Proposed Protocol for Field Testing of Endurance Fitness of Young Labrador Retrievers
Abstract
1. Introduction
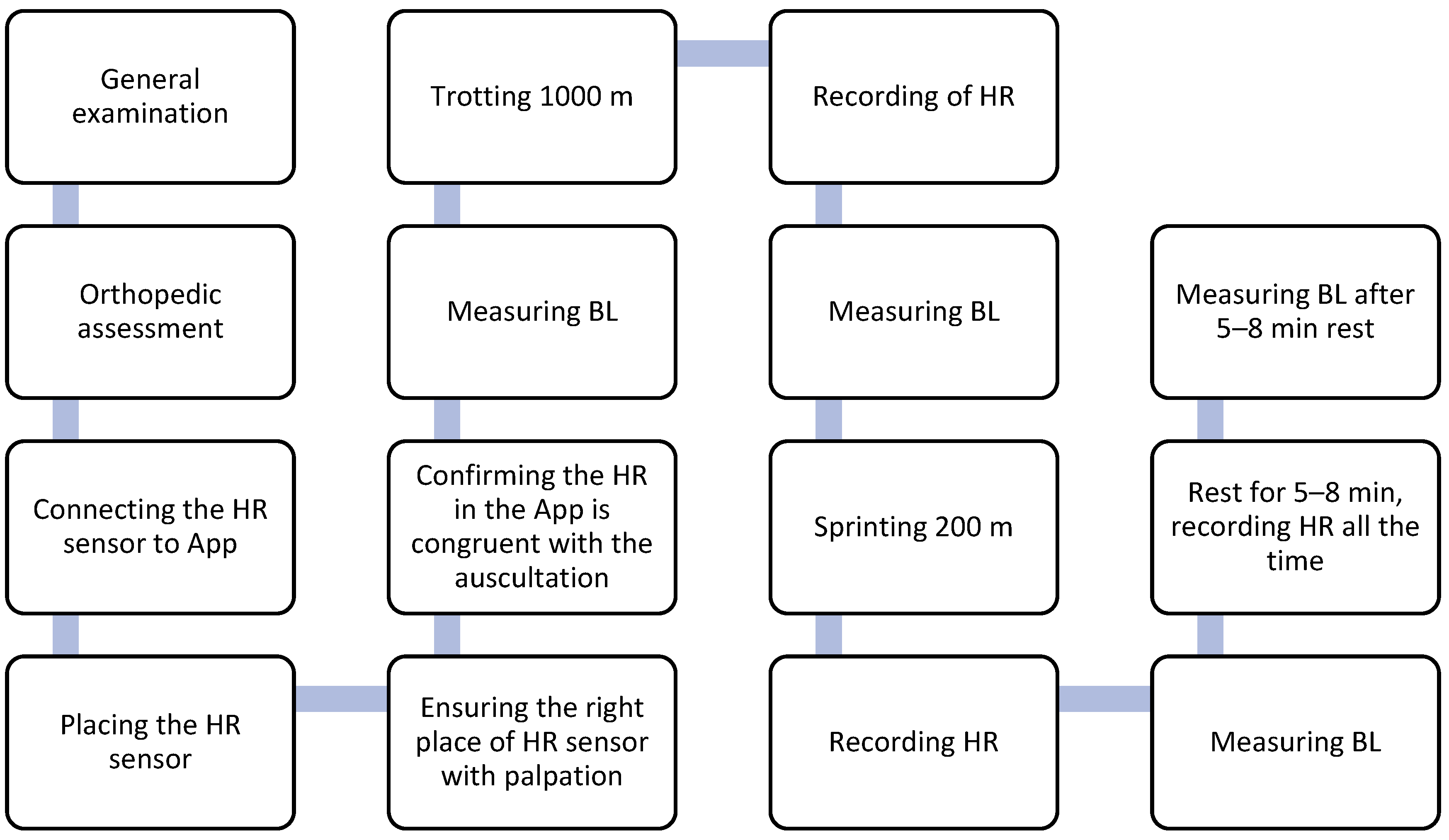
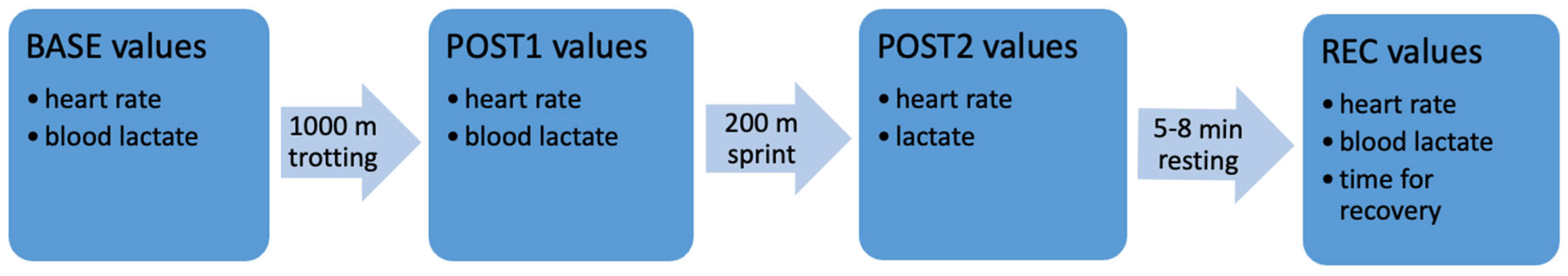
2. Procedure
2.1. Study Protocol
2.2. Description of Measurement Technique
3. Expected Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, W.R.; Gordon, N.F.; Pescatello, L.S. American College of Sports Medicine. In ACSM’s Guidelines for Exercise Testing and Prescription, 8th ed.; Lippincot Williams & Wilkins: Philadelphia, PA, USA, 2010; ISBN 978-0-78817-6903-7. [Google Scholar]
- Jones, A.M.; Carter, H. The effect of endurance training on parameters of aerobic fitness. Sports Med. 2000, 29, 373–386. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christensen, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of assessing cardiorespiratory fitness in clinical vital signs: A scientific statement from the American Heart Association. Circulation 2016, 134, 653–699. [Google Scholar] [CrossRef]
- Hargrieves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–823. [Google Scholar] [CrossRef]
- Hogan, M.C.; Gladden, L.B.; Kurdak, S.S.; Poole, D.C. Increased [lactate] in working dog muscle reduces tension development independent of pH. Med. Sci. Sports Exerc. 1995, 27, 371–377. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Verheyden, B.; Aubert, A.E.; Fagard, R.H. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J. Hum. Hypertens. 2010, 24, 175–182. [Google Scholar] [CrossRef]
- Kuwahara, M.; Hiraga, A.; Kai, M.; Tsubone, H.; Sugano, S. Influence of training on autonomic nervous function in horses: Evaluation by power spectral analysis of heart rate variability. Equine Vet. J. Suppl. 1999, 30, 178–180. [Google Scholar] [CrossRef]
- Betros, C.L.; McKeever, N.M.; Manso Filho, H.C.; Malinowski, K.; McKeever, K.H. Effect of training on intrinsic and resting heart rate and plasma volume inn young and old horses. Comp. Exerc. Physiol. 2013, 9, 43–50. [Google Scholar] [CrossRef]
- Wagner, J.A.; Horvath, S.M.; Dahms, T.E. Cardiovascular, respiratory, and metabolic adjustments to exercise in dogs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 403–407. [Google Scholar] [CrossRef]
- Morgan, K.; Hughes, A.O.; Philipp, R. Reliability of a test of cardiovascular fitness. Int. J. Epidemiol. 1984, 13, 32–37. [Google Scholar] [CrossRef]
- Treiber, F.A.; Musante, L.; Hartdagan, S.; Davis, H.; Levy, M.; Strong, W.B. Validation of a heart rate monitor with children in laboratory and field settings. Med. Sci. Sports Exerc. 1989, 21, 338–342. [Google Scholar] [CrossRef]
- Boddy, K.N.; Roche, B.M.; Schwartz, D.S.; Nakayama, T.; Hamlin, R.I. Evaluation of the six-minute walk test in dogs. Am. J. Vet. Res. 2004, 65, 311–333. [Google Scholar] [CrossRef]
- Ferasin, L.; Marcora, S. A pilot study to assess the feasibility of a submaximal exercise test to measure individual response to cardiac medication in dogs with acquired heart failure. Vet. Res. Commun. 2007, 31, 725–737. [Google Scholar] [CrossRef]
- Swimmer, R.A.; Rozanski, E.A. Evaluation of the 6 minute walk test (6MWT) in pet dogs. J. Vet. Intern. Med. 2011, 25, 405–406. [Google Scholar] [CrossRef]
- Coelho, C.S.; Adam, G.L.; Agra de Omena E Silva, G.; Silva de Carvalho, R.; Cuña de Souza, V.R.; Fazio, F. Heart rate monitoring in Mangalarga Marchador horses during a field marcha Test. J. Equine Vet. Sci. 2019, 79, 50–53. [Google Scholar] [CrossRef]
- Salier Eriksson, J.; Olsson, K.S.E.; Rosdahl, H.; Schantz, P. Heart rate methods can be valid for estimating intensity spectrums of oxygen uptake in field exercise. Front. Physiol. 2021, 112, 345–355. [Google Scholar] [CrossRef]
- Shull, S.A.; Rich, S.K.; Gillette, R.L.; Manfredi, J.M. Heart Rate Changes Before, During, and After Treadmill Walking Exercise in Normal Dogs. Front. Vet. Sci. 2021, 8, 641871. [Google Scholar] [CrossRef]
- Muñoz, A.; Riber, C.; Santisteban, R.; Rubio, M.D.; Agüera, E.I.; Castejón, F.M. Cardiovascular and metabolic adaptations in horses competing in cross-country events. J. Vet. Med. Sci. 1999, 61, 13–20. [Google Scholar] [CrossRef]
- Essner, A.; Sjöström, R.; Ahlgren, E.; Lindmark, B. Validity and reliability of Polar® RS800CX heart rate monitor, measuring heart rate in dogs during standing position and at trot on a treadmill. Physiol. Behav. 2013, 114, 1–5. [Google Scholar] [CrossRef]
- Essner, A.; Sjöström, R.; Ahlgren, E.; Gustås, P.; Edge-Hughes, L.; Zetterberg, L.; Hellström, K. Comparison of Polar ® RS800CX heart rate monitor and electrocardiogram for measuring inter-beat intervals in healthy dogs. Physiol. Behav. 2015, 138, 247–253. [Google Scholar] [CrossRef]
- Bunc, V.; Heller, J.; Leso, J. Kinetics of heart rate responses to exercise. J. Sports Sci. 1988, 6, 39–48. [Google Scholar] [CrossRef]
- Short, K.R.; Sedlock, D.A. Excess postexercise oxygen consumption and recovery rate in trained and untrained subjects. J. Appl. Physiol. 1985, 83, 153–159. [Google Scholar] [CrossRef]
- Sugawara, J.; Murakami, H.; Maeda, S.; Kuno, S.; Matsuda, M. Change in post-exercise vagal reactivation with exercise training and detraining in young men. Eur. J. Appl. Physiol. 2001, 85, 259–263. [Google Scholar] [CrossRef]
- Watson, A.M.; Brickson, S.L.; Prawda, E.R.; Sanfilippo, J.L. Short-term heart rate recovery is related to aerobic fitness in elite intermittent sport athletes. J. Strength Cond. Res. 2017, 31, 1055–1061. [Google Scholar] [CrossRef]
- Wilson, G.; McGowan, C.M. Recovery heart rates as a predictor of race position in race-fit national hunt racehorses. Comp. Exerc. Physiol. 2019, 12, 307–312. [Google Scholar] [CrossRef]
- Lindner, A.; Esser, M.; López, R.; Boffi, F. Relationship between resting and recovery heart rate in horses. Animals 2020, 10, 120. [Google Scholar] [CrossRef]
- Londeree, B.R. Effect of training on lactate/ventilatory thresholds: A meta-analysis. Med. Sci. Sports Exerc. 1997, 29, 837–843. [Google Scholar] [CrossRef]
- Heuberger, J.A.A.C.; Gal, P.; Stuurman, F.E.; de Muinck Keizer, W.A.S.; Mejia Miranda, Y.; Cohen, A.F. Repeatability and predictive value of lactate threshold concepts in endurance sports. PLoS ONE 2018, 13, e0206846. [Google Scholar] [CrossRef]
- Bentley, D.J.; McNaughton, L.R.; Thompson, D.; Vleck, V.E.; Batterham, A.M. Peak power output, the lactate threshold, and time trial performance in cyclists. Med. Sci. Sports Exerc. 2001, 33, 2077–2081. [Google Scholar] [CrossRef]
- Tanner, R.K.; Fuller, K.L.; Ross, M.L. Evaluation of three portable blood lactate analysers: Lactate Pro, Lactate Scout and Lactate Plus. Eur. J. Appl. Physiol. 2010, 109, 551–559. [Google Scholar] [CrossRef]
- Evans, D.L.; Rainger, J.E.; Hodgson, D.R.; Eaton, M.D.; Rose, R.J. The effects of intensity and duration of training on blood lactate concentrations during and after exercise. Eq. Vet. J. 1995, 18, 422–425. [Google Scholar] [CrossRef]
- Baltzer, W.I.; Firshman, A.M.; Stang, B.; Warnock, J.J.; Gorman, E.; McKenzie, E.C. The effect of agility exercise on eicosanoid excretion, oxidant status, and plasma lactate in dogs. BMC Vet. Res. 2012, 8, 249. [Google Scholar] [CrossRef]
- Belić, M.; Radin, L.; Bottegaro, N.B.; Ljubić, B.B.; Benić, M.; Stanin, D.; Vrbanac, Z. Reliability of lactate scout portable analyzer in agility dogs during multiple measurements. Acta Vet. 2016, 66, 549–555. [Google Scholar] [CrossRef]
- Restan, A.Z.; Zacche, E.; da Silva, S.B.; Cerqueira, J.A.; Carfiofi, A.C.; Queiroz-Neto, A.; Camacho, A.A.; Ferraz, G.C. Lactate and glucose thresholds and heart rate deflection points for Beagles during intense exercise. Am. J. Vet. Res. 2019, 80, 284–293. [Google Scholar] [CrossRef]
- Arokoski, J.; Miettinen, P.V.A.; Säämänen, A.M.; Haapanen, K.; Parviainen, M.; Tammi, M.; Helminen, H.J. Effects of aerobic long distance running training (up to 40 km·day−1) of 1-year duration on blood and endocrine parameters of female beagle dogs. Eur. J. Appl. Physiol. 1993, 67, 321–329. [Google Scholar] [CrossRef]
- Ferasin, L.; Dodkin, S.J.; Amodio, A.; Murray, J.K.; Papasouliotis, K. Evaluation of a portable lactate analyzer (Lactate Scout) in dogs. Vet. Clin. Path. 2007, 36, 36–39. [Google Scholar] [CrossRef]
- Weller, I.M.; Thomas, S.G.; Cox, M.H.; Corey, P.N. A study to validate the Canadian aerobic fitness test. Can. J. Public Health 1992, 83, 120–124. [Google Scholar] [CrossRef]
- Castro-Piñero, J.; Artero, E.G.; España-Romero, V.; Ortega, F.B.; Sjöström, M.; Suni, J.; Ruiz, J.R. Criterion-related validity of field-based fitness tests in youth: A systematic review. Br. J. Sports Med. 2010, 44, 934–943. [Google Scholar] [CrossRef]
- Ortega, F.B.; Cadenas-Sánches, C.; Sánches-Delgado, G.; Mora-Gonzáles, J.; Martínez-Téllez, B.; Atero, E.G.; Castro-Piñero, J.; Labayen, I.; Chillón, P.; Löf, M.; et al. Systematic review and proposal of a field-based physical fitness—Test battery in preschool children: The PREFIT battery. Sports Med. 2014, 4, 533–555. [Google Scholar] [CrossRef]
- Mänttäri, A.; Suni, J.; Sievänen, H.; Husu, P.; Vähä-Ypyä, H.; Valkeinen, H.; Tokola, K.; Vasankari, T. Six-minute walk test: A tool for predicting maximal aerobic power (VO2max) in healthy adults. Clin. Physiol. Funct. Imaging 2018, 38, 1038–1045. [Google Scholar] [CrossRef]
- Tomkinson, G.R.; Lang, J.J.; Blanchard, J.; Léger, L.A.; Tremblay, M.S. The 20-m shuttle run: Assessment and interpretation of data in relation to youth aerobic fitness and health. Pediatr. Exerc. Sci. 2019, 31, 152–163. [Google Scholar] [CrossRef]
- Castro-Piñero, J.; Marin-Jimenez, N.; Fernandez-Santos, J.R.; Martin-Acosta, F.; Segura-Jimenez, V.; Izquierdo-Gomez, R.; Ruiz, J.R.; Cuenca-Garcia, M. Criterion-related validity of field-based fitness tests in adults: A systematic review. J. Clin. Med. 2021, 10, 3743. [Google Scholar] [CrossRef]
- Nevill, A.M.; Ramsbottom, R.; Sandercock, G.; Bocachica-González, C.E.; Ramírez-Vélez, R.; Tomkinson, G. Developing a new curvilinear allometric model to improve the fit and validity of the 20-m shuttle run tests as a predictor of cardiorespiratory fitness in adults and youth. Sports Med. 2020, 51, 1581–1589. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Ramirez-Lechuga, J.; Ortega, F.B.; Castro-Piñero, J.; Benitez, J.M.; Arauzo-Azofra, A.; Sanches, C.; Sjöström, M.; Castillo, M.J.; Gutierrez, A.; et al. HELENA Study Group. Artificial neural network-based equation for estimating VO2max from the 20 m shuttle run test in adolescents. Artif. Intell. Med. 2008, 44, 233–245. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Silva, G.; Oliveira, N.; Ribeiro, J.C.; Oliveira, J.F.; Mota, J. Criterion-related validity of the 20-m shuttle run test in youths aged 13-19 years. J. Sports Sci. 2009, 27, 899–906. [Google Scholar] [CrossRef]
- Artero, E.G.; España-Romero, V.; Castro-Piñero, J.; Ortega, F.B.; Suni, J.; Castillo-Garzon, M.J.; Ruiz, J.R. Reliability of field-based fitness tests in youth. Int. J. Sports Med. 2011, 32, 159–169. [Google Scholar] [CrossRef]
- Committee on Fitness Measures and Health Outcomes in Youth. Food and Nutrition Board: Institute of Medicine. Health-Related Fitness Measures for Youth: Cardiorespiratory Endurance. In Fitness Measures and Health Outcomes in Youth; Pate, R., Oria, M., Pillsbury, L., Eds.; National Academies Press: Washington, DC, USA, 2012; pp. 111–152. ISBN 13:978-0-309-26284-2. [Google Scholar]
- Tomkinson, G.R.; Léger, L.A.; Olds, T.S.; Cazorla, G. Secular trends in the performance of children and adolescents (1980-2000). An analysis of 55 studies of the 20m shuttle run test in 11 countries. Sports Med. 2003, 33, 285–300. [Google Scholar] [CrossRef]
- Mayorga-Vega, D.; Bocanegra-Parrilla, R.; Ornelas, M.; Viciana, J. Criterion-related validity of the distance- and time-based walk/run field tests for estimating cardiorespiratory fitness: A systematic review and meta-analysis. PLoS ONE 2016, 1, e0151671. [Google Scholar] [CrossRef]
- Levy, I.; Hall, C.; Trentacosta, N.; Percival, M. A preliminary retrospective survey of injuries occurring in dogs participating in canine agility. Vet. Comp. Orthop. Traumatol. 2019, 22, 321–324. [Google Scholar] [CrossRef]
- Cullen, K.L.; Dickey, J.P.; Bent, L.R.; Thomason, J.J.; Moëns, N.M. Survey-based analysis of risk factors for injury among dogs participating in agility training and competition events. J. Am. Vet. Med. Assoc. 2013, 243, 1019–1024. [Google Scholar] [CrossRef]
- Zanghi, B.M.; Robbins, P.J.; Ramos, M.T.; Otto, C.M. Working Dogs Drinking a Nutrient-Enriched Water Maintain Cooler Body Temperature and Improved Pulse Rate Recovery After Exercise. Front. Vet. Sci. 2018, 28, 202. [Google Scholar] [CrossRef]
- Essner, A.; Kjellerstedt, C.; Hesbach, A.L.; Svensson, K.; Igelström, H. Dog Handler Beliefs regarding Barriers and Facilitators to Canine Health Promotion and Injury Prevention in Swedish Working Dog Trials and Competitions. Vet. Sci. 2022, 9, 242. [Google Scholar] [CrossRef]
- Inkilä, L.; Hyytiäinen, H.K.; Hielm-Björkman, A.; Junnila, J.; Bergh, A.; Boström, A. Part II of finnish agility dog survey: Agility-related injuries and risk factors for injury in competition-level agility dogs. Animals 2022, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Lilja-Maula, L.I.O.; Laurila, H.P.; Syrjä, P.; Lappalainen, A.K.K.; Krafft, E.; Clerx, C.; Rajamäki, M.M. Long-term outcome and use of 6-minute walk test in West Highland Terriers with idiopathic pulmonary fibrosis. J. Vet. Intern. Med. 2014, 28, 379–385. [Google Scholar] [CrossRef]
- Acosta, A.R.; Van Wie, E.; Stoughton, W.B.; Bettis, A.K.; Barnett, H.H.; LaBrie, N.R.; Baloq-Alvarez, C.J.; Nghiem, P.P.; Cummings, K.J.; Kornegay, J.N. Use of the six-minute walk test to characterize golden retriever muscular dystrophy. Neuromuscul. Disord. 2016, 26, 865–872. [Google Scholar] [CrossRef]
- Lopedote, M.; Valentini, S.; Musella, V.; Vilar, J.M.; Spinella, G. Changes in pulse rate, respiratory rate and rectal temperature in working dogs before and after three different field trials. Animals 2020, 10, 733. [Google Scholar] [CrossRef]
- Farr, B.D.; Ramos, M.T.; Otto, C.M. The Penn Vet Working Dog Center Fit to Work Program: A formalized method for assessing and developing foundational canine physical fitness. Front. Vet. Sci. 2020, 7, 470. [Google Scholar] [CrossRef]
- Dahl, S.; Cotrel, C.; Leleu, C. Optimal active recovery intensity in standardbreds after submaximal work. Equine Vet. J. Suppl. 2006, 36, 102–105. [Google Scholar] [CrossRef]
- Ferasin, L.; Marcora, S. Reliability of an incremental exercise test to evaluate acute blood lactate, heart rate and body temperature responses in Labrador retrievers. J. Comp. Physiol. B. 2009, 179, 839–845. [Google Scholar] [CrossRef]
- Moraes, V.S.; Soares, J.K.I.; Cabidelli, J.F.; Fadini, A.N.B.; Ribeiro, P.A.; Pinheiro, R.M.; Conti, L.M.; Souza, V.R.C.; Coelho, C.S. Effect of resistance training on electrocardiographic and blood parameters of police dogs. Comp. Exerc. Physiol. 2017, 13, 217–226. [Google Scholar] [CrossRef]
- Mach, R.; Wiegel, P.S.; Bach, J.P.; Beyerbach, M.; Kreienbrock, L.; Nolte, I. Evaluation of a treadmill-based submaximal fitness test in pugs, and collecting breed-specific information on brachycephalic obstructive airway syndrome. Animals 2022, 12, 1585. [Google Scholar] [CrossRef]
- Finley, P.S.; Fountain, J.J.; Finley, D.P. Road racing and youth running: Cross country coaches’ perspectives. Sport J. 2017, 19, 627. [Google Scholar]
- Penry, J.T.; Wilcox, A.R.; Yun, J. Validity and reliability analysis of Cooper’s 12-minute run and the multistage shuttle run in healthy adults. J. Strength Cond. Res. 2011, 25, 597–605. [Google Scholar] [CrossRef]
- Bandyopadhyay, A. Validity of Cooper’s 12-minute run test for estimation of maximum oxygen uptake in male university students. Biol. Sport 2015, 32, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Fraipont, A.; Van Erck, E.; Ramery, E.; Fortier, G.; Lekeux, P.; Art, T. Assessing fitness in endurance horses. Can. Vet. J. 2012, 53, 311–314. [Google Scholar]
- Léguillette, R.; Bond, S.L.; Lawlor, K.; Haan, T.; Weber, L.M. Comparison of physiological demands in Warmblood show jumping horses over a standardized 1.10 m jumping course versus a standardized exercise test on a track. BMC Vet. Res. 2020, 16, 182. [Google Scholar] [CrossRef]
- Banse, H.E.; Sides, R.H.; Ruby, B.C.; Bayly, W.M. Effects of endurance training on VO2max and submaximal blood lactate concentrations of untrained sled dogs. Equine Comp. Exerc. Physiol. 2007, 4, 89–94. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Panzera, S.; Giannetto, C.; Fazio, F. Effect of moderate treadmill exercise on some physiological parameters in untrained beagle dogs. Exp. Anim. 2012, 61, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Takken, T.; Bongers, B.C.; van Brussel, M.; Haapala, E.A.; Hulzebos, E.H.J. Cardiopulmonary exercise testing in pediatrics. Ann. Am. Thorac. Soc. 2017, 14, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, B.S.; Vincent, H.K.; Chi, X.; Filipp, S.L.; Mercado, R.; Modave, F.; Guo, Y.; Gurka, M.J.; Bernier, A. Simple tests of cardiorespiratory fitness in a pediatric population. PLoS ONE 2020, 15, e0238863. [Google Scholar] [CrossRef]
- Gunn, H.M. Heart weight and running ability. J. Anat. 1989, 167, 225–233. [Google Scholar] [PubMed]
- Von Dehn, B. Pediatric clinical pathology. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Rørtveit, R.; Sævik, B.K.; Eggertsdóttir, A.V.; Skancke, E.; Lingaas, F.; Thoresen, S.I.; Jansen, J.H. Age-related changes in hematologic and serum biochemical variables in dogs aged 16-60 days. Vet. Clin. Pathol. 2015, 44, 47–57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Söderlund, E.-E.; Kyröläinen, H.; Laitinen-Vapaavuori, O.M.; Hyytiäinen, H.K. Proposed Protocol for Field Testing of Endurance Fitness of Young Labrador Retrievers. Methods Protoc. 2023, 6, 61. https://doi.org/10.3390/mps6040061
Söderlund E-E, Kyröläinen H, Laitinen-Vapaavuori OM, Hyytiäinen HK. Proposed Protocol for Field Testing of Endurance Fitness of Young Labrador Retrievers. Methods and Protocols. 2023; 6(4):61. https://doi.org/10.3390/mps6040061
Chicago/Turabian StyleSöderlund, Ella-Erika, Heikki Kyröläinen, Outi M. Laitinen-Vapaavuori, and Heli K. Hyytiäinen. 2023. "Proposed Protocol for Field Testing of Endurance Fitness of Young Labrador Retrievers" Methods and Protocols 6, no. 4: 61. https://doi.org/10.3390/mps6040061
APA StyleSöderlund, E.-E., Kyröläinen, H., Laitinen-Vapaavuori, O. M., & Hyytiäinen, H. K. (2023). Proposed Protocol for Field Testing of Endurance Fitness of Young Labrador Retrievers. Methods and Protocols, 6(4), 61. https://doi.org/10.3390/mps6040061





