Effect of Dietary Fiber Supplementation on Metabolic Endotoxemia: A Protocol for Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
:1. Introduction
1.1. Description of the Intervention
1.2. How the Intervention Might Work
1.3. Why It Is Important to Do This Review
1.4. Objective
2. Methods
2.1. Criteria for Considering Studies for This Review
2.1.1. Type of Studies
2.1.2. Types of Participants
2.1.3. Types of Intervention
2.1.4. Comparators/Control
3. Outcomes
3.1. Types of Outcome Measure
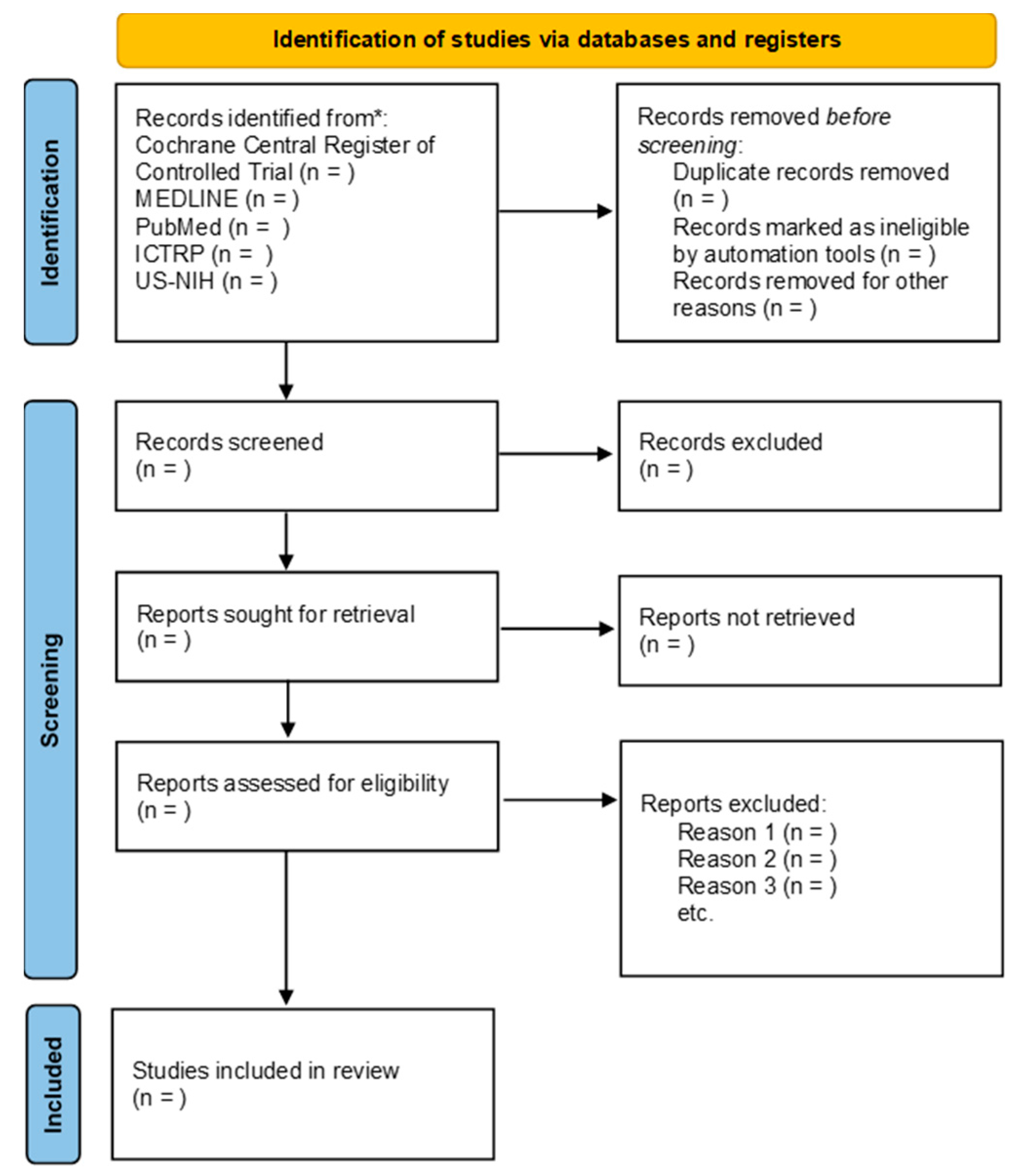
3.2. Search Methods for Identification of Studies
3.2.1. Electronic Search
- Cochrane Central Register of Controlled Trial (CENTRAL, current issues) in the Cochrane Library;
- MEDLINE Ovid SP;
- PubMed;
- World Health Organization International Clinical Trials Registry Platforms;
- US National Institutes of Health Ongoing Trials Register Clinicaltrials.gov.
3.2.2. Searching Other Resources
3.3. Data Collection and Analysis
3.4. Selection of Studies
3.5. Data Extraction and Management
- Participant characteristics (e.g., age, gender, genotype, phenotype, pancreatic status);
- trial characteristics and design (e.g., RCTs or quasi-RCT);
- interventions and comparator (e.g., type of fiber-prebiotic, dose, duration);
- outcome data—reported separately for each outcome.
3.6. Assessment Risk of Bias in the Included Studies
3.7. Measures of Treatment Effect
3.8. Unit of Analysis Issue
3.9. Dealing with Missing Data
3.10. Assessment of Heterogeneity
3.11. Assessment of Reporting Biases
3.12. Data Synthesis
3.13. Subgroup Analysis and Investigation of Heterogeneity
3.14. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef] [PubMed]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef] [PubMed]
- Tir, A.M.D.; Labor, M.; Plavec, D. The Effects of Physical Activity on Chronic Subclinical Systemic Inflammation. Arch. Ind. Hyg. Toxicol. 2017, 68, 276–286. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-Grade Inflammation, Diet Composition and Health: Current Research Evidence and Its Translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Attina, T.; Spickett, C.M.; Webb, D.J. A High-Fat Meal Induces Low-Grade Endotoxemia: Evidence of a Novel Mechanism of Postprandial Inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef]
- Pendyala, S.; Walker, J.M.; Holt, P.R. A High-Fat Diet Is Associated with Endotoxemia That Originates from the Gut. Gastroenterology 2012, 142, 1100–1101. [Google Scholar] [CrossRef]
- Amar, J.; Burcelin, R.; Ruidavets, J.B.; Cani, P.D.; Fauvel, J.; Alessi, M.C.; Chamontin, B.; Ferriéres, J. Energy Intake Is Associated with Endotoxemia in Apparently Healthy Men. Am. J. Clin. Nutr. 2008, 87, 1219–1223. [Google Scholar] [CrossRef]
- Brown, B.I. Nutritional Management of Metabolic Endotoxemia: A Clinical Review. Altern. Ther. Health Med. 2017, 23, 42–54. [Google Scholar]
- Xiao, S.; Fei, N.; Pang, X.; Shen, J.; Wang, L.; Zhang, B.; Zhang, M.; Zhang, X.; Zhang, C.; Li, M.; et al. A Gut Microbiota-Targeted Dietary Intervention for Amelioration of Chronic Inflammation Underlying Metabolic Syndrome. FEMS Microbiol. Ecol. 2014, 87, 357–367. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Konjac Mannan (Glucomannan) and Reduction of Body Weight (ID 854, 1556, 3725), Reduction of Post-Prandial Glycaemic Responses (ID 1559), Maintenance of Normal Blood Glucose Concentration. EFSA J. 2010, 8, 1798. [Google Scholar] [CrossRef]
- Collins, S.M.; Gibson, G.R.; Kennedy, O.B.; Walton, G.; Rowland, I.; Commane, D.M. Development of a Prebiotic Blend to Influence in Vitro Fermentation Effects, with a Focus on Propionate, in the Gut. FEMS Microbiol. Ecol. 2021, 97, fiab101. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Rosendale, D.; Blatchford, P.; Stoklosinski, H.; Coad, J. Variability in Gut Microbiota Response to an Inulin-Type Fructan Prebiotic within an in Vitro Three-Stage Continuous Colonic Model System. Bioact. Carbohydr. Diet. Fibre 2017, 11, 26–37. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Meyer, R.W.; Greenhawt, M.; Pali-Schöll, I.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; et al. Role of Dietary Fiber in Promoting Immune health—An EAACI Position Paper. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 3185–3198. [Google Scholar] [CrossRef]
- Bailey, M.A.; Thompson, S.V.; Mysonhimer, A.R.; Bennett, J.N.; Vanhie, J.J.; De Lisio, M.; Burd, N.A.; Khan, N.A.; Holscher, H.D. Dietary Fiber Intake and Fecal Short-Chain Fatty Acid Concentrations Are Associated with Lower Plasma Lipopolysaccharide-Binding Protein and Inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G369–G377. [Google Scholar] [CrossRef]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in Plasma Endotoxin Concentrations and the Expression of Toll-like Receptors and Suppressor of Cytokine Signaling-3 in Mononuclear Cells after a High-Fat, High-Carbohydrate Meal: Implications for Insulin Resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef]
- Parnell, J.A.; Klancic, T.; Reimer, R.A. Oligofructose Decreases Serum Lipopolysaccharide and Plasminogen Activator Inhibitor-1 in Adults with Overweight/obesity. Obesity 2017, 25, 510–513. [Google Scholar] [CrossRef]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-abadi, M. Oligofructose-Enriched Inulin Improves Some Inflammatory Markers and Metabolic Endotoxemia in Women with Type 2 Diabetes Mellitus: A Randomized Controlled Clinical Trial. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the Prebiotic Concept: Lessons from an Exploratory, Double Blind Intervention Study with Inulin-Type Fructans in Obese Women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef]
- Morel, F.B.; Dai, Q.; Ni, J.; Thomas, D.; Parnet, P.; Fança-Berthon, P. α-Galacto-Oligosaccharides Dose-Dependently Reduce Appetite and Decrease Inflammation in Overweight Adults. J. Nutr. 2015, 145, 2052–2059. [Google Scholar] [CrossRef]
- Guerville, M.; Boudry, G. Gastrointestinal and Hepatic Mechanisms Limiting Entry and Dissemination of Lipopolysaccharide into the Systemic Circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G1–G15. [Google Scholar] [CrossRef]
- Akiba, Y.; Maruta, K.; Takajo, T.; Narimatsu, K.; Said, H.; Kato, I.; Kuwahara, A.; Kaunitz, J.D. Lipopolysaccharides Transport during Fat Absorption in Rodent Small Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G1070–G1087. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; McClements, D.J. Impact of Dietary Fibers [Methyl Cellulose, Chitosan, and Pectin] on Digestion of Lipids under Simulated Gastrointestinal Conditions. Food Funct. 2014, 5, 3083–3095. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-Dependent and -Independent Effects of Dietary Fibre on Human Health. Br. J. Pharmacol. 2020, 177, 1363–1381. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Rösch, C.; Schols, H.A.; Faas, M.M.; de Vos, P. Resistant Starches Differentially Stimulate Toll-like Receptors and Attenuate Proinflammatory Cytokines in Dendritic Cells by Modulation of Intestinal Epithelial Cells. Mol. Nutr. Food Res. 2015, 59, 1814–1826. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan Oligosaccharide: Biological Activities and Potential Therapeutic Applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Flores-Lopez, A.G.; Aguilar-Lopez, M.; Sanchez-Tapia, M.; Medina-Vera, I.; Dıaz, D.; Tovar, A.R.; Torres, N. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects with Metabolic Syndrome. J. Am. Heart Assoc. 2019, 8, e012401. [Google Scholar] [CrossRef]
- Fernandes, R.; do Rosario, V.A.; Mocellin, M.C.; Kuntz, M.G.F.; Trindade, E.B.S.M. Effects of Inulin-Type Fructans, Galacto-Oligosaccharides and Related Synbiotics on Inflammatory Markers in Adult Patients with Overweight or Obesity: A Systematic Review. Clin. Nutr. 2017, 36, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Maleki, V.; Jafari-Vayghyan, H.; Vaghef-Mehrabany, E.; Alizadeh, M. Metabolic Endotoxemia and Cardiovascular Disease: A Systematic Review about Potential Roles of Prebiotics and Probiotics. Clin. Exp. Pharmacol. Physiol. 2020, 47, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 [Updated February 2021]; 3O Cochrane Training: London, UK, 2021. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 88, 105906. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranneh, Y.; Fadel, A.; Md Akim, A.; Idris, I.; Ilesanmi-Oyelere, B.L.; Ismail, L.C. Effect of Dietary Fiber Supplementation on Metabolic Endotoxemia: A Protocol for Systematic Review and Meta-Analysis of Randomized Clinical Trials. Methods Protoc. 2023, 6, 84. https://doi.org/10.3390/mps6050084
Ranneh Y, Fadel A, Md Akim A, Idris I, Ilesanmi-Oyelere BL, Ismail LC. Effect of Dietary Fiber Supplementation on Metabolic Endotoxemia: A Protocol for Systematic Review and Meta-Analysis of Randomized Clinical Trials. Methods and Protocols. 2023; 6(5):84. https://doi.org/10.3390/mps6050084
Chicago/Turabian StyleRanneh, Yazan, Abdulmannan Fadel, Abdah Md Akim, Iskandar Idris, Bolaji Lilian Ilesanmi-Oyelere, and Leila Cheikh Ismail. 2023. "Effect of Dietary Fiber Supplementation on Metabolic Endotoxemia: A Protocol for Systematic Review and Meta-Analysis of Randomized Clinical Trials" Methods and Protocols 6, no. 5: 84. https://doi.org/10.3390/mps6050084
APA StyleRanneh, Y., Fadel, A., Md Akim, A., Idris, I., Ilesanmi-Oyelere, B. L., & Ismail, L. C. (2023). Effect of Dietary Fiber Supplementation on Metabolic Endotoxemia: A Protocol for Systematic Review and Meta-Analysis of Randomized Clinical Trials. Methods and Protocols, 6(5), 84. https://doi.org/10.3390/mps6050084







