Health Benefits of Montmorency Tart Cherry Juice Supplementation in Adults with Mild to Moderate Ulcerative Colitis: A Protocol for a Placebo Randomized Controlled Trial
Abstract
:1. Introduction
1.1. Rationale
1.2. Aims and Objectives
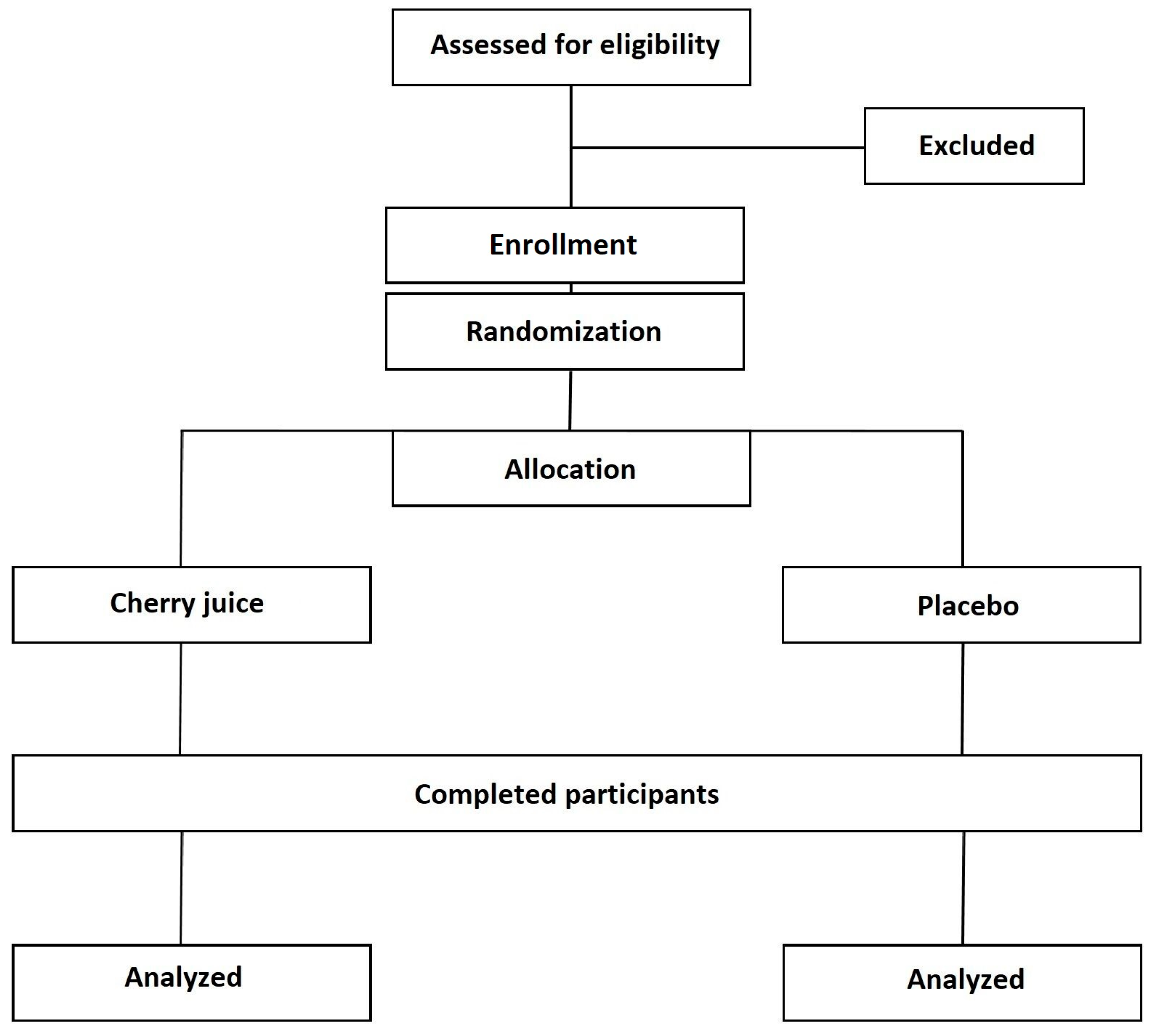
2. Experimental Design
2.1. Study Design and Setting
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Sample Size
2.5. Participants and Recruitment
3. Procedures
3.1. Intervention and Control Groups
3.1.1. Montmorency Tart Cherry
3.1.2. Placebo
3.2. Data Collection
3.2.1. Questionnaires
3.2.2. Biological Samples
3.2.3. Blood Samples
3.3. Data Management
3.4. Statistical Analysis
3.5. Ethical Approval and Registration
3.6. Dissemination
3.7. Safety Reporting
4. Expected Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis.-A-Mon. 2019, 65, 100851. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflam-matory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Freeman, K.; Ryan, R.; Parsons, N.; Taylor-Phillips, S.; Willis, B.H.; Clarke, A. The incidence and prevalence of inflammatory bowel disease in UK primary care: A retrospective cohort study of the IQVIA Medical Research Database. BMC Gastroenterol. 2021, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. Inflammatory Bowel Disease: An Expanding Global Health Problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Longobardi, T.; Finlayson, G.; Blanchard, J.F. Direct medical cost of managing IBD patients: A Canadian popula-tion-based study. Inflamm. Bowel Dis. 2012, 18, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.W.; Matheeuwsen, M.; Lönnkvist, M.H.; Parker, C.E.; Wildenberg, M.E.; Gecse, K.B.; D’Haens, G.R. Faculty Opinions recommendation of Systematic review: Predictive biomarkers of therapeutic response in inflammatory bowel disease-personalised medicine in its infancy. Aliment. Pharmacol. Ther. 2020, 48, 1213–1231. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.J.; Rhee, K.H.; Kim, Y.H.; Hong, S.N.; Kim, K.H.; Seo, S.I.; Cha, J.M.; Park, S.Y.; Jeong, S.K.; et al. A 30-year trend analysis in the epidemiology of inflamma-tory bowel disease in the Songpa-Kangdong district of Seoul, Korea in 1986–2015. J. Crohn’s Colitis 2019, 13, 1410–1417. [Google Scholar] [CrossRef]
- Luces, C.; Bodger, K. Economic burden of inflammatory bowel disease: A UK perspective. Expert Rev. Pharm. Outcomes Res. 2006, 6, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Stawowczyk, E.; Kawalec, P. A Systematic Review of the Cost-Effectiveness of Biologics for Ulcerative Colitis. PharmacoEconomics 2017, 36, 419–434. [Google Scholar] [CrossRef]
- Bernklev, T.; Jahnsen, J.; Lygren, I.; Henriksen, M.; Vatn, M.; Moum, B. Health-related Quality of Life in Patients with Inflammatory Bowel Disease Measured with the Short Form-36: Psychometric Assessments and a Comparison with General Population Norms. Inflamm. Bowel Dis. 2005, 11, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Graff, L.A.; Walker, J.R.; Lix, L.; Clara, I.; Rawsthorne, P.; Rogala, L.; Miller, N.; Jakul, L.; McPhail, C.; Ediger, J.; et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin. Gastroenterol. Hepatol. 2006, 4, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Neurath, M.F.; Wirtz, S. The Intestinal Microbiota in Inflammatory Bowel Disease. ILAR J. 2015, 56, 192–204. [Google Scholar] [CrossRef]
- Longobardi, T.; Jacobs, P.; Bernstein, C.N. Work losses related to inflammatory bowel disease in the United States: Results from the National Health Interview Survey. Am. J. Gastroenterol. 2003, 98, 1064–1072. [Google Scholar] [PubMed]
- Biedermann, L.; Mwinyi, J.; Scharl, M.; Frei, P.; Zeitz, J.; Kullak-Ublick, G.A.; Vavricka, S.R.; Fried, M.; Weber, A.; Humpf, H.-U.; et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—An open pilot study. J. Crohns. Colitis. 2013, 7, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Cruz-Correa, M.R.; Gasche, C.; Jass, J.R.; Lichtenstein, G.R.; Montgomery, E.A.; Riddell, R.H.; Rutter, M.D.; Ullman, T.A.; Velayos, F.S.; et al. Colorectal cancer prevention in in-flammatory bowel disease and the role of 5-aminosalicylic acid: A clinical review and update. Inflamm. Bowel Dis. 2008, 14, 265–274. [Google Scholar] [CrossRef]
- Kruis, W.; Schreiber, S.; Theuer, D.; Brandes, J.-W.; Schütz, E.; Howaldt, S.; Krakamp, B.; Hämling, J.; Mönnikes, H.; Koop, I.; et al. Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses. Gut 2001, 49, 783–789. [Google Scholar] [CrossRef]
- Chumanevich, A.A.; Chaparala, A.; Witalison, E.E.; Tashkandi, H.; Hofseth, A.B.; Lane, C.; Pena, E.; Liu, P.; Pittman, D.L.; Nagarkatti, P.; et al. Looking for the best anti-colitis medi-cine: A comparative analysis of current and prospective compounds. Oncotarget 2017, 8, 228–237. [Google Scholar] [CrossRef]
- Siegel, C.A.; Marden, S.M.; Persing, S.M.; Larson, R.J.; Sands, B.E. Risk of Lymphoma Associated With Combination Anti–Tumor Necrosis Factor and Immunomodulator Therapy for the Treatment of Crohn’s Disease: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2009, 7, 874–881. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Grisham, M.B.; Granger, D.N. Neutrophil-mediated mucosal injury: Role of reactive oxygen metabolites. Am. J. Dig. Dis. 1988, 33, 6S–15S. [Google Scholar] [CrossRef]
- Babbs, C.F. Oxygen radicals in ulcerative colitis. Free. Radic. Biol. Med. 1992, 13, 169–181. [Google Scholar] [CrossRef]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A Pilot Study to Evaluate the Safety and Efficacy of an Oral Dose of (−)-Epigallocatechin-3-Gallate–Rich Polyphenon E in Patients With Mild to Moderate Ulcerative Colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Montrose, D.C.; Horelik, N.A.; Madigan, J.P.; Stoner, G.D.; Wang, L.-S.; Bruno, R.S.; Park, H.J.; Giardina, C.; Rosenberg, D.W. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis 2010, 32, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Spalinger, M.R.; Gottier, C.; Biedermann, L.; Zeitz, J.; Lang, S.; Weber, A.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins modulate cytokine ex-pression in the intestine of patients with ulcerative colitis. PLoS ONE 2016, 11, e0154817. [Google Scholar] [CrossRef]
- Piberger, H.; Oehme, A.; Hofmann, C.; Dreiseitel, A.; Sand, P.G.; Obermeier, F.; Schoelmerich, J.; Schreier, P.; Krammer, G.; Rogler, G. Bilberries and their anthocyanins ameliorate experimental colitis. Mol. Nutr. Food Res. 2011, 55, 1724–1729. [Google Scholar] [CrossRef]
- Nemzer, B.; Vargas, L.; Xia, X.; Sintara, M.; Feng, H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Carbonero, F. Impact of tart cherries polyphenols on the hu-man gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Delday, M.; Mulder, I.; Logan, E.T.; Grant, G. Bacteroides thetaiotaomicron Ameliorates Colon Inflammation in Preclinical Models of Crohn’s Disease. Inflamm. Bowel Dis. 2018, 25, 85–96. [Google Scholar] [CrossRef]
- Sinclair, J.; Dillon, S.; Bottoms, L. Perceptions, beliefs and behaviors of nutritional and supplementary practices in inflammatory bowel disease. Sport Sci. Health 2022, 18, 1301–1310. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus Jr, E.V.; Danese, S.; Colombel, J.F.; Törüner, M.; Schreiber, S. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef]
- Bjarnason, I.; Sission, G.; Hayee, B.H. A randomised, double-blind, placebo-controlled trial of a multi-strain probi-otic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology 2019, 27, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; IBD Guidelines eDelphi Consensus Group. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.; Stainton, P.; Dillon, S.; Taylor, P.J.; Richardson, C.; Bottoms, L.; Hobbs, S.J.; Shadwell, G.; Liles, N.; Allan, R. The efficacy of a tart cherry drink for the treatment of patellofemoral pain in recreationally active individuals: A placebo randomized control trial. Sport Sci. Health 2022, 18, 1491–1504. [Google Scholar] [CrossRef]
- Sinclair, J.; Bottoms, L.; Dillon, S.; Allan, R.; Shadwell, G.; Butters, B. Effects of Montmorency tart cherry and blueberry juice on car-diometabolic and other health-related outcomes: A three-arm placebo randomized controlled trial. Int. J. Environ. Res. Public Health 2022, 19, 5317. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2011, 51, 909–916. [Google Scholar] [CrossRef]
- Chai, S.C.; Jerusik, J.; Davis, K.; Wright, R.S.; Zhang, Z. Effect of Montmorency tart cherry juice on cognitive per-formance in older adults: A randomized controlled trial. Food Funct. 2019, 10, 4423–4431. [Google Scholar] [CrossRef]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016, 55, 1695–1705. [Google Scholar]
- Guyatt, G.; Mitchell, A.; Irvine, E.J.; Singer, J.; Williams, N.; Goodacre, R.; Tompkins, C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989, 96, 804–810. [Google Scholar] [CrossRef]
- Moradkhani, A.; Beckman, L.J.; Tabibian, J.H. Health-related quality of life in inflammatory bowel disease: Psychosocial, clinical, socioeconomic, and demographic predictors. J. Crohn’s Colitis 2013, 7, 467–473. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.S.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Varbobitis, I.; Kokkotis, G.; Gizis, M.; Perlepe, N.; Laoudi, E.; Bletsa, M.; Bekiari, D.; Koutsounas, I.; Kounadis, G.; Xourafas, V.; et al. The IBD-F Patient Self-Assessment Scale Accurately Depicts the Level of Fatigue and Predicts a Negative Effect on the Quality of Life of Patients With IBD in Clinical Remission. Inflamm. Bowel Dis. 2020, 27, 826–835. [Google Scholar] [CrossRef]
- Flora, S.; Marques, A.; Hipólito, N.; Morais, N.; Silva, C.G.; Januário, F.; Cruz, J. Test-retest reliability, agreement and con-struct validity of the International Physical Activity Questionnaire short-form (IPAQ-sf) in people with COPD. Respir. Med. 2023, 206, 107087. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J. Psychosom. Res. 2022, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. The influence of tart cherry (Prunus cerasus, cv Montmoren-cy) concentrate supplementation for 3 months on cardiometabolic risk factors in middle-aged adults: A randomised, place-bo-controlled trial. Nutrients 2021, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Hillman, A.R.; Chrismas, B.C.R. Thirty Days of Montmorency Tart Cherry Supplementation Has No Effect on Gut Microbiome Composition, Inflammation, or Glycemic Control in Healthy Adults. Front. Nutr. 2021, 8, 733057. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Roberts, M.; Bottoms, L. Effects of Montmorency tart cherry supplementation on cardio-metabolic mark-ers in metabolic syndrome participants: A pilot study. J. Funct. Foods 2019, 57, 286–298. [Google Scholar] [CrossRef]
- Costa, F.; Mumolo, M.G.; Bellini, M.A.; Romano, M.R.; Ceccarelli, L.; Arpe, P.; Sterpi, C.; Marchi, S.; Maltinti, G. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig. Liver Dis. 2003, 35, 642–647. [Google Scholar] [CrossRef]
- McGough, J.J.; Faraone, S.V. Estimating the size of treatment effects: Moving beyond p values. Psychiatry 2009, 6, 21–29. [Google Scholar] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1989. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinclair, J.; Dillon, S.; Allan, R.; Brooks-Warburton, J.; Desai, T.; Lawson, C.; Bottoms, L. Health Benefits of Montmorency Tart Cherry Juice Supplementation in Adults with Mild to Moderate Ulcerative Colitis: A Protocol for a Placebo Randomized Controlled Trial. Methods Protoc. 2023, 6, 76. https://doi.org/10.3390/mps6050076
Sinclair J, Dillon S, Allan R, Brooks-Warburton J, Desai T, Lawson C, Bottoms L. Health Benefits of Montmorency Tart Cherry Juice Supplementation in Adults with Mild to Moderate Ulcerative Colitis: A Protocol for a Placebo Randomized Controlled Trial. Methods and Protocols. 2023; 6(5):76. https://doi.org/10.3390/mps6050076
Chicago/Turabian StyleSinclair, Jonathan, Stephanie Dillon, Robert Allan, Johanne Brooks-Warburton, Terun Desai, Charlotte Lawson, and Lindsay Bottoms. 2023. "Health Benefits of Montmorency Tart Cherry Juice Supplementation in Adults with Mild to Moderate Ulcerative Colitis: A Protocol for a Placebo Randomized Controlled Trial" Methods and Protocols 6, no. 5: 76. https://doi.org/10.3390/mps6050076
APA StyleSinclair, J., Dillon, S., Allan, R., Brooks-Warburton, J., Desai, T., Lawson, C., & Bottoms, L. (2023). Health Benefits of Montmorency Tart Cherry Juice Supplementation in Adults with Mild to Moderate Ulcerative Colitis: A Protocol for a Placebo Randomized Controlled Trial. Methods and Protocols, 6(5), 76. https://doi.org/10.3390/mps6050076






