Two Methods for the Isolation and Cultivation of Porcine Primary Corneal Cells
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
- Bovine serum albumin (BSA) (PanReac AppliChem ITW Reagent, Darmstadt, Germany; Cat. No. A1391,0500).
- 8-well chamber slides (Thermo Scientific™ Nunc™ Lab-TekII™, Rochester, NY, USA; Cat. No. 154534).
- CnT-Prime (CellnTec-Advanced Cell Systems, Bern, Switzerland; Cat. No. CnT-PR)
- Collagenase 100 mg (0.171 U/mg lyo.) (Sigma-Aldrich, Taufkirchen, Germany; Cat. No. 10103578001).
- Culture flask with ventilation screw cap T25 (Greiner Bio-One Gmbh, Frickenhausen, Germany; Cat. No. 690175).
- Disposable cup 100 mL (Sarstedt, Nümbrecht, Germany; Cat. No. 75.563).
- Disposable safety scalpel #11 (Aesculap AG, Tuttlingen, Germany; Cat. No. BA811SU).
- Dulbecco’s Phosphate Buffered Saline (PBS) (Gibco™ Thermo-Fisher, Karlsruhe, Germany; Cat. No. 10010056).
- Eppendorf Safe-Lock tube 2 mL (Eppendorf, Hamburg, Germany; Cat. No. 0030121880).
- Ethanol 70% (VWR, Darmstadt, Germany; Cat. No. 97065-058).
- Tube 15 mL (Greiner Bio-One, Frickenhausen, Germany; Cat. No. 188271).
- Fetal bovine serum (FBS) (Thermo-Fischer, Karlsruhe, Germany; Cat. No. 10100147).
- FluorSave™ (Merck Milipore, Darmstadt, Germany; Cat. No. 345789).
- Iodine (Braunol®, B. Braun, Melsungen, Germany; Cat. No. 190970).
- MultiMACS cDNA Synthesis Kit (Miltenyi Biotec, Köln, Germany; Cat. No. 130-094-410).
- Paraformaldehyde (PFA) (Merck Milipore, Darmstadt, Germany; Cat No. 1040051000).
- Penicillin–streptomycin 10,000 U/mL (Thermo-Fischer, Karlsruhe, Germany; Cat. No. 15140148).
- iTaq Universal SYBR® Green Supermix (Bio-Rad, Feldkirchen, Germany; Cat. No. 1725124).
- Trypsin/EDTA (Gibco™ Thermo-Fischer, Karlsruhe, Germany; Cat. No. 25200072).
- Triton X-100 (Sigma-Aldrich, Taulfkirchen, Germany; Cat. No. 9002-93-1).
- 6-well plate (Greiner Cellstar®, Frickenhausen, Germany; Cat. No. M9062-100EA).
- 12-well plate (Nerbe plus, Winsen/Luhe, Germany; Cat. No. 14-020-0012).
- 4′,6-diamidino-2-phenylindole (DAPI) (Thermo-Fischer, Karlsruhe, Germany; Cat. No. D1306).
2.2. Equipment
- Olympus R1 SLI Cell counter (Olympus coorporation, Tokyo, Japan; Cat. No. K23009240).
- Centrifuge VWR Mega Star 3.0R (VWR, Darmstadt, Germany; Cat. No. 521.1752).
- Heracell 150i CO2 incubator (Thermo-Fischer, Karlsruhe, Germany; Cat. No. 50116050).
- MultiMACS™ M96 Separator (Miltenyi Biotec, Köln, Germany; Cat. No. 130-091-937).
- VWR Thermal Shake Touch (VWR, Darmstadt, Germany; Cat. No. 89232-908).
- Thermal cycler Bio-Rad CFX96™ Real-Time System (Bio-Rad, Feldkirchen, Germany; Cat. No. 1845097).
- Zeiss Axio Observer (CarlZeiss, Oberkochen, Germany; Cat. No. 491916-0002-000).
- Zeiss Axio Imager Z1 Apotome Microscope with Mrm digital camera (CarlZeiss, Oberkochen, Germany; Cat. No. p1739).
3. Procedure
3.1. Corneal Cell Culture and Splitting
3.1.1. Checklist (6 h)
- To guarantee sterile conditions and avoid contaminations, clean and disinfect the laminar-air-flow hood with 70% ethanol.
- Disinfect and autoclave tools (e.g., scissors and forceps) before use.
- Prepare the following medium in advance under a laminar-air-flow hood, under sterile conditions: CnT-Prime, 2% penicillin–streptomycin. Fetal bovine serum (FBS) can be added in different concentrations (e.g., 10%).
- Adjust all substances to room temperature before use.
3.1.2. Corneal Cell Outgrowth Protocol (2 h)
- Collect enucleated porcine eyes from a local abattoir just after sacrification of the animals and store them on ice during transportation to the laboratory. Avoid direct contact between the eyes and the ice. At the laboratory, store the eyes at 4 °C until use. Process the eyes as soon as possible. The longer the storage at 4 °C, the lower the tissue quality.
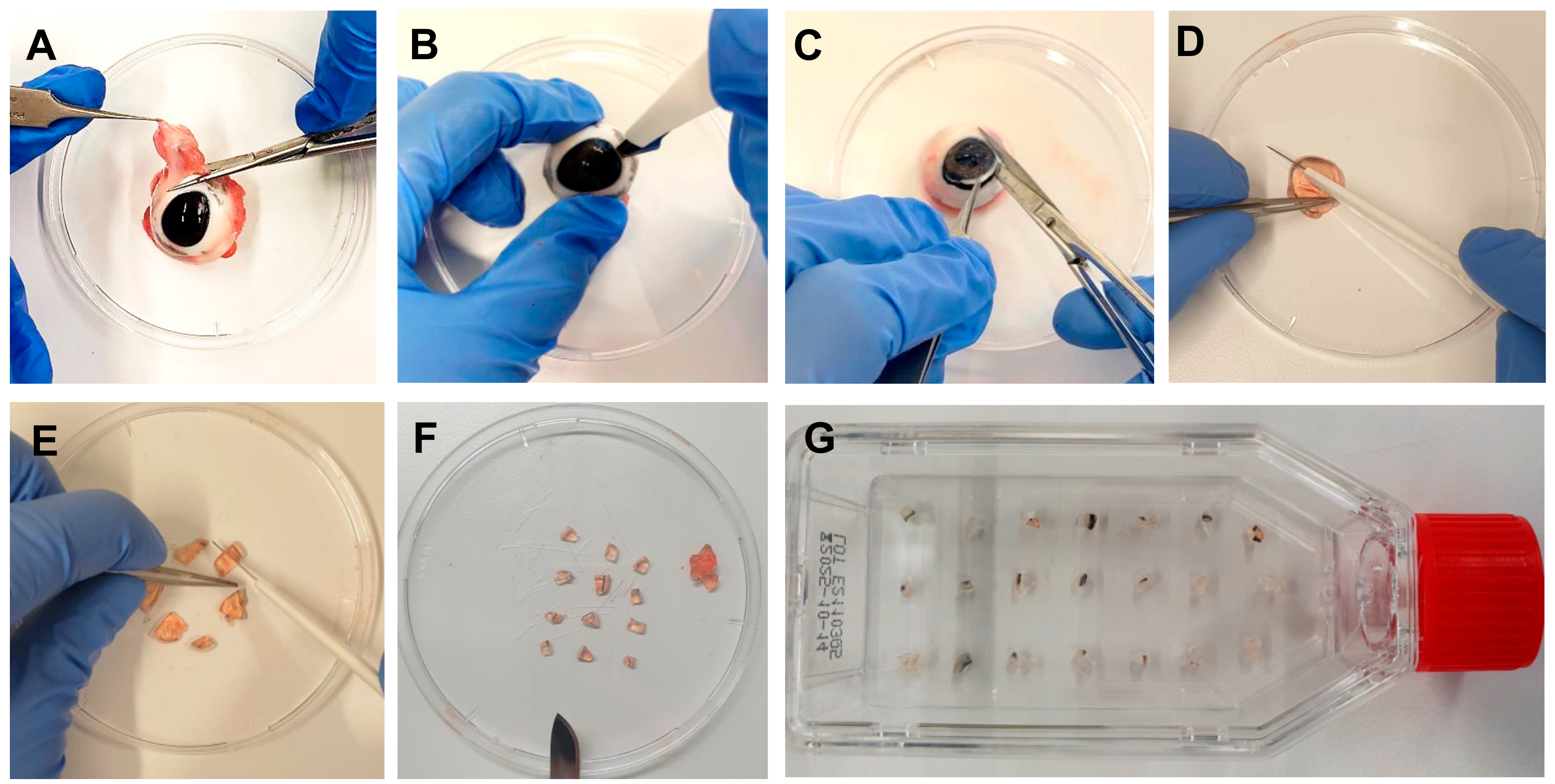
- Remove the eyes from the refrigerator and remove muscles and tissues around the eye with scissors and forceps (Figure 1A). Do not harm the eyeball. One eye is needed to isolate explants before sufficient cultivation of one flask.
- Disinfect the eye in iodine 5% (v/v) diluted in distilled water for 5 min in a disposable cup.
- Wash the eye 5 times in phosphate-buffered saline (PBS) with 2% penicillin-streptomycin (PS).
- In a petri dish, extract the pig cornea from the eye with a scalpel, scissors, and forceps by cutting circularly around the cornea, leaving a thin part of the sclera around it (Figure 1B,C).
- Discard the rest of the eye, clean the cornea in PBS with PS, first cut it in half, and then cut each half into three parts. Extract the center of the cornea, leaving a strip of approximately 2 mm of corneal tissue and 2 mm of sclera (Figure 1D,F).
- Place the explants of one eye in a T25 culture flask with a ventilation screw cap (Figure 1G). Use 15–20 explants per culture flask (cells grow most effectively when they are closer to other corneal explants).
CRITICAL STEP: Wait 5 min until the cornea explants adhere to the surface of the flask and carefully add 1 mL of culture medium (CnT-Prime + 2% PS), one drop per explant, so that the explants do not detach from the flask. Only attached explants assure outgrowth. Floating explants should be removed. 10% FBS can opitionally be added to the culture medium.
- Incubate the flask in an incubator at 37 °C with 5% CO2 overnight.
PAUSE STEP
CRITICAL STEP: On the next day, carefully add 3 mL of culture medium to the flask. Pay attention and make sure that the explants do not move.
PAUSE STEP
- Incubate the flask in an incubator at 37 °C with 5% CO2 for 4–5 weeks and change the culture medium twice weekly. To change the medium, aspirate carefully the old medium to avoid detachment of corneal explants and slowly add 4 mL of new culture medium.
3.1.3. Splitting Cells (30 min)
- Remove the corneal explants from the culture flask with the help of forceps.
- Remove the culture medium with a Pasteur pipette and rinse with 4 mL of PBS.
- Remove PBS and add 1 mL of trypsin/EDTA (T/E), and then incubate the flask in an incubator at 37 °C with 5% CO2 for 5 min.
- Mechanically detach the cells by tapping on the side of the bottle.
- Add 2 mL of T/E-stopping solution (CnT-Prime + 2% PS + 10% FBS).
- Pipette the entire content of the flask into a 15 mL tube and centrifuge it at room temperature, 300× g for 5 min.
CRITICAL STEP: Carefully aspirate the supernatant and resuspend the cell content with 1 mL of culture medium (CnT-Prime + 2% PS with or without FBS depending on whether the explants were previously incubated with serum or not).
- Count cells, dilute the cells accordingly, and seed them in well plates. The cell numbers are defined according to the area inside the culture vessel (Table 1).
3.1.4. Corneal Cell Extraction with Collagenase Protocol (5 h)
- Collect enucleated porcine eyes from a local abattoir just after the sacrifice of the animals and store them in a plastic bag in a foam box refrigerated with ice during the transportation to the laboratory. Do not place the eyes directly onto the ice. At the laboratory, store the eyes at 4 °C until use. Make sure that the eyes are used as soon as possible. The longer the storage at 4 °C, the lower the tissue quality.
- Select at least 2 and up to 10 pig eyes with intact corneas.
- Remove the eyes from the refrigerator and discard muscles and tissue around the eye with scissors and forceps. Do not harm the eyeballs.
- Disinfect the eye with iodine 5% (v/v) diluted in distilled water for 5 min in a disposable cup.
- Wash the eye 5 min in PBS with 2% PS.
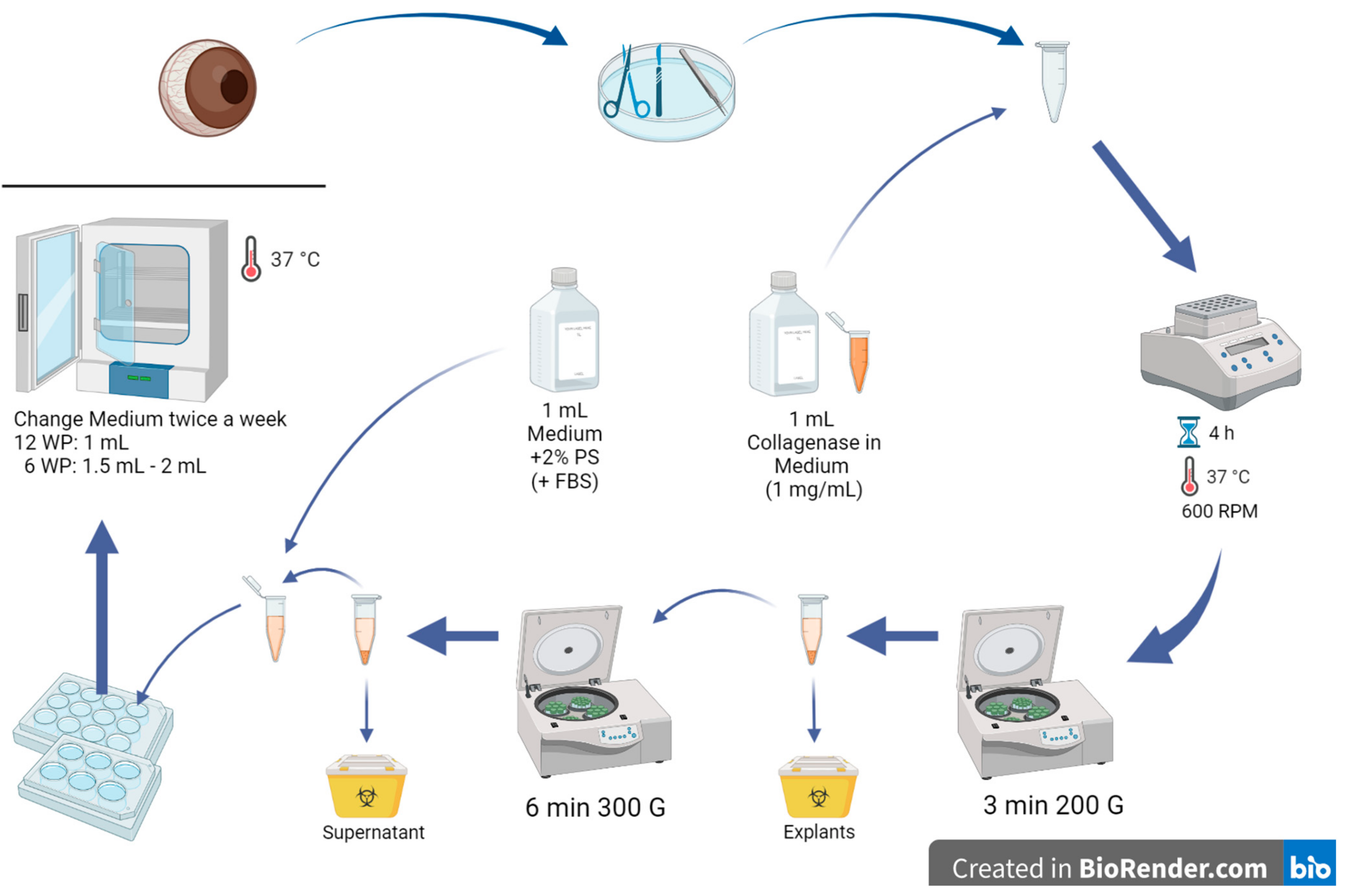
- In a petri dish, extract the pig cornea from the eye with a scalpel, scissors, and forceps by cutting circularly around the cornea, leaving a thin part of the sclera around it (Figure 2).
- Cut the corneas into small pieces and put them in a 2 mL Eppendorf tube (2–3 pig corneas per tube); make sure to clean your instruments with 70% alcohol from time to time.
- Add 1 mL of collagenase with a concentration of 1 mg/mL in medium to the tube with cornea explants.
- Place the Eppendorf tubes on a shaker for 4 h (37 °C, 600 RPM).
- Centrifuge the tubes for 3 min at 20 °C and 200✕ g.
CRITICAL STEP: Under the hood, remove the supernatant (with the diluted cells in it) and transfer it into another Eppendorf tube. Dispose of the explants.
- Centrifuge the tubes again for 6 min at 20 °C and 300✕ g.
- Remove the supernatant and dispose of it.
- Add 1 mL medium (CnT-Prime + 2% PS) to the remaining cells in the Eppendorf tube. 10% FBS can opitionally be added to the culture medium.
- Sow the extracted epithelium cells in a 6- or 12-well plate (content of one Eppendorf tube per well) and incubate the well plate in an incubator at 37 °C with 5% CO2 overnight. Advice: 12-well plates work better than 6-well plates because the cells may achieve more cell-cell contact in a smaller area (6-well plates may be too big in area for only 2–3 eyes per well).
PAUSE STEP.
- Change the medium twice a week.
3.1.5. Splitting Cells (30 min)
- Remove the culture medium with a Pasteur pipette, pump, and then rinse with 500 µL of PBS.
- Remove PBS and add 200 µL of trypsin/EDTA, incubate the 12-well plate in an incubator at 37 °C with 5% CO2 for 3–5 min.
- Mechanically detach the cells by tapping on the side of the well plate.
- Add the double amount (400 µL) of T/E-stopping solution (CnT-Prime + 2% PS + 10% FBS).
- Pipette the entire content of the well plate into a 15 mL tube and centrifuge it at room temperature, 300× g for 5 min.
CRITICAL STEP: Carefully aspirate the supernatant and resuspend the cell content with 1 mL of culture medium (CnT-Prime + 2% PS with or without FBS depending on whether the explants were incubated with serum or not previously).
- Count cells with an automated cell counter and add the quantity according to the envisaged experiment (Table 1).
3.2. Immunostaining
3.3. Cell Quantification
3.4. Quantitative Real-Time PCR
3.5. Statistical Analysis
4. Expected Results
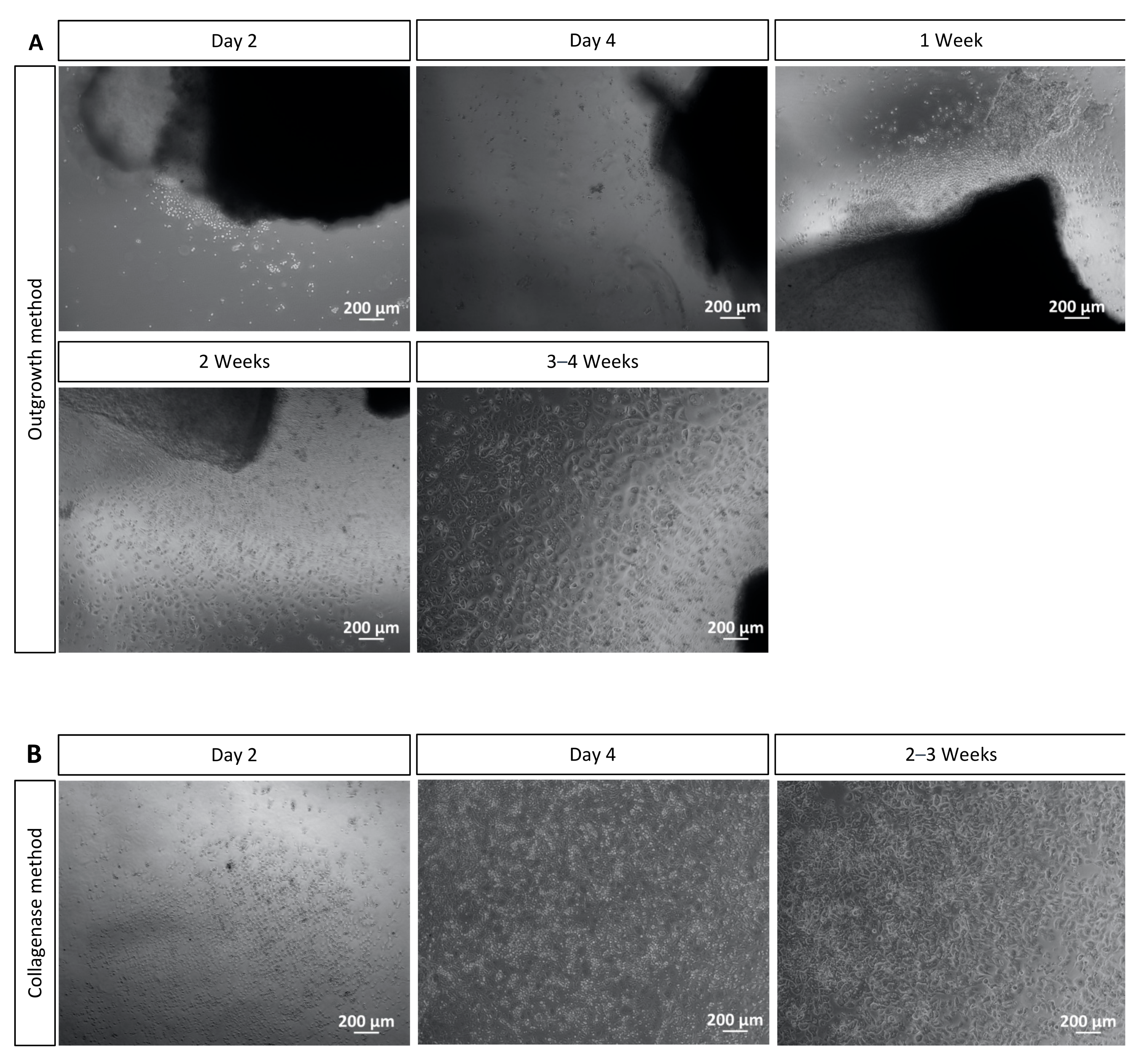
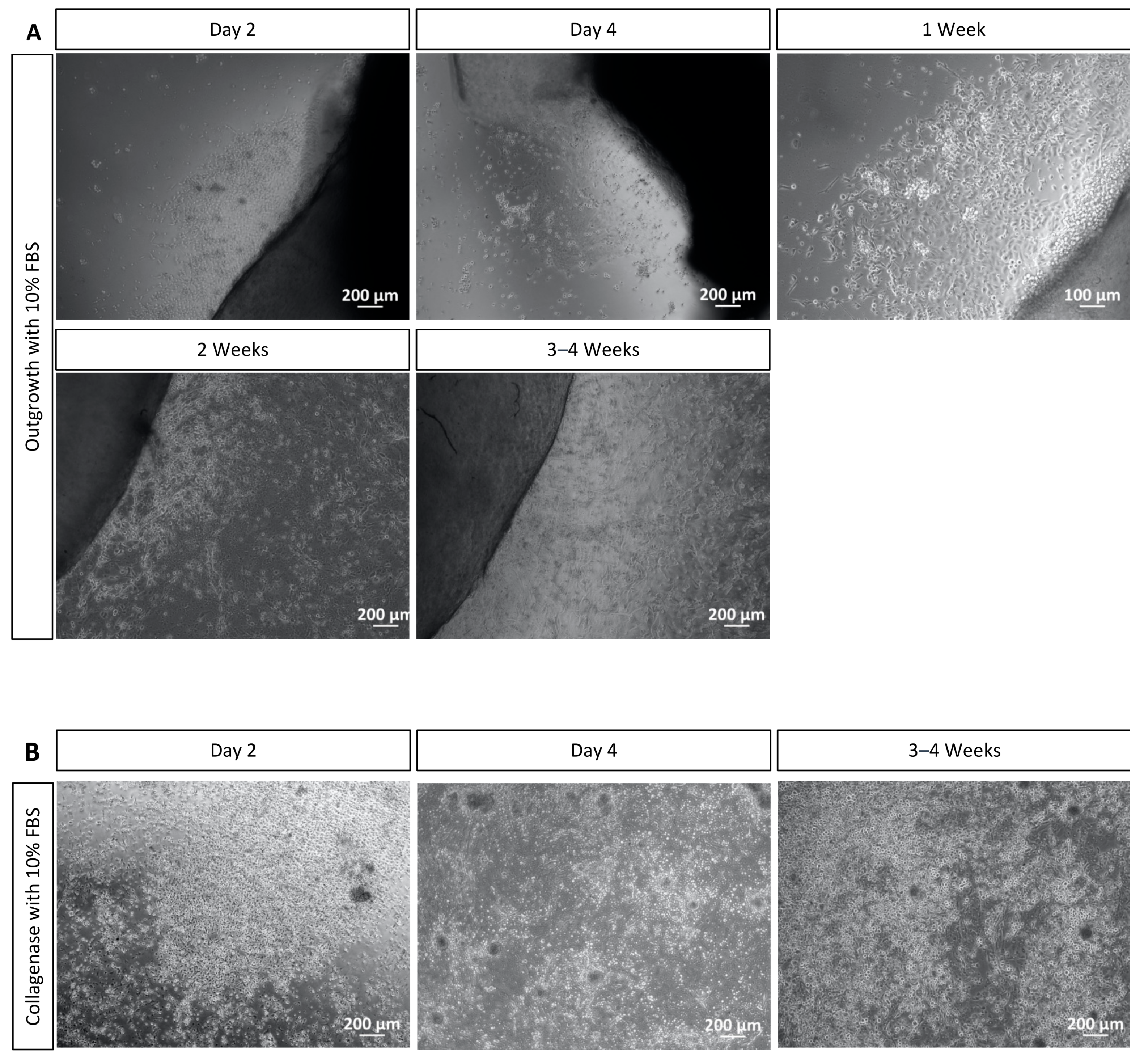
4.1. Outgrowth and Collagenase Methods
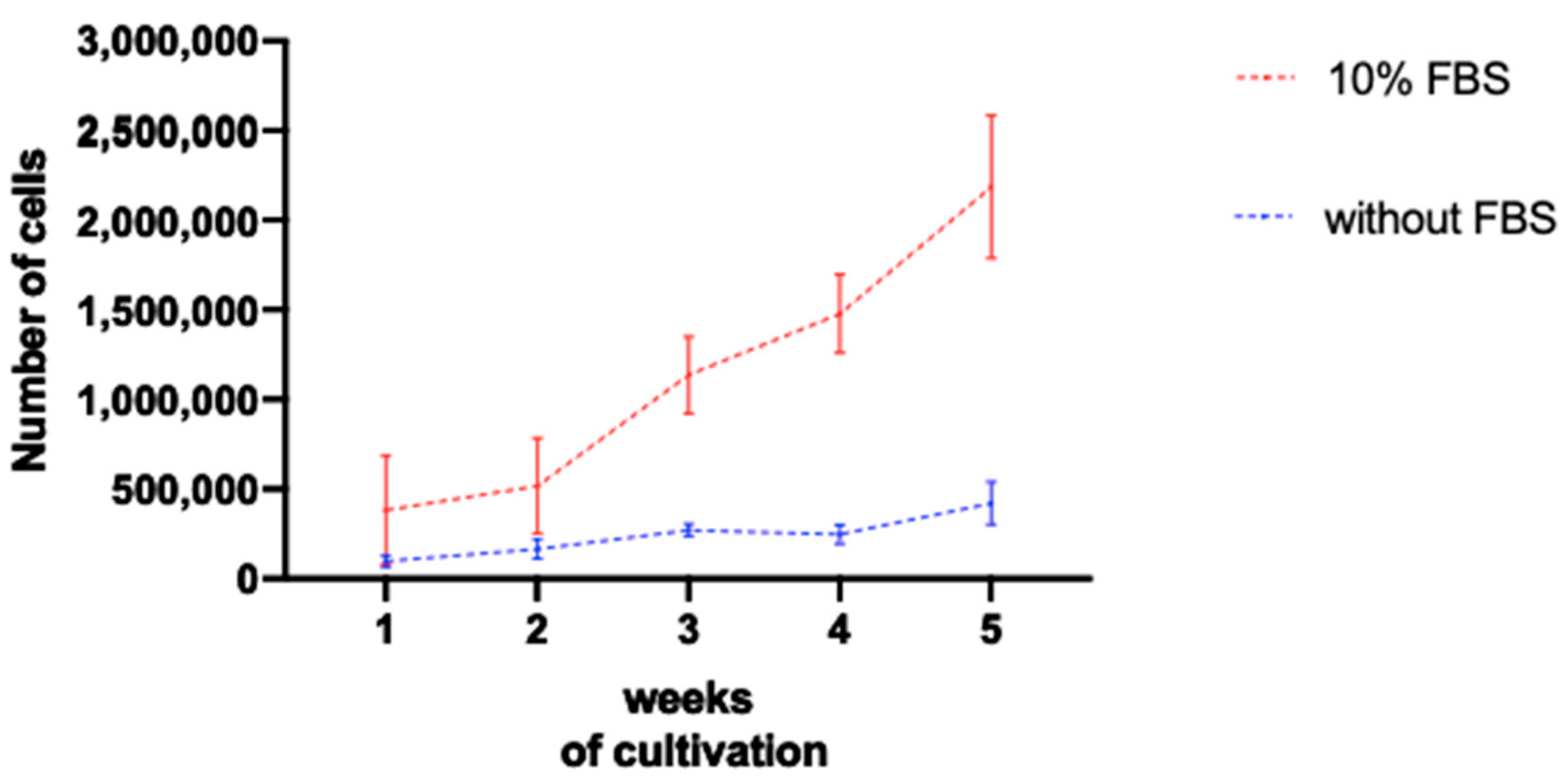
4.2. Cell Cultures Cultivated with Serum Present Higher Amount of Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510, Erratum in Ocul. Surf. 2019, 17, 842. [Google Scholar] [CrossRef] [PubMed]
- Wentz-Hunter, K.; Cheng, E.L.; Ueda, J.; Sugar, J.; Yue, B.Y.J.T. Keratocan Expression Is Increased in the Stroma of Keratoconus Corneas. Mol. Med. 2001, 7, 470–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Ban, Y.; Cooper, L.J.; Fullwood, N.J.; Nakamura, T.; Tsuzuki, M.; Koizumi, N.; Dota, A.; Mochida, C.; Kinoshita, S. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp. Eye Res. 2003, 76, 735–743. [Google Scholar] [CrossRef]
- Shi, L.; Stachon, T.; Käsmann-Kellner, B.; Seitz, B.; Szentmáry, N.; Latta, L. Keratin 12 mRNA expression could serve as an early corneal marker for limbal explant cultures. Cytotechnology 2020, 72, 239–245. [Google Scholar] [CrossRef][Green Version]
- Chaurasia, S.; Kaur, H.; de Medeiros, F.W.; Smith, S.D.; Wilson, S.E. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009, 89, 133–139. [Google Scholar] [CrossRef][Green Version]
- Castro-Muñozledo, F.; Meza-Aguilar, D.G.; Domínguez-Castillo, R.; Hernández-Zequinely, V.; Sánchez-Guzmán, E. Vimentin as a Marker of Early Differentiating, Highly Motile Corneal Epithelial Cells. J. Cell. Physiol. 2016, 232, 818–830. [Google Scholar] [CrossRef]
- Espana, E.M.; He, H.; Kawakita, T.; Di Pascuale, M.A.; Raju, V.K.; Liu, C.-Y.; Tseng, S.C.G. Human Keratocytes Cultured on Amniotic Membrane Stroma Preserve Morphology and Express Keratocan. Investig. Opthalmol. Vis. Sci. 2003, 44, 5136–5141. [Google Scholar] [CrossRef][Green Version]
- Fukuda, K. Corneal fibroblasts: Function and markers. Exp. Eye Res. 2020, 200, 108229. [Google Scholar] [CrossRef]
- Porth, J.M.; Deiotte, E.; Dunn, M.; Bashshur, R. A Review of the Literature on the Global Epidemiology of Corneal Blindness. Cornea 2019, 38, 1602–1609. [Google Scholar] [CrossRef]
- Hamrah, P.; Sahin, A.; Dastjerdi, M.H.; Shahatit, B.M.; Bayhan, H.A.; Dana, R.; Pavan-Langston, D. In Vivo Confocal Microscopic Changes of the Corneal Epithelium and Stroma in Patients with Herpes Zoster Ophthalmicus. Am. J. Ophthalmol. 2015, 159, 1036–1044.e1. [Google Scholar] [CrossRef][Green Version]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef]
- Yoon, J.J.; Ismail, S.; Sherwin, T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J. Stem Cells 2014, 6, 391–403. [Google Scholar] [CrossRef]
- Bremond-Gignac, D.; Copin, H.; Benkhalifa, M. Corneal epithelial stem cells for corneal injury. Expert Opin. Biol. Ther. 2018, 18, 997–1003. [Google Scholar] [CrossRef]
- Yuan, S.; Fan, G. Stem cell-based therapy of corneal epithelial and endothelial diseases. Regen. Med. 2015, 10, 495–504. [Google Scholar] [CrossRef][Green Version]
- Hurst, J.; Mueller-Buehl, A.M.; Hofmann, L.; Kuehn, S.; Herms, F.; Schnichels, S.; Joachim, S.C. iNOS-inhibitor driven neuroprotection in a porcine retina organ culture model. J. Cell. Mol. Med. 2020, 24, 4312–4323. [Google Scholar] [CrossRef][Green Version]
- Netto, A.R.T.; Hurst, J.; Bartz-Schmidt, K.-U.; Schnichels, S. Porcine Corneas Incubated at Low Humidity Present Characteristic Features Found in Dry Eye Disease. Int. J. Mol. Sci. 2022, 23, 4567. [Google Scholar] [CrossRef]
- Menduni, F.; Davies, L.N.; Madrid-Costa, D.; Fratini, A.; Wolffsohn, J.S. Characterisation of the porcine eyeball as an in-vitro model for dry eye. Contact Lens Anterior Eye 2018, 41, 13–17. [Google Scholar] [CrossRef][Green Version]
- Ruiz-Ederra, J.; Garcia, M.; Hernandez, M.; Urcola, H.; Hernandez-Barbachano, E.; Araiz, J.; Vecino, E. The pig eye as a novel model of glaucoma. Exp. Eye Res. 2005, 81, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Arranz-Valsero, I.; Soriano-Romaní, L.; García-Posadas, L.; López-García, A.; Diebold, Y. IL-6 as a corneal wound healing mediator in an in vitro scratch assay. Exp. Eye Res. 2014, 125, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKay, T.B.; Guo, X.; Hutcheon, A.E.K.; Karamichos, D.; Ciolino, J.B. Methods for Investigating Corneal Cell Interactions and Extracellular Vesicles In Vitro. Curr. Protoc. Cell Biol. 2020, 89, e114. [Google Scholar] [CrossRef]
- Kasper, M.; Moll, R.; Stosiek, P.; Karsten, U. Patterns of cytokeratin and vimentin expression in the human eye. Histochem. 1988, 89, 369–377. [Google Scholar] [CrossRef]
- Dunlevy, J.R.; Beales, M.P.; Berryhill, B.L.; Cornuet, P.K.; Hassell, J.R. Expression of the Keratan Sulfate Proteoglycans Lumican, Keratocan and Osteoglycin/Mimecan During Chick Corneal Development. Exp. Eye Res. 2000, 70, 349–362. [Google Scholar] [CrossRef]
- Zito-Abbad, E.; Borderie, V.M.; Baudrimont, M.; Bourcier, T.; Laroche, L.; Chapel, C.; Uzel, J.-L. Corneal Epithelial Cultures Generated from Organ-Cultured Limbal Tissue: Factors Influencing Epithelial Cell Growth. Curr. Eye Res. 2006, 31, 391–399. [Google Scholar] [CrossRef]
- Okumura, N.; Kusakabe, A.; Hirano, H.; Inoue, R.; Okazaki, Y.; Nakano, S.; Kinoshita, S.; Koizumi, N. Density-gradient centrifugation enables the purification of cultured corneal endothelial cells for cell therapy by eliminating senescent cells. Sci. Rep. 2015, 5, 15005. [Google Scholar] [CrossRef][Green Version]
- Nakatsu, M.N.; Deng, S.X. Enrichment of human corneal epithelial stem/progenitor cells by magnetic bead sorting using SSEA4 as a negative marker. Methods Mol Biol. 2013, 1014, 71–77. [Google Scholar] [CrossRef] [PubMed]
- García-Posadas, L.; Arranz-Valsero, I.; López-García, A.; Soriano-Romaní, L.; Diebold, Y. A New Human Primary Epithelial Cell Culture Model to Study Conjunctival Inflammation. Investig. Opthalmol. Vis. Sci. 2013, 54, 7143–7152. [Google Scholar] [CrossRef][Green Version]
- Yu, F.-S.X.; Yin, J.; Xu, K.; Huang, J. Growth factors and corneal epithelial wound healing. Brain Res. Bull. 2010, 81, 229–235. [Google Scholar] [CrossRef][Green Version]
- Joris, F.; Manshian, B.B.; Peynshaert, K.; De Smedt, S.C.; Braeckmans, K.; Soenen, S.J. Assessing nanoparticle toxicity in cell-based assays: Influence of cell culture parameters and optimized models for bridging the in vitro–in vivo gap. Chem. Soc. Rev. 2013, 42, 8339–8359. [Google Scholar] [CrossRef][Green Version]
| Place | Quantity of Cells |
|---|---|
| 24-well plate | 100,000 |
| 96-well plate | 25,000 |
| Chamber slide | 25,000–50,000 |
| Primary Antibody | Manufacturer and Dilution | Secondary Antibody | Manufacturer and Dilution |
|---|---|---|---|
| Cytokeratin-3 (epithelial cell marker) | Biorbyt (Cambridge, UK) Orb5866 1:50 | AlexaFluor 488 anti rabbit | Thermo Fischer (Karlsruhe, Germany) A11008 1:500 |
| Occludin (tight junction marker) | Santa Cruz (Heidelberg, Germany sc-133256 1:50 | AlexaFluor 488 anti mouse | Thermo Fischer (Karlsruhe, Germany) A11001 1:500 |
| Vimentin (undifferentiated epithelial cell and mesenchymal cell marker) | Sigma (Taufkirchen, Germany) V2258 1:100 | AlexaFluor 555 anti mouse | Thermo Fischer (Karlsruhe, Germany) A21422 1:500 |
| Keratocan (keratocyte marker) | Santa Cruz (Heidelberg, Germany) sc-33243 1:50 | AlexaFluor anti goat | Thermo Fischer (Karlsruhe, Germany) A11055 1:500 |
| Lumican (keratocyte marker) | Santa Cruz (Heidelberg, Germany) sc-27718 1:50 | AlexaFluor anti goat | Thermo Fischer (Karlsruhe, Germany) A11055 1:500 |
| ZO-1 (tight junction marker) | Santa Cruz (Heidelberg, Germany) sc-33725 1:50 | AlexaFluor anti rat | Invitrogen (Darmstadt, Germany) 11006 1:500 |
| Primer | Forward | Reverse |
|---|---|---|
| GAPDH | GGGCATGAACCATGAGAAGT | AAGCAGGGATGATGTTCTGG |
| ACTB | CACGCCATCCTGCGTCTGGA | AGCACCGTGTTGGCGTAGAG |
| CK12 | TGGTCTCATCGCAAGTTCAG | TAAAGACCAACATGGCCACA |
| Keratocan | TCCCAGGGAGTGTTTCTGTC | GCATTCTCGAATGGCTTCTC |
| Outgrowth Method | Collagenase Method |
|---|---|
|
|
|
|
| |
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netto, A.R.T.; Hrusa, M.D.; Bartz-Schmidt, K.-U.; Schnichels, S.; Hurst, J. Two Methods for the Isolation and Cultivation of Porcine Primary Corneal Cells. Methods Protoc. 2023, 6, 50. https://doi.org/10.3390/mps6030050
Netto ART, Hrusa MD, Bartz-Schmidt K-U, Schnichels S, Hurst J. Two Methods for the Isolation and Cultivation of Porcine Primary Corneal Cells. Methods and Protocols. 2023; 6(3):50. https://doi.org/10.3390/mps6030050
Chicago/Turabian StyleNetto, Alice Rocha Teixeira, Marc Dieter Hrusa, Karl-Ulrich Bartz-Schmidt, Sven Schnichels, and José Hurst. 2023. "Two Methods for the Isolation and Cultivation of Porcine Primary Corneal Cells" Methods and Protocols 6, no. 3: 50. https://doi.org/10.3390/mps6030050
APA StyleNetto, A. R. T., Hrusa, M. D., Bartz-Schmidt, K.-U., Schnichels, S., & Hurst, J. (2023). Two Methods for the Isolation and Cultivation of Porcine Primary Corneal Cells. Methods and Protocols, 6(3), 50. https://doi.org/10.3390/mps6030050









