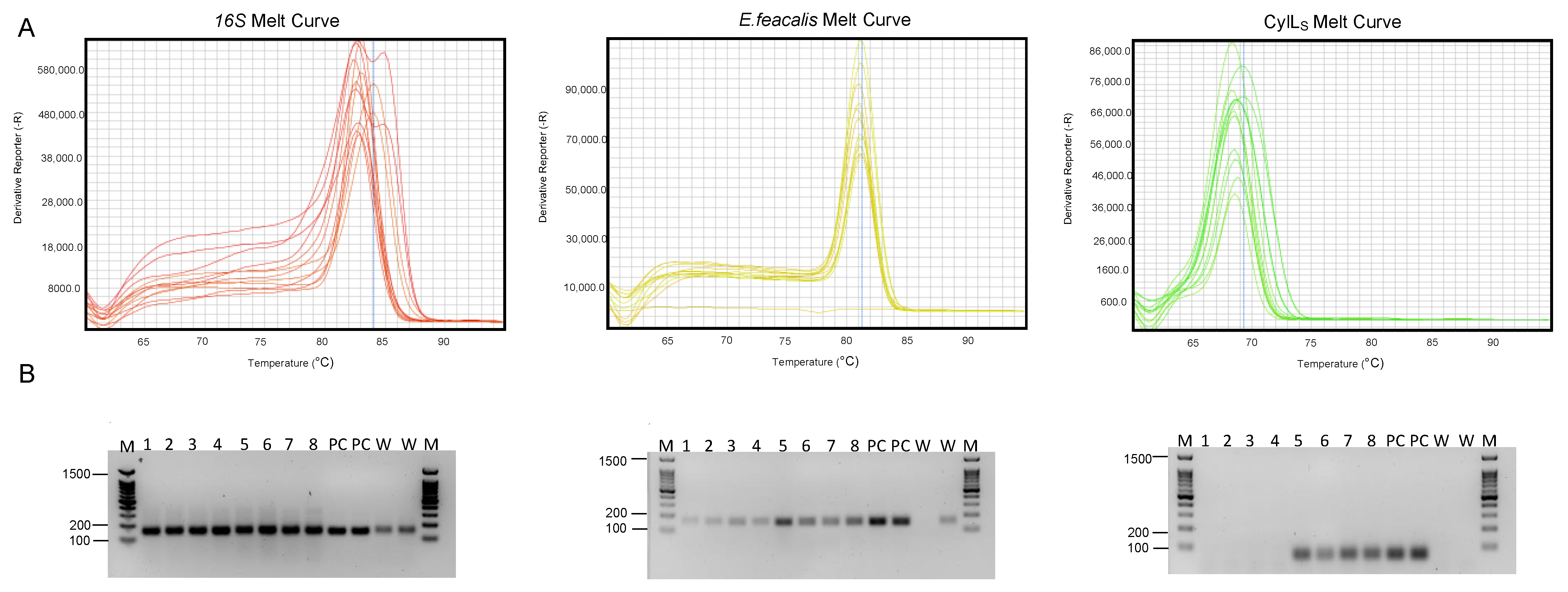
3.3.1. Melting Curve
- After the completion of the qPCR amplification cycles, initiate the melting curve analysis on the qPCR instrument.
- Set the temperature range for the melting curve analysis. This typically involves heating the samples from the annealing temperature to a higher temperature, allowing for the DNA to denature.
- The instrument will measure the fluorescence at each temperature increment as the DNA denatures. The resulting data will be used to generate the melting curve.
- Once the analysis is complete, review the melting curve graph. Look for distinctive peaks that correspond to the specific DNA products.
- Compare the melting curve peaks with the expected melting temperatures (Tm) of the target amplicons using the positive and negative controls. This will help confirm the specificity of the amplification.
CRITICAL STEP Any unexpected peaks, irregularities, or deviations from the expected Tm values should be investigated further, and if necessary, adjustments to the PCR conditions should be made for optimization.
3.3.2. Gel Electrophoresis
- Prepare the agarose gel at 2% using agarose and buffer (TAE). Add SYBR Safe to the gel mix before casting the gel. Cast the gel and allow it to solidify.
- Mix the qPCR products with loading dye in a 1:1 ratio.
- Load 3.5 μL of the DNA ladder and 5 μL of the qPCR samples onto the gel wells.
- Run the gel at 130 V for 20 min.
- After the electrophoresis is complete, visualize the DNA bands under a UV transilluminator.
- Compare the size of the DNA bands with the expected sizes of the qPCR products (see Table 1). This helps to verify the presence of the correct amplicons.
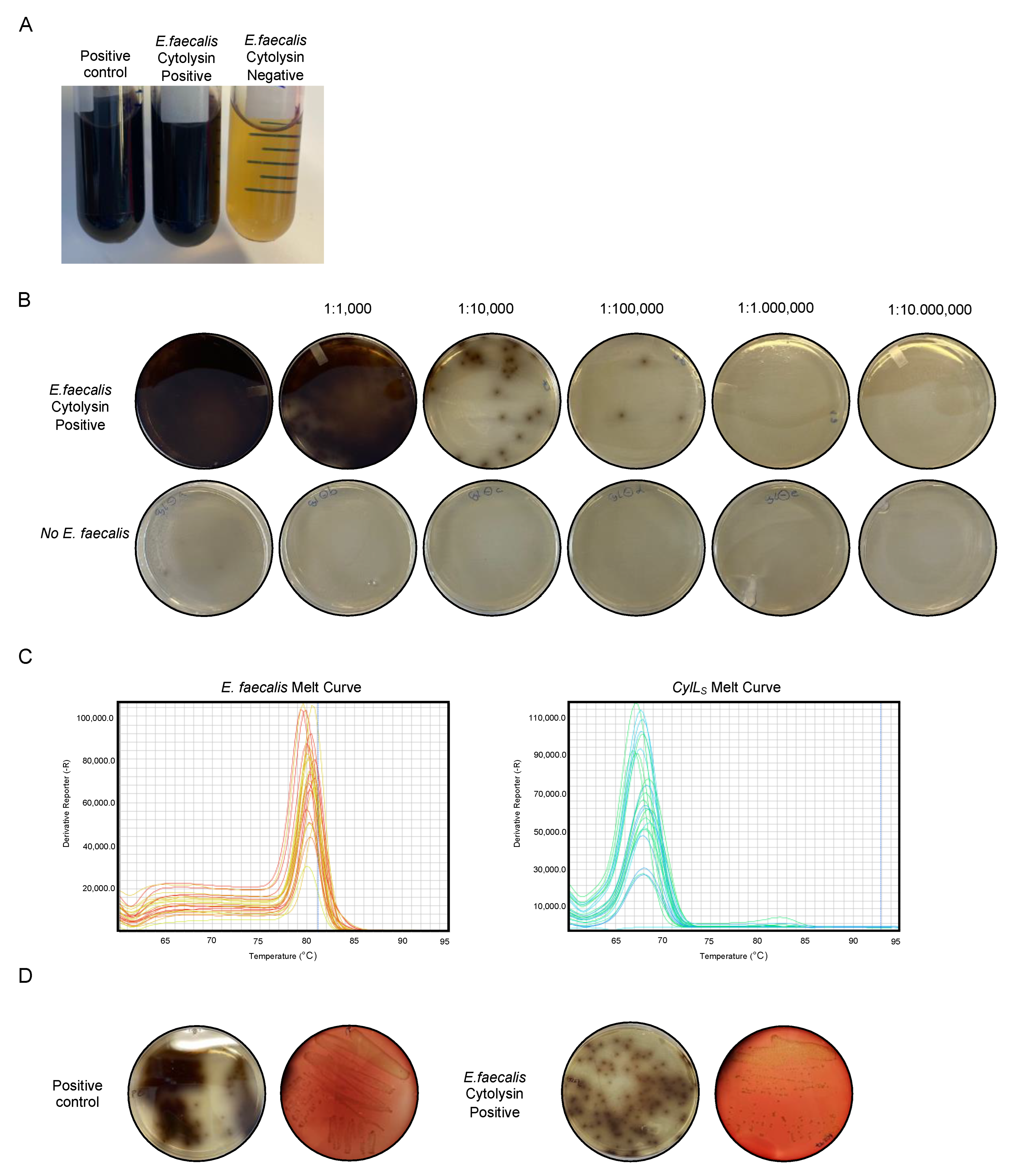
3.4. Detection of E. faecalis Cytolysin-Positive Strains from Colonies
- Mix 43 g of Enterococcosel Broth in 1 L of MilliQ water for broth preparation, and autoclave the mixture for sterilization.
- Prepare Enterococcosel Agar Plates: Mix 43 g of Enterococcosel Broth with 15 g of Agar in 1 L of MilliQ water, and autoclave the mixture for sterilization.
- Weigh 10 mg of human stool samples and place into 5 mL of Enterococcosel Broth.
- Vortex the mixture until the media is visibly turbulent and well-mixed. Prepare different dilution stocks: 1/1000, 1/10,000, 1/100,000, 1/1,000,000, and 1/10,000,000.
- Plate 200 µL of the diluted stocks using 4 mm glass sterile beads for equal distribution on the plate.
- Incubate the plates overnight at 37 °C.
- Pick colonies that appear on the plates, and perform qPCR using E. faecalis-specific and CylLS-specific primers.
- If the qPCR results indicate positivity for the cytolysin gene, select the same colony from the plate, and incubate it with 5 mL of Enterococcosel Broth overnight.
- Inoculate onto blood agar plates to facilitate the identification of beta-hemolysis caused by the cytolytic toxins released by the bacteria.
3.5. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
- To establish the limit of detection (LOD) and limit of quantitation (LOQ) for the assay, specific criteria were employed. For the detection of E. faecalis, a cut-off Ct value of ≤30 was utilized. Similarly, for the detection of CylLS, a cut-off Ct value of ≤32 was employed. These Ct value thresholds were determined based on the Ct values from positive and negative controls.
- To ensure the accuracy and reliability of the assay, two types of controls were used. The positive control for the cytolysin-positive E. faecalis strain was obtained from a human stool that was cultured on agar plates to have single colonies. We consistently employ this control to determine variations in qPCR, confirm the reliability of our primers, and validate the quality of our samples.
- Conversely, a negative control consisting of water was employed to assess and mitigate any potential contamination during the experimental process. This control is vital in confirming the absence of false-positive results.
- By adhering to these LOD and LOQ thresholds and utilizing appropriate controls, the assay’s sensitivity and specificity were rigorously evaluated, ensuring the validity of the results obtained.
4. Expected Results and Discussion
4.1. Reproducible DNA Extraction from Human Fecal Samples
4.2. Reproducible Ct Values in Real-Time Quantitative PCR from Human Fecal Samples
4.3. Detection of E. faecalis Cytolysin-Positive Strains from Colonies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | Alcohol-Associated Liver Disease |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| Ct | Cycle Threshold |
| CylLS | Cytolysin small subunit |
| LOD | Limit of Detection |
| LOQ | Limit of Quantitation |
| qPCR | Quantitative Polymerase Chain Reaction |
| RT-qPCR | Real-Time Quantitative Polymerase Chain Reaction |
| TAE | Tris-Acetate-EDTA |
| Tm | Melting Temperature |
| UV | Ultraviolet |
References
- Asrani, S.K.; Mellinger, J.; Arab, J.P.; Shah, V.H. Reducing the Global Burden of Alcohol-Associated Liver Disease: A Blueprint for Action. Hepatology 2021, 73, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Aberg, F.; Byrne, C.D.; Pirola, C.J.; Mannisto, V.; Sookoian, S. Alcohol consumption and metabolic syndrome: Clinical and epidemiological impact on liver disease. J. Hepatol. 2023, 78, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Rametta, R.; Dongiovanni, P. Genetic and Epigenetic Modifiers of Alcoholic Liver Disease. Int. J. Mol. Sci. 2018, 19, 3857. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Bataller, R. Advancing alcohol-related liver disease: From novel biomarkers to refining selection for liver transplantation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Schnabl, B. The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Fairfield, B.; Schnabl, B. Gut dysbiosis as a driver in alcohol-induced liver injury. JHEP Rep. 2021, 3, 100220. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Cabré, N.; Hartmann, P.; Llorente, C.; Kouno, T.; Wang, Y.; Zeng, S.; Kim, H.Y.; Zhang, X.; Kisseleva, T.; Iyer, S.; et al. IgY antibodies against cytolysin reduce ethanol-induced liver disease in mice. Hepatology 2023, 78, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Demir, M.; Duan, Y.; Martin, A.; Schnabl, B. Cytolysin-positive Enterococcus faecalis is not increased in patients with non-alcoholic steatohepatitis. Liver Int. 2020, 40, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Lang, S.; Schierwagen, R.; Klein, S.; Praktiknjo, M.; Trebicka, J.; Schnabl, B. Fecal cytolysin does not predict disease severity in acutely decompensated cirrhosis and acute-on-chronic liver failure. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 474–481. [Google Scholar] [CrossRef]
- Ryu, H.; Henson, M.; Elk, M.; Toledo-Hernandez, C.; Griffith, J.; Blackwood, D.; Noble, R.; Gourmelon, M.; Glassmeyer, S.; Santo Domingo, J.W. Development of quantitative PCR assays targeting the 16S rRNA genes of Enterococcus spp. and their application to the identification of enterococcus species in environmental samples. Appl. Environ. Microbiol. 2013, 79, 196–204. [Google Scholar] [CrossRef]
- Haas, W.; Shepard, B.D.; Gilmore, M.S. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 2002, 415, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Horz, H.P.; Vianna, M.E.; Gomes, B.P.; Conrads, G. Evaluation of universal probes and primer sets for assessing total bacterial load in clinical samples: General implications and practical use in endodontic antimicrobial therapy. J. Clin. Microbiol. 2005, 43, 5332–5337. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer | Sequence | Product Size (bp) |
|---|---|---|---|
| E. faecalis [13] | F R | 5′-CGCTTCTTTCCTCCCGAGT-3′ 5′-GCCATGCGGCATAAACTG-3′ | 142 |
| E.faecalis Cytolysin small subunit (CylLS) [14] | F R | 5-GTAAAATAAGTAAAATCAAGAAAACTATTACTC-3 5-CAAAAGAAGGACCAACAAGTTCTAATT-3 | 61 |
| 16S rRNA [15] | F R | 5-GTGSTGCAYGGYTGTCGTCA-3 5-ACGTCRTCCMCACCTTCCTC-3 | 200 |
| Investigator 1 | Investigator 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Replicate | Sample | DNA (ng/uL) | 260/280 | 260/230 | Replicate | Sample | DNA (ng/uL) | 260/280 | 260/230 | |
| 1 | 1A | 153 | 2.05 | 1.65 | 1 | 1B | 236.3 | 2.1 | 1.59 | |
| 2A | 92.2 | 2.1 | 1.88 | 2B | 86 | 2.1 | 1.73 | |||
| 3A | 84.5 | 2.12 | 2.3 | 3B | 492.6 | 2.1 | 2.17 | |||
| 4A | 292 | 1.98 | 1.03 | 4B | 284 | 2.08 | 1.92 | |||
| 2 | 1A | 409.4 | 2.09 | 1.46 | 2 | 1B | 152.9 | 1.93 | 0.92 | |
| 2A | 114.8 | 2.1 | 1.9 | 2B | 902.7 | 1.97 | 1.8 | |||
| 3A | 1159.1 | 1.94 | 1.36 | 3B | 1413.3 | 2.16 | 2.29 | |||
| 4A | 480.1 | 2.03 | 1.28 | 4B | 272 | 1.8 | 0.83 | |||
| 3 | 1A | 232.4 | 2.11 | 1.47 | 3 | 1B | 289.3 | 2.01 | 1.24 | |
| 2A | 151.6 | 2.15 | 1.88 | 2B | 142 | 2.15 | 1.73 | |||
| 3A | 2045.3 | 2.14 | 2.26 | 3B | 2200.2 | 2.13 | 2.28 | |||
| 4A | 246.8 | 2.07 | 1.14 | 4B | 135.1 | 2.09 | 1.373 | |||
| Investigator 1 | Investigator 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Replicate | Sample | Cт E. faecalis | Cт CylLS | Cт 16S | Replicate | Sample | Cт E. faecalis | Cт CylLS | Cт 16S |
| 1 | 1 | 36.798 | Undetermined | 12.392 | 1 | 1 | 30.374 | Undetermined | 10.263 |
| 1 | Undetermined | 36.919 | 12.268 | 1 | 30.318 | Undetermined | 10.708 | ||
| 2 | 35.089 | Undetermined | 13.145 | 2 | 31.379 | 34.200 | 11.423 | ||
| 2 | 35.911 | Undetermined | 13.207 | 2 | 29.385 | Undetermined | 11.999 | ||
| 3 | 21.983 | 28.645 | 12.453 | 3 | 17.598 | 26.769 | 7.001 | ||
| 3 | 21.936 | 28.482 | 12.504 | 3 | 17.227 | 26.388 | 7.521 | ||
| 4 | 24.393 | 26.505 | 12.906 | 4 | 23.211 | Undetermined | 11.728 | ||
| 4 | 24.493 | 26.370 | 12.939 | 4 | 22.555 | 26.952 | 11.173 | ||
| Positive control 1 | 11.945 | 15.558 | 12.026 | Positive control 1 | 11.725 | 15.558 | 9.965 | ||
| Positive control 2 | 11.977 | 15.461 | 12.070 | Positive control 2 | 12.804 | 15.461 | 7.696 | ||
| Negative Control | Undetermined | Undetermined | 31.529 | Negative Control | 33.474 | Undetermined | 36.040 | ||
| Negative Control | Undetermined | 36.830 | 30.843 | Negative Control | 37.150 | Undetermined | 31.099 | ||
| 2 | 1 | 37.005 | Undetermined | 12.901 | 2 | 1 | 33.814 | 37.106 | 13.160 |
| 1 | 36.418 | Undetermined | 12.936 | 1 | 33.641 | 33.427 | 13.903 | ||
| 2 | 33.991 | Undetermined | 12.123 | 2 | 34.525 | 34.890 | 13.935 | ||
| 2 | 35.720 | Undetermined | 12.175 | 2 | 35.179 | 36.381 | 12.222 | ||
| 3 | 19.944 | 27.239 | 12.200 | 3 | 22.255 | 26.421 | 12.411 | ||
| 3 | 19.883 | 27.256 | 11.978 | 3 | 22.532 | 26.788 | 11.842 | ||
| 4 | 22.651 | 24.848 | 12.669 | 4 | 28.119 | 26.855 | 13.983 | ||
| 4 | 22.640 | 24.868 | 12.700 | 4 | 27.506 | 27.175 | 13.882 | ||
| Positive control 1 | 11.704 | 15.611 | 12.396 | Positive control 1 | 14.212 | 13.181 | 30.131 | ||
| Positive control 2 | 11.411 | 15.614 | 12.505 | Positive control 2 | 13.993 | 13.557 | 29.480 | ||
| Negative Control | Undetermined | Undetermined | 31.164 | Negative Control | 35.373 | Undetermined | 12.468 | ||
| Negative Control | 36.216 | Undetermined | 31.511 | Negative Control | 35.931 | Undetermined | 12.427 | ||
| 3 | 1 | 37.113 | Undetermined | 13.059 | 3 | 1 | 32.038 | 35.604 | 15.222 |
| 1 | 36.873 | Undetermined | 12.919 | 1 | 34.958 | Undetermined | 15.650 | ||
| 2 | 33.919 | 36.075 | 12.697 | 2 | 33.430 | 34.193 | 15.333 | ||
| 2 | Undetermined | 35.888 | 12.664 | 2 | 33.989 | Undetermined | 14.092 | ||
| 3 | 18.491 | 27.679 | 11.946 | 3 | 27.323 | 30.231 | 15.636 | ||
| 3 | 18.555 | 27.459 | 11.837 | 3 | 24.076 | 28.918 | 14.322 | ||
| 4 | 22.607 | 26.641 | 14.437 | 4 | 27.831 | 25.903 | 16.883 | ||
| 4 | 22.582 | 26.562 | 14.497 | 4 | 30.392 | 28.336 | 17.249 | ||
| Positive control 1 | 9.402 | 15.272 | 13.242 | Positive control 1 | 13.046 | 12.979 | 13.487 | ||
| Positive control 2 | 9.408 | 15.155 | 13.265 | Positive control 2 | 13.175 | 12.901 | 13.332 | ||
| Negative Control | 32.194 | 37.043 | 30.759 | Negative Control | 36.891 | 32.877 | 30.982 | ||
| Negative Control | Undetermined | 36.288 | 30.606 | Negative Control | 34.509 | 36.565 | 30.959 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabré, N.; Yang, Y.; Wang, Y.; Schnabl, B. Development of a Quantitative PCR Method for Detecting Enterococcus faecalis Cytolysin in Human Stool Samples. Methods Protoc. 2023, 6, 107. https://doi.org/10.3390/mps6060107
Cabré N, Yang Y, Wang Y, Schnabl B. Development of a Quantitative PCR Method for Detecting Enterococcus faecalis Cytolysin in Human Stool Samples. Methods and Protocols. 2023; 6(6):107. https://doi.org/10.3390/mps6060107
Chicago/Turabian StyleCabré, Noemí, Yongqiang Yang, Yanhan Wang, and Bernd Schnabl. 2023. "Development of a Quantitative PCR Method for Detecting Enterococcus faecalis Cytolysin in Human Stool Samples" Methods and Protocols 6, no. 6: 107. https://doi.org/10.3390/mps6060107







