Building Accurate Intracellular Polarity Maps through Multiparametric Microscopy
Abstract
1. Introduction
2. Experimental Design
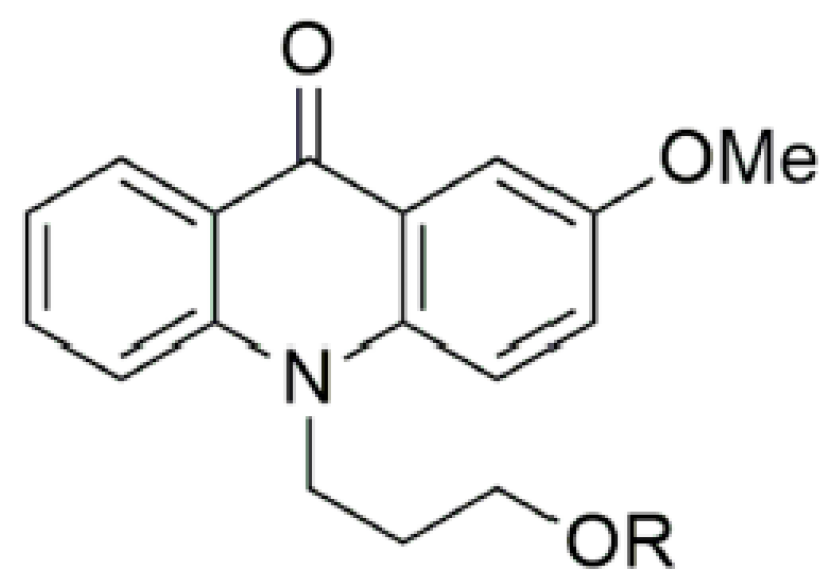
2.1. Requirements of Luminescence Probes
2.2. Requirements of Equipment
3. Procedure
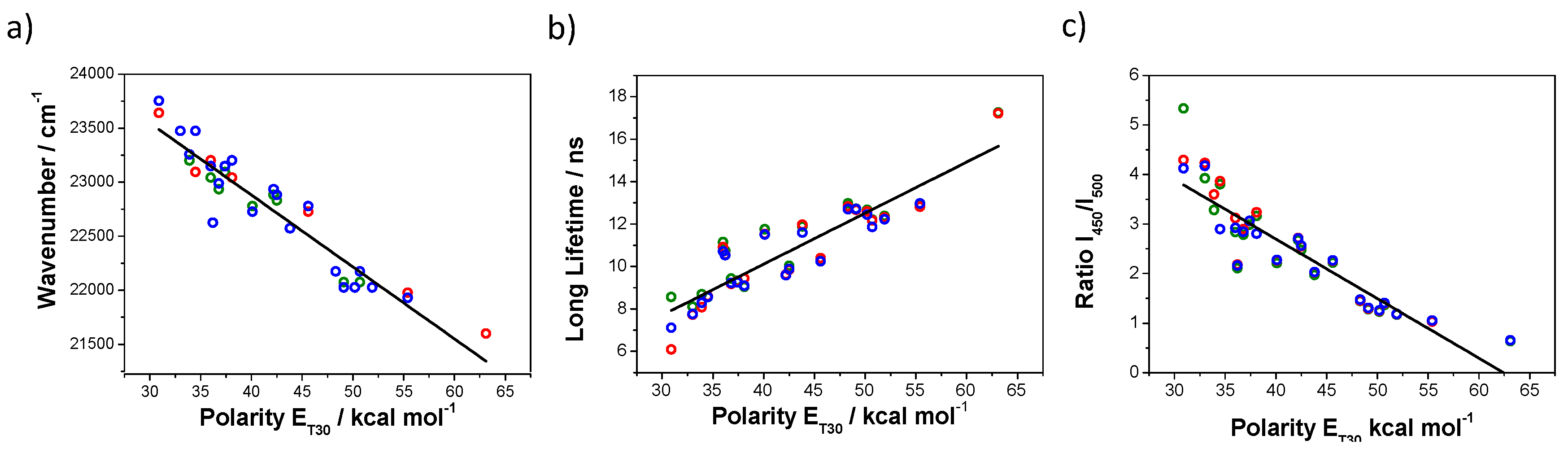
3.1. Step 1: Calibration with Solvent Polarity
3.2. Step 2: Multiparametric Calibration Curve Fitting
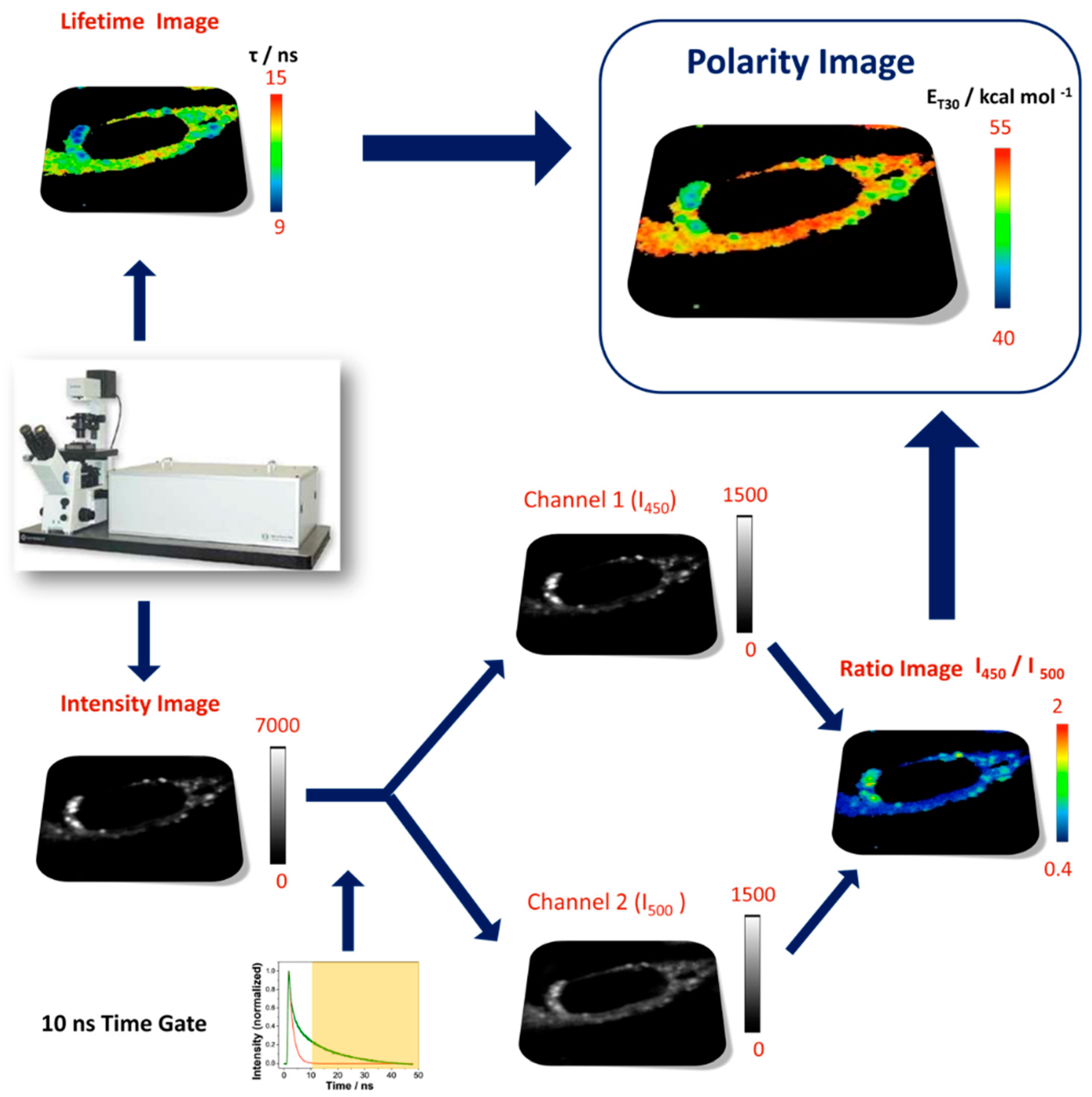
3.3. Step 3: Multiparametric Fluorescence Imaging
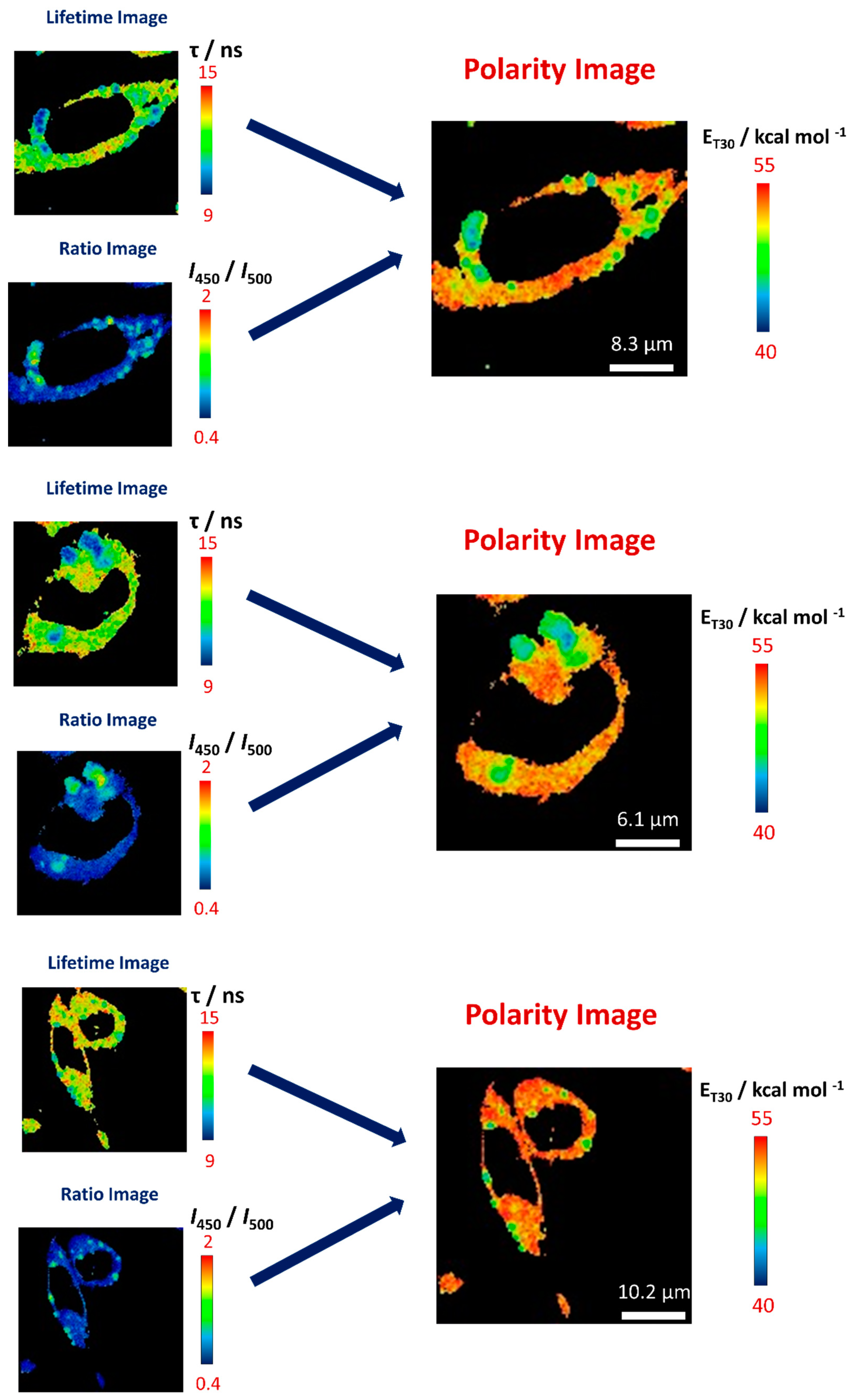
3.4. Step 4: Building Polarity Maps
4. Method Validation—Intracellular Polarity Maps in Different Cellular Microenvironments
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Cao, J.; He, Y.; Yang, J.H.; Kim, T.; Peng, X.; Kim, J.S. Macro-/micro-environment-sensitive chemosensing and biological imaging. Chem. Soc. Rev. 2014, 43, 4563–4601. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, J.; Xu, F.; Peng, X.; Mu, H.; Wang, J.; Xiong, X. Ratiometric Fluorescence Imaging of Cellular Polarity: Decrease in Mitochondrial Polarity in Cancer Cells. Angew. Chem. Int. Ed. 2015, 54, 2510–2514. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, X.; Ge, J.; Li, F.; Wang, X.; Wang, J.; Shuang, S.; Dong, C. A lysosome-targeting and polarity-specific fluorescent probe for cancer diagnosis. Chem. Commun. 2019, 55, 4703–4706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, J.; Mu, H.; Zhu, T.; Zhang, Z.; Du, J.; Peng, X. d-PET-controlled “off-on” Polarity-sensitive Probes for Reporting Local Hydrophilicity within Lysosomes. Sci. Rep. 2016, 6, 35627. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.L.; Tecedor, L.; Chang, M.; Davidson, B.L. Clarifying lysosomal storage diseases. Trends Neurosci. 2011, 34, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Yu, W.H.; Kumar, A.; Lee, S.; Mohan, P.S.; Peterhoff, C.M.; Wolfe, D.M.; Martinez-Vicente, M.; Massey, A.C.; Sovak, G.; et al. Lysosomal Proteolysis and Autophagy Require Presenilin 1 and Are Disrupted by Alzheimer-Related PS1 Mutations. Cell 2010, 141, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Juvekar, V.; Jo, J.H.; Kim, H.M. Combining hydrophilic and hydrophobic environment sensitive dyes to detect a wide range of cellular polarity. Chem. Sci. 2020, 11, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Signore, G.; Nifosì, R.; Albertazzi, L.; Storti, B.; Bizzarri, R. Polarity-Sensitive Coumarins Tailored to Live Cell Imaging. J. Am. Chem. Soc. 2010, 132, 1276–1288. [Google Scholar] [CrossRef]
- Owyong, T.C.; Subedi, P.; Deng, J.; Hinde, E.; Paxman, J.J.; White, J.M.; Chen, W.; Heras, B.; Wong, W.W.H.; Hong, Y. A Molecular Chameleon for Mapping Subcellular Polarity in an Unfolded Proteome Environment. Angew. Chem. Int. Ed 2020, 59, 10129–10135. [Google Scholar] [CrossRef]
- Ashoka, A.H.; Ashokkumar, P.; Kovtun, Y.P.; Klymchenko, A.S. Solvatochromic Near-Infrared Probe for Polarity Mapping of Biomembranes and Lipid Droplets in Cells under Stress. J. Phys. Chem. Lett. 2019, 10, 2414–2421. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Chen, Y.; Zheng, J.; Zhang, J.; Li, R.; Wan, H.; Yin, J.; Yuan, Z.; Chen, H. A family of push-pull bio-probes for tracking lipid droplets in living cells with the detection of heterogeneity and polarity. Anal. Chim. Acta 2020, 1096, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, M.C.; Peña-Ruiz, T.; Herrero-Foncubierta, P.; Miguel, D.; Giron, M.D.; Salto, R.; Cuerva, J.M.; Navarro, A.; Garcia-Fernandez, E.; Orte, A. Orthogonal cell polarity imaging by multiparametric fluorescence microscopy. Sens. Actuator B Chem. 2020, 309, 127770. [Google Scholar] [CrossRef]
- Ruedas-Rama, M.J.; Alvarez-Pez, J.M.; Crovetto, L.; Paredes, J.M.; Orte, A. FLIM Strategies for Intracellular Sensing. In Advanced Photon Counting; Kapusta, P., Wahl, M., Erdmann, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 15, pp. 191–223. [Google Scholar]
- Monteith, A.; Marszalec, W.; Chan, P.; Logan, J.; Yu, W.; Schwarz, N.; Wokosin, D.; Hockberger, P. Imaging of Mitochondrial and Non-Mitochondrial Responses in Cultured Rat Hippocampal Neurons Exposed to Micromolar Concentrations of TMRM. PLoS ONE 2013, 8, e58059. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, E.; Pernagallo, S.; González-Vera, J.A.; Ruedas-Rama, M.J.; Díaz-Mochón, J.J.; Orte, A. Time-Gated Luminescence Acquisition for Biochemical Sensing: miRNA Detection. In Fluorescence in Industry; Pedras, B., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 18, pp. 213–267. [Google Scholar]
- Okkelman, I.A.; Neto, N.; Papkovsky, D.B.; Monaghan, M.G.; Dmitriev, R.I. A deeper understanding of intestinal organoid metabolism revealed by combining fluorescence lifetime imaging microscopy (FLIM) and extracellular flux analyses. Redox Biol. 2020, 30, 101420. [Google Scholar] [CrossRef] [PubMed]
- González-Vera, J.A.; Bouzada, D.; Bouclier, C.; Eugenio Vázquez, M.; Morris, M.C. Lanthanide-based peptide biosensor to monitor CDK4/cyclin D kinase activity. Chem. Commun. 2017, 53, 6109–6112. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, M.C.; Herrero-Foncubierta, P.; Castro, S.; Resa, S.; Alvarez-Pez, J.M.; Miguel, D.; Cuerva, J.M.; Garcia-Fernandez, E.; Orte, A. Coupled Excited-State Dynamics in N-Substituted 2-Methoxy-9-Acridones. Front. Chem. 2019, 7, 129. [Google Scholar] [CrossRef]
- Gonzalez-Vera, J.A.; Fueyo-Gonzalez, F.; Alkorta, I.; Peyressatre, M.; Morris, M.C.; Herranz, R. Highly solvatochromic and tunable fluorophores based on a 4,5-quinolimide scaffold: Novel CDK5 probes. Chem. Commun. 2016, 52, 9652–9655. [Google Scholar] [CrossRef]
- Fueyo-González, F.; González-Vera, J.A.; Alkorta, I.; Infantes, L.; Jimeno, M.L.; Aranda, P.; Acuña-Castroviejo, D.; Ruiz-Arias, A.; Orte, A.; Herranz, R. Environment-Sensitive Probes for Illuminating Amyloid Aggregation In Vitro and in Zebrafish. ACS Sens. 2020, 5, 2792–2799. [Google Scholar] [CrossRef]
- Esposito, A.; Venkitaraman, A.R. Enhancing Biochemical Resolution by Hyperdimensional Imaging Microscopy. Biophys. J. 2019, 116, 1815–1822. [Google Scholar] [CrossRef]
- Danylchuk, D.I.; Sezgin, E.; Chabert, P.; Klymchenko, A.S. Redesigning Solvatochromic Probe Laurdan for Imaging Lipid Order Selectively in Cell Plasma Membranes. Anal. Chem. 2020, 92, 14798–14805. [Google Scholar] [CrossRef]
- Marcus, Y. The properties of organic liquids that are relevant to their use as solvating solvents. Chem. Soc. Rev. 1993, 22, 409–416. [Google Scholar] [CrossRef]
- Catalán, J. Toward a Generalized Treatment of the Solvent Effect Based on Four Empirical Scales: Dipolarity (SdP, a New Scale), Polarizability (SP), Acidity (SA), and Basicity (SB) of the Medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Bevington, P.R.; Robinson, D.K. Data Reduction and Error Analysis for the Physical Sciences, 3rd ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Garcia, M.C.; Herrero-Foncubierta, P.; Garcia-Fernandez, E.; Orte, A. Building Accurate Intracellular Polarity Maps through Multiparametric Microscopy. Methods Protoc. 2020, 3, 78. https://doi.org/10.3390/mps3040078
Gonzalez-Garcia MC, Herrero-Foncubierta P, Garcia-Fernandez E, Orte A. Building Accurate Intracellular Polarity Maps through Multiparametric Microscopy. Methods and Protocols. 2020; 3(4):78. https://doi.org/10.3390/mps3040078
Chicago/Turabian StyleGonzalez-Garcia, M. Carmen, Pilar Herrero-Foncubierta, Emilio Garcia-Fernandez, and Angel Orte. 2020. "Building Accurate Intracellular Polarity Maps through Multiparametric Microscopy" Methods and Protocols 3, no. 4: 78. https://doi.org/10.3390/mps3040078
APA StyleGonzalez-Garcia, M. C., Herrero-Foncubierta, P., Garcia-Fernandez, E., & Orte, A. (2020). Building Accurate Intracellular Polarity Maps through Multiparametric Microscopy. Methods and Protocols, 3(4), 78. https://doi.org/10.3390/mps3040078






