Investigating the Role of Telomere and Telomerase Associated Genes and Proteins in Endometrial Cancer
Abstract
:1. Introduction
2. Experimental Design
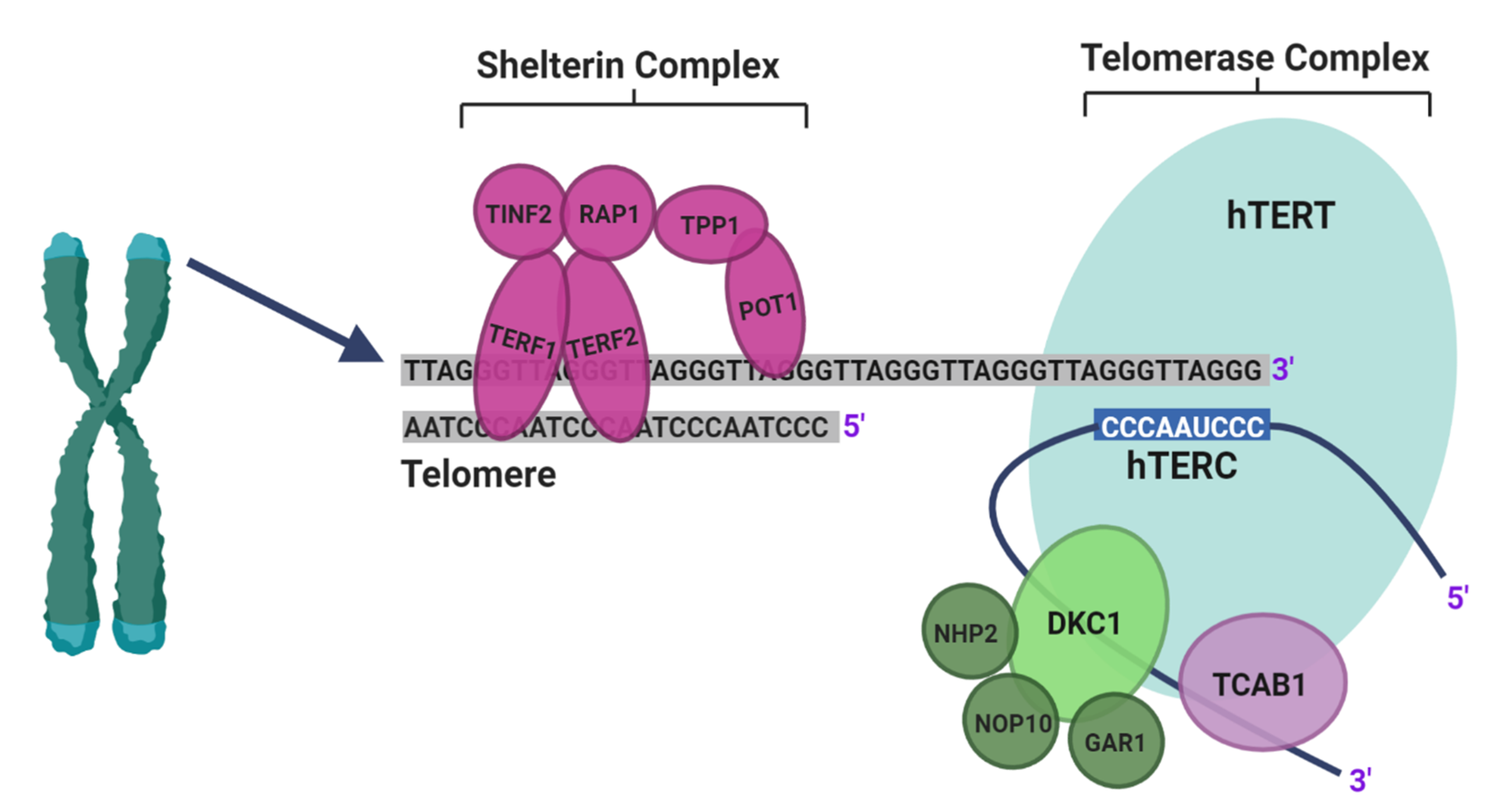
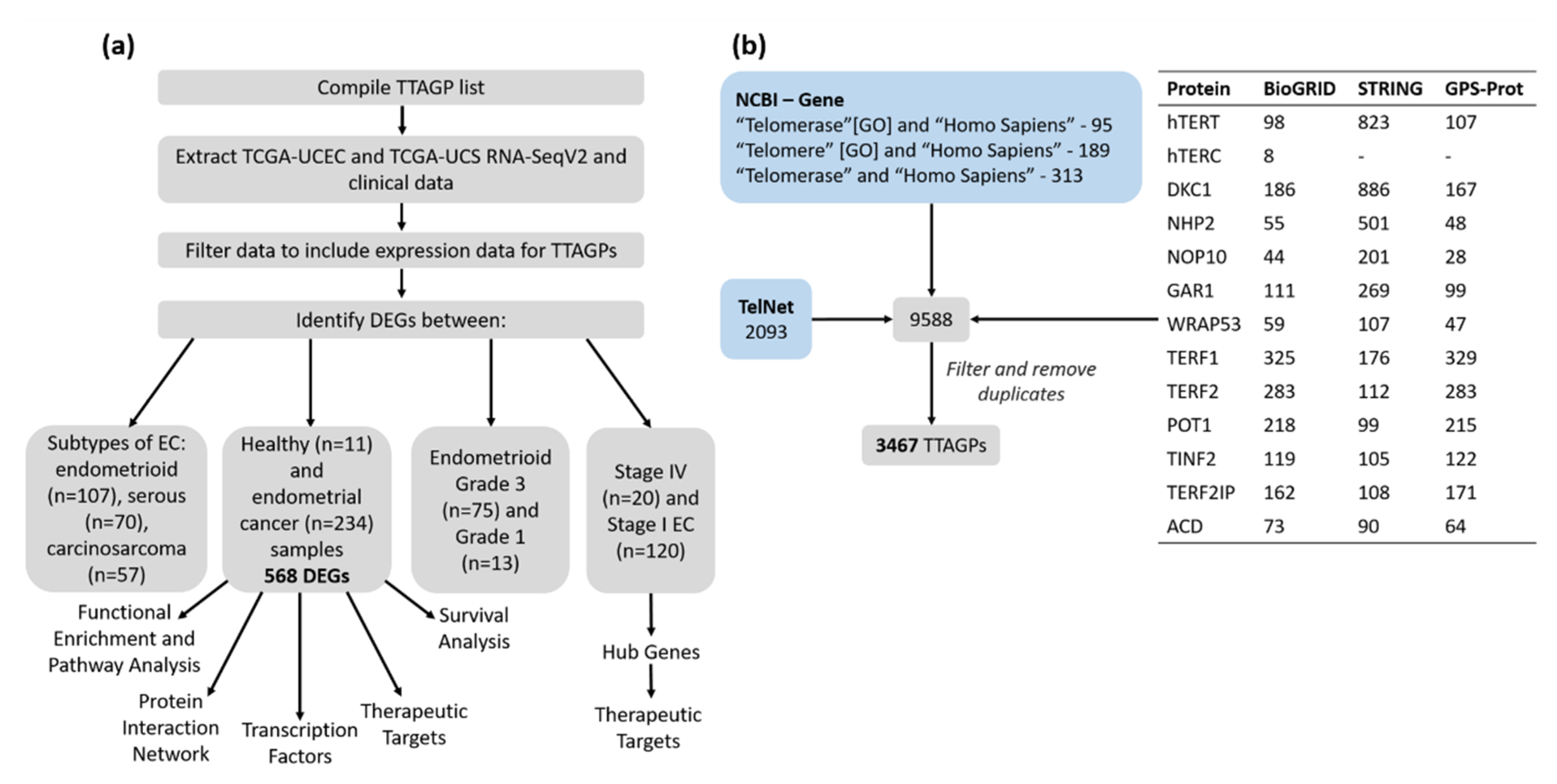
2.1. Identification of TTAGPs
2.2. TCGA Data Cohort
2.3. Identification of Differentially Expressed Genes (DEGs)
2.4. Functional Enrichment and Pathway Analysis
2.5. Protein–Protein Interaction (PPI) Network
2.6. Identification of Key Transcription Factors (TFs)
2.7. Therapeutic Targets
2.8. Survival Analysis
3. Results
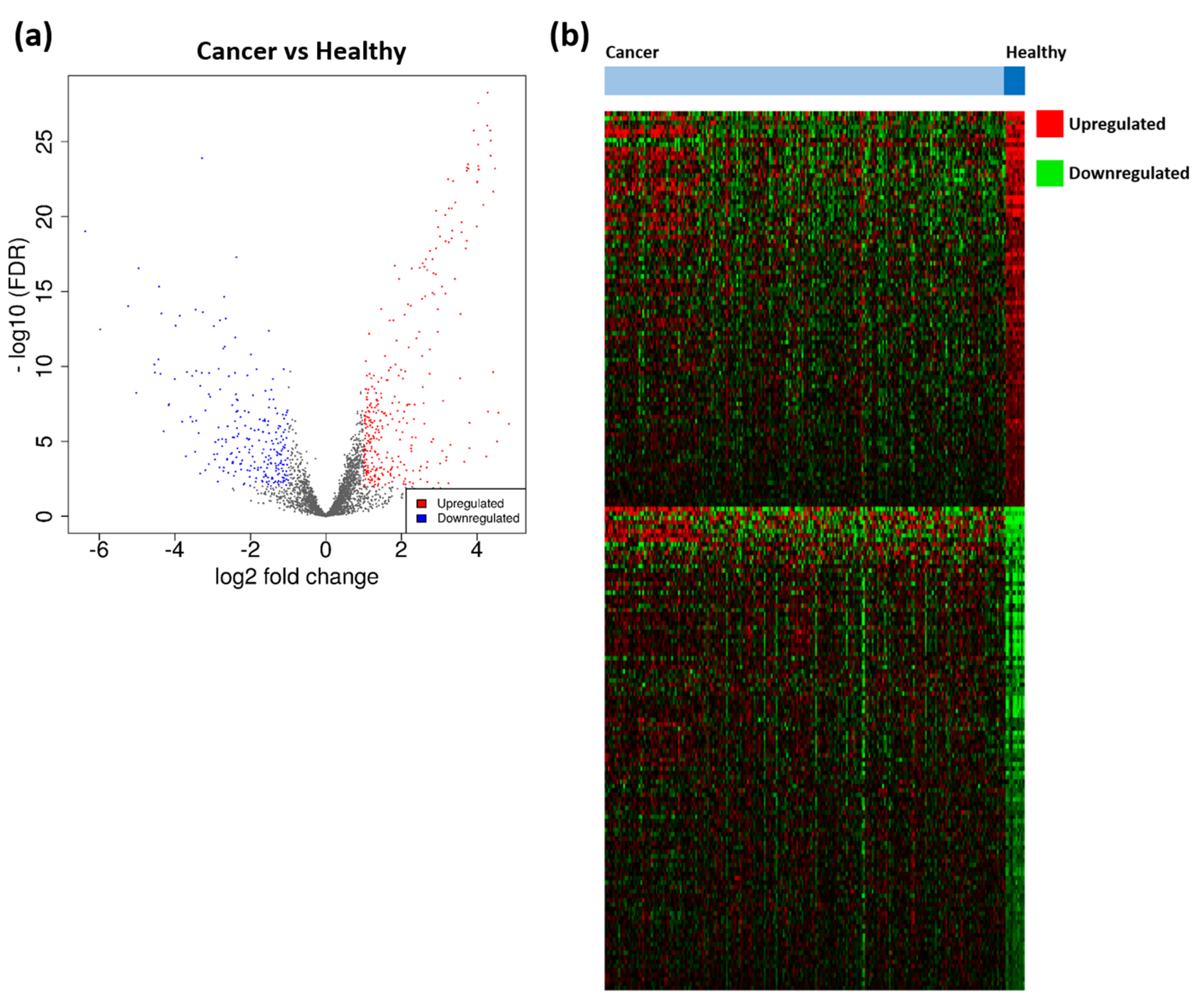
3.1. Identification of TTAGPs and EC-Associated DEGs
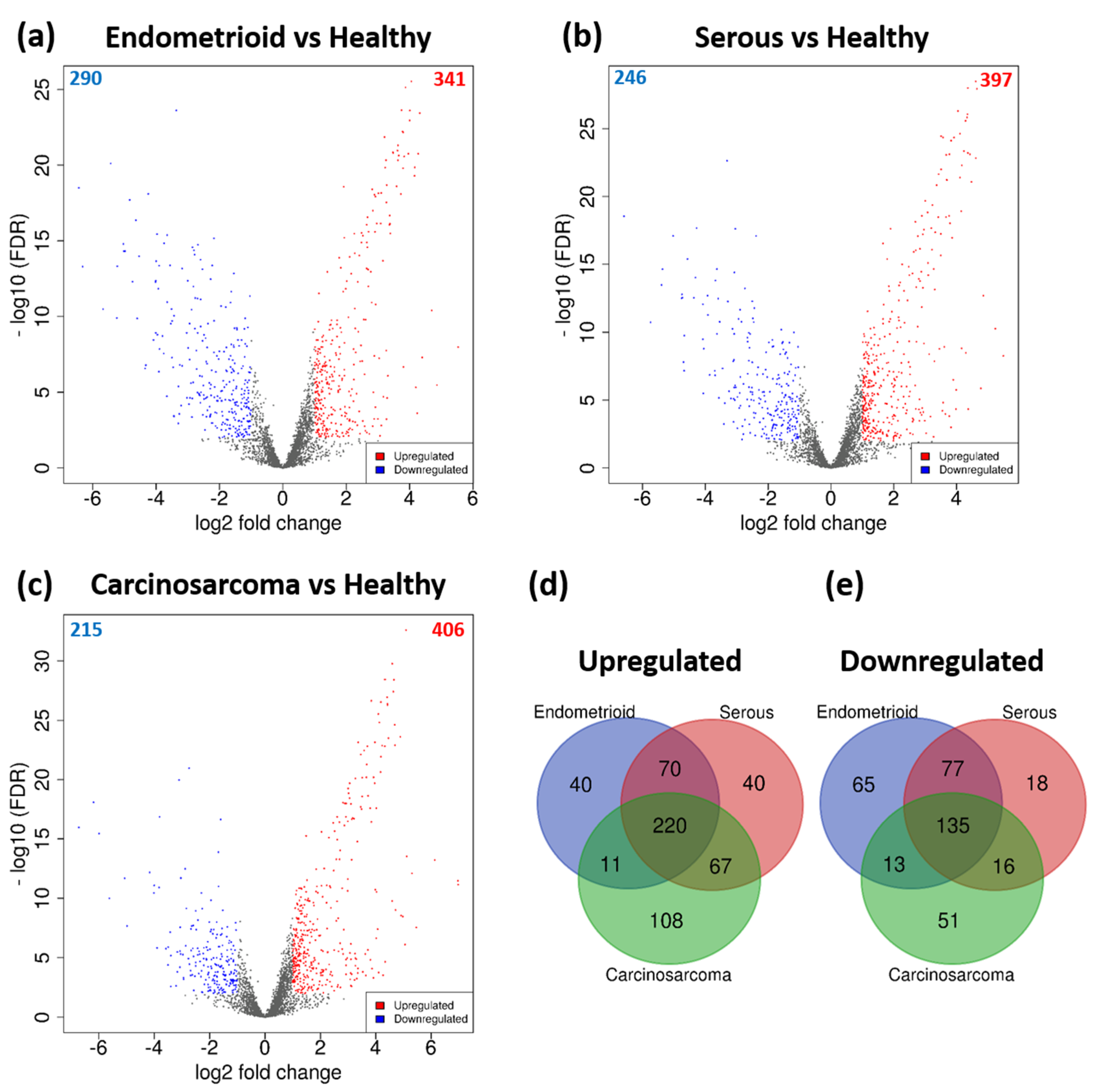
3.2. DEGs Associated with Histological Subtypes of EC
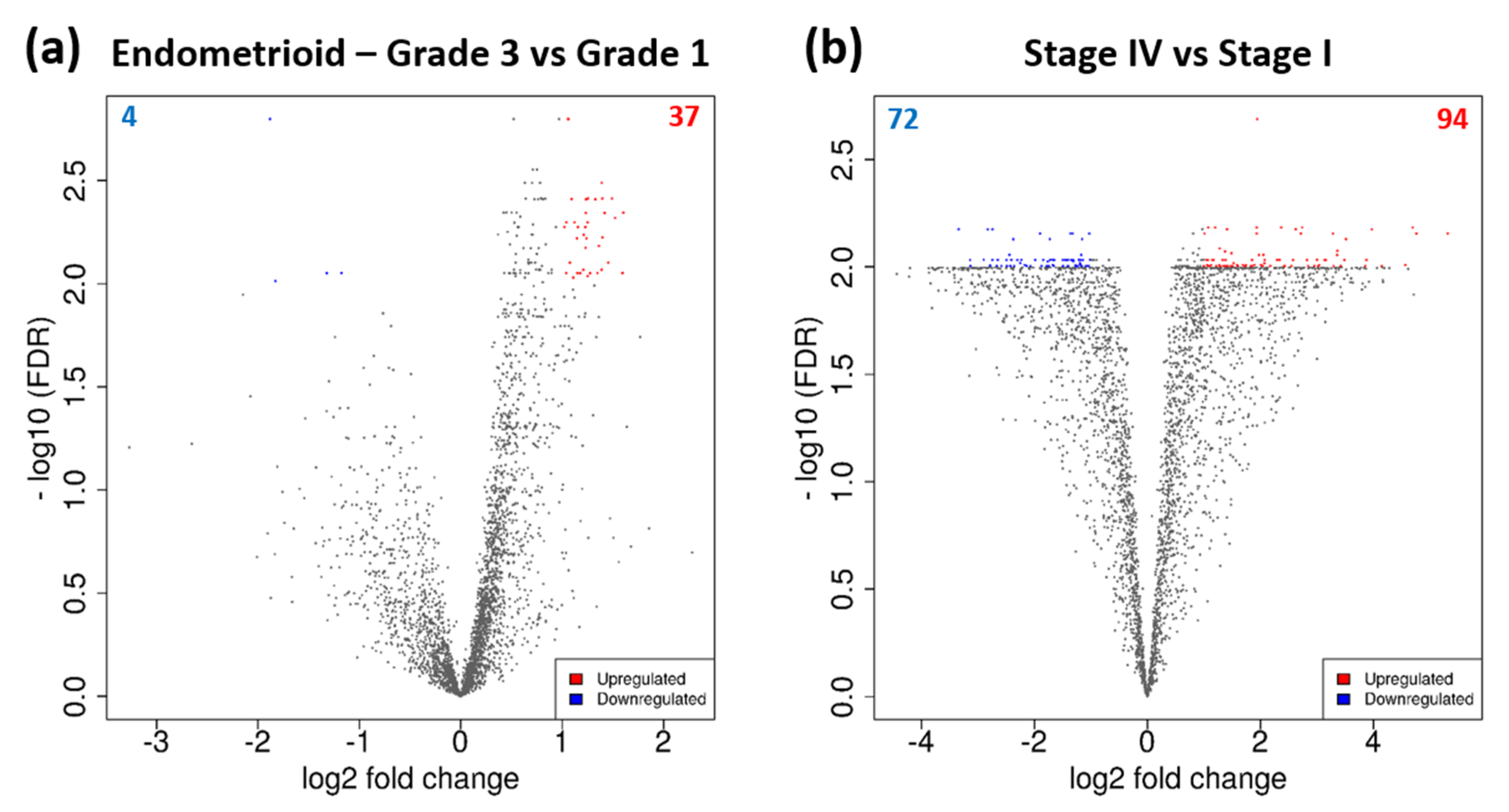
3.3. DEGs Associated with Tumour Grade and Clinical Stage
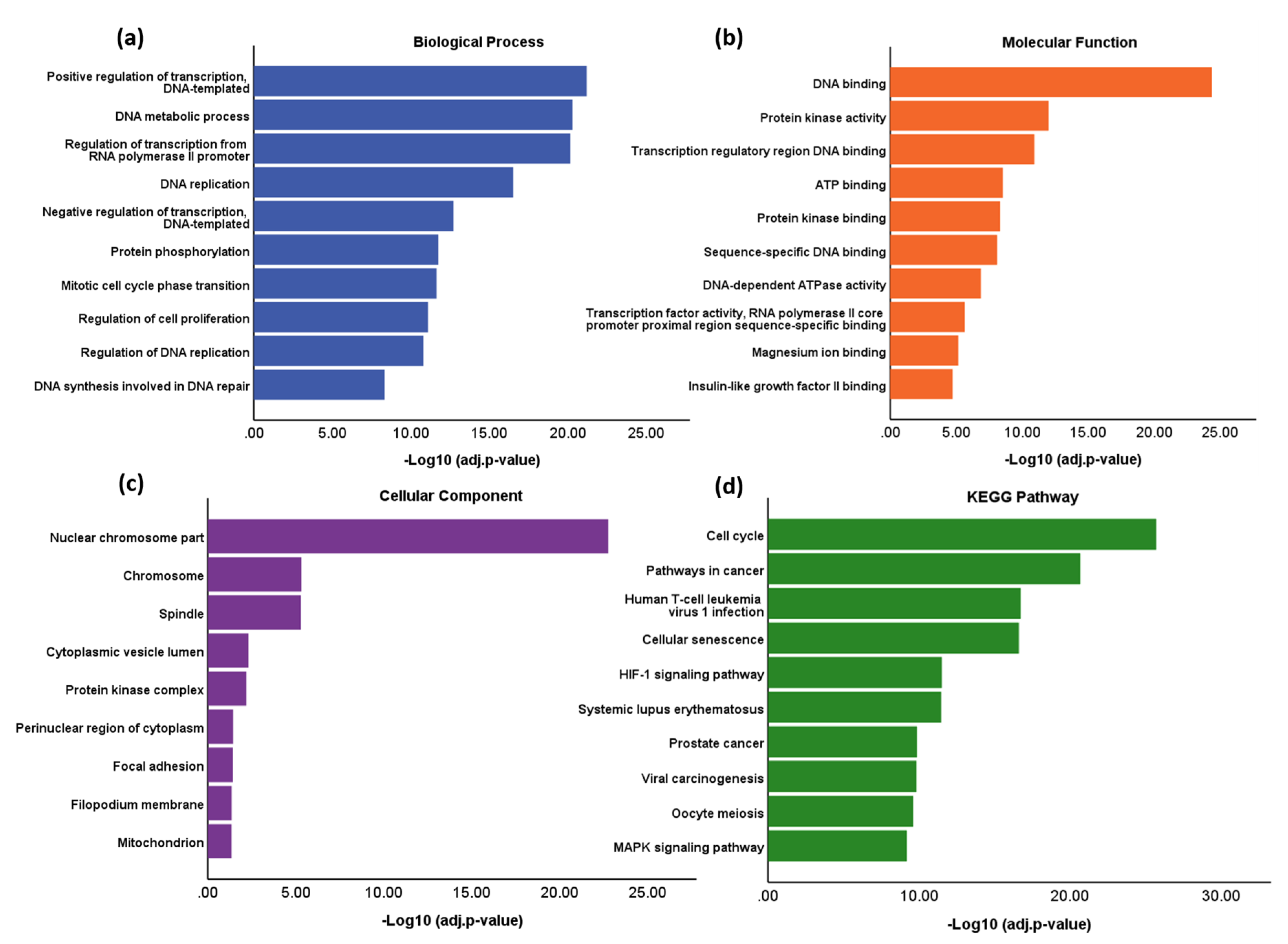
3.4. Functional Enrichment and Pathway Analysis
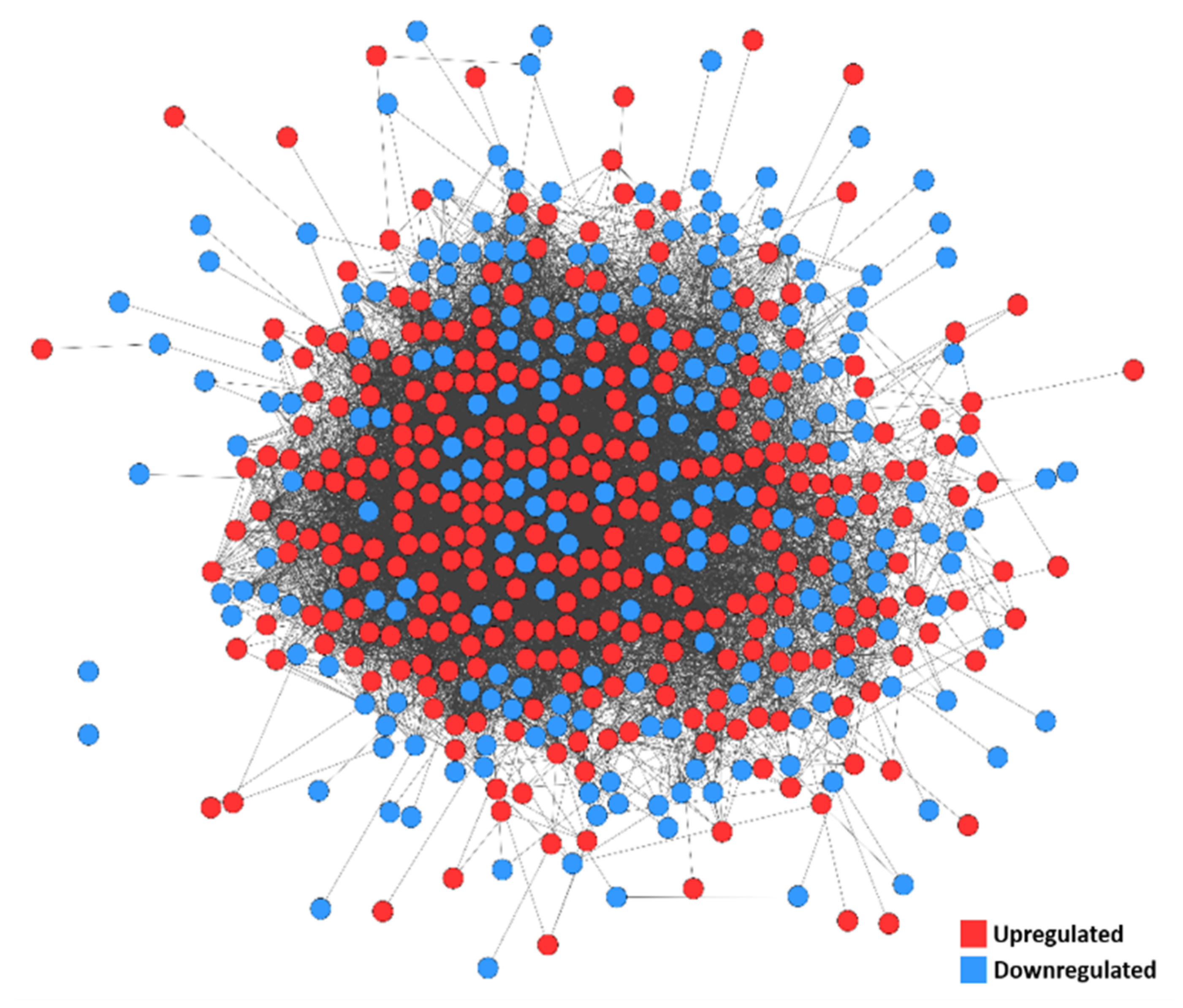
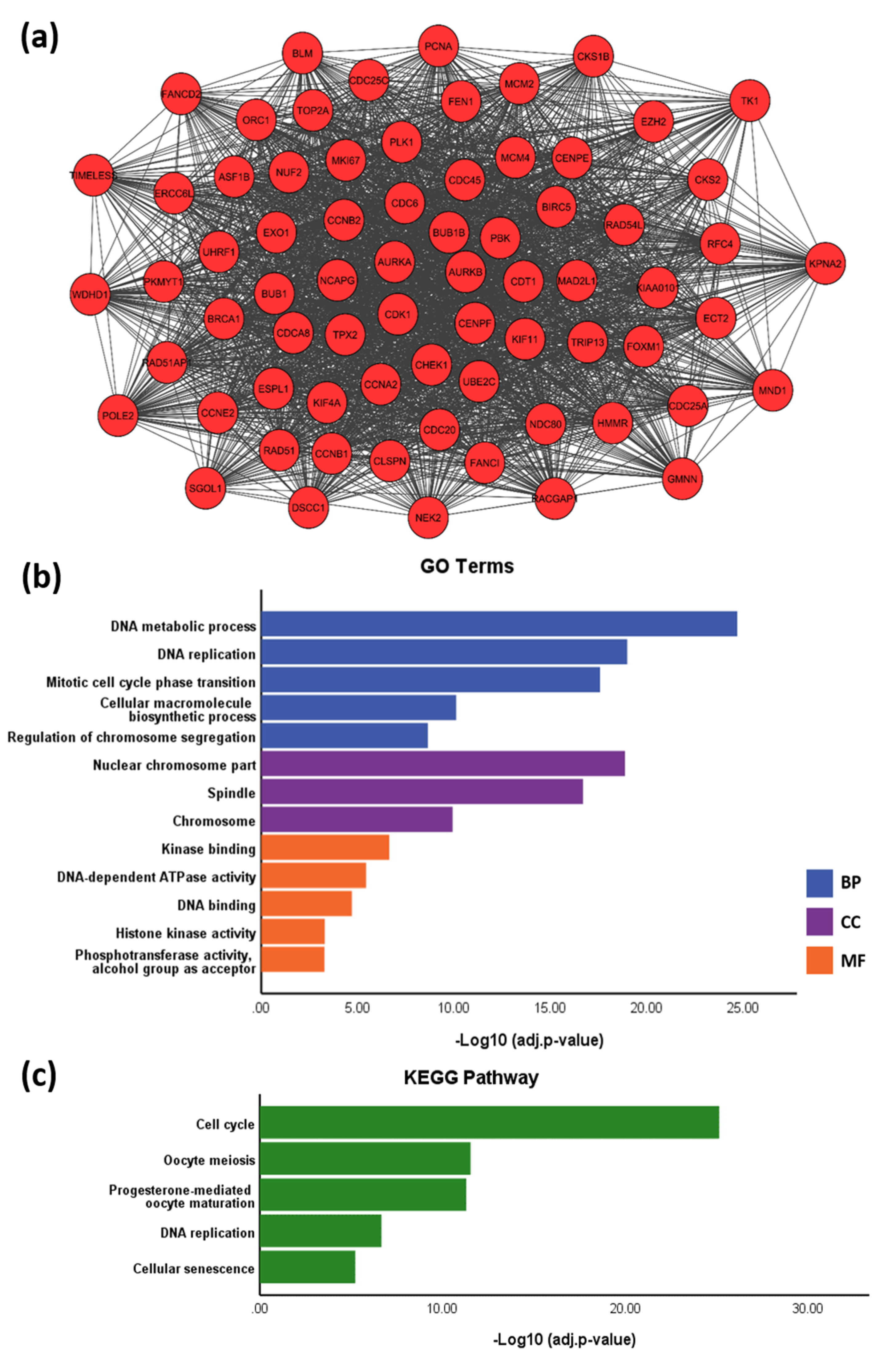
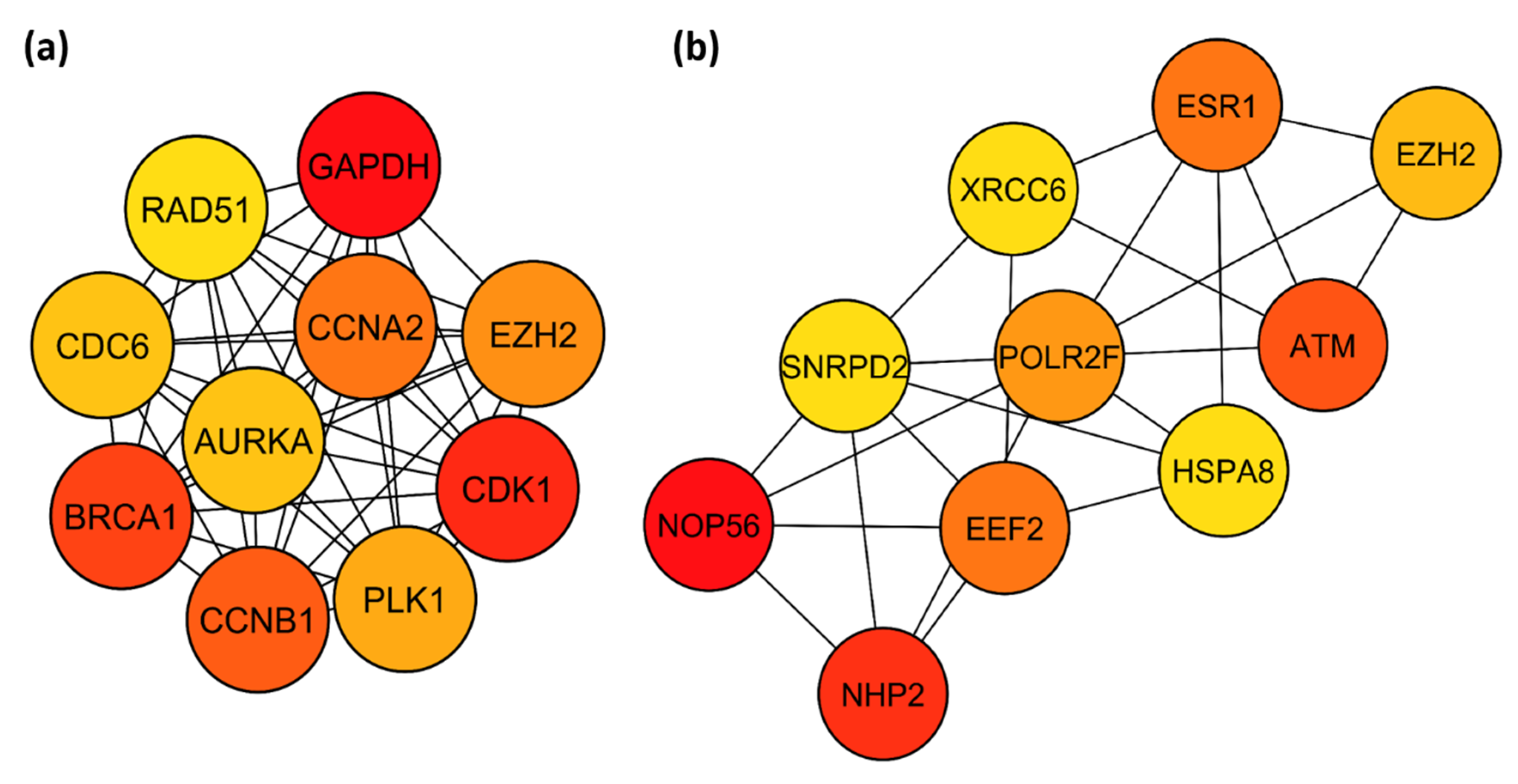
3.5. PPI Network
3.6. Identification of Key TFs
3.7. Therapeutic Targets
3.8. Survival Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Uterine Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer (accessed on 9 November 2019).
- Audet-Delage, Y.; Gregoire, J.; Caron, P.; Turcotte, V.; Plante, M.; Ayotte, P.; Simonyan, D.; Villeneuve, L.; Guillemette, C. Estradiol metabolites as biomarkers of endometrial cancer prognosis after surgery. J. Steroid Biochem. Mol. Biol. 2018, 178, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitson, S.J.; Evans, D.G.; Crosbie, E.J. Identifying High-Risk Women for Endometrial Cancer Prevention Strategies: Proposal of an Endometrial Cancer Risk Prediction Model. Cancer Prev. Res. 2017, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billingsley, C.C.; Cansino, C.; O’Malley, D.M.; Cohn, D.E.; Fowler, J.M.; Copeland, L.J.; Backes, F.J.; Salani, R. Survival outcomes of obese patients in type II endometrial cancer: Defining the prognostic impact of increasing BMI. Gynecol. Oncol. 2016, 140, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Buhtoiarova, T.N.; Brenner, C.A.; Singh, M. Endometrial Carcinoma: Role of Current and Emerging Biomarkers in Resolving Persistent Clinical Dilemmas. Am. J. Clin. Pathol. 2016, 145, 8–21. [Google Scholar] [CrossRef]
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. Cancer of the corpus uteri. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 37–50. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, E.H. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005, 579, 859–862. [Google Scholar] [CrossRef] [Green Version]
- von Zglinicki, T.; Saretzki, G.; Ladhoff, J.; d’Adda di Fagagna, F.; Jackson, S.P. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 2005, 126, 111–117. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Role of telomeres and telomerase in cancer. Semin. Cancer Biol. 2011, 21, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016, 8, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Alnafakh, R.A.; Adishesh, M.; Button, L.; Saretzki, G.; Hapangama, D.K. Telomerase and Telomeres in Endometrial Cancer. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Hapangama, D.K.; Kamal, A.; Saretzki, G. Implications of telomeres and telomerase in endometrial pathology. Hum. Reprod. Update 2017, 23, 166–187. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: Old actors and new players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef] [Green Version]
- Clark, G.M.; Osborne, C.K.; Levitt, D.; Wu, F.; Kim, N.W. Telomerase activity and survival of patients with node-positive breast cancer. J. Natl. Cancer Inst. 1997, 89, 1874–1881. [Google Scholar] [CrossRef]
- Nault, J.C.; Ningarhari, M.; Rebouissou, S.; Zucman-Rossi, J. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 544–558. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Hapangama, D.K.; Turner, M.A.; Drury, J.A.; Quenby, S.; Saretzki, G.; Martin-Ruiz, C.; Von Zglinicki, T. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum. Reprod. 2008, 23, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Kyo, S.; Takakura, M.; Kohama, T.; Inoue, M. Telomerase activity in human endometrium. Cancer Res. 1997, 57, 610–614. [Google Scholar]
- Tanaka, M.; Kyo, S.; Takakura, M.; Kanaya, T.; Sagawa, T.; Yamashita, K.; Okada, Y.; Hiyama, E.; Inoue, M. Expression of telomerase activity in human endometrium is localized to epithelial glandular cells and regulated in a menstrual phase-dependent manner correlated with cell proliferation. Am. J. Pathol. 1998, 153, 1985–1991. [Google Scholar] [CrossRef]
- Valentijn, A.J.; Saretzki, G.; Tempest, N.; Critchley, H.O.; Hapangama, D.K. Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Hum. Reprod. 2015, 30, 2816–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hapangama, D.K.; Kamal, A.M.; Bulmer, J.N. Estrogen receptor β: The guardian of the endometrium. Hum. Reprod. Update 2015, 21, 174–193. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.D.; Boggess, J.F.; LaMarque, L.R.; Meyer, W.R.; Murray, M.J.; Fritz, M.A.; Lessey, B.A. A prospective, randomized study of endometrial telomerase during the menstrual cycle. J. Clin. Endocrinol. Metab. 2001, 86, 3912–3917. [Google Scholar] [CrossRef]
- Kim, J.J.; Chapman-Davis, E. Role of progesterone in endometrial cancer. Semin. Reprod. Med. 2010, 28, 81–90. [Google Scholar] [CrossRef] [Green Version]
- TelNet. TelNet. Available online: http://www.cancertelsys.org/telnet (accessed on 7 May 2020).
- NCBI. Gene. Available online: www.ncbi.nlm.nih.gov/gene/ (accessed on 7 May 2020).
- Stark, C.; Breitkreutz, B.J.; Reguly, T.; Boucher, L.; Breitkreutz, A.; Tyers, M. BioGRID: A general repository for interaction datasets. Nucleic Acids Res 2006, 34, D535–D539. [Google Scholar] [CrossRef] [Green Version]
- BioGRID. BioGRID. Available online: https://thebiogrid.org/ (accessed on 4 June 2020).
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- STRING. STRING. Available online: https://string-db.org (accessed on 4 June 2020).
- Fahey, M.E.; Bennett, M.J.; Mahon, C.; Jäger, S.; Pache, L.; Kumar, D.; Shapiro, A.; Rao, K.; Chanda, S.K.; Craik, C.S.; et al. GPS-Prot: A web-based visualization platform for integrating host-pathogen interaction data. BMC Bioinform. 2011, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- GPS-Prot. GPS-Prot. Available online: http://gpsprot.org/ (accessed on 4 June 2020).
- Braun, D.M.; Chung, I.; Kepper, N.; Deeg, K.I.; Rippe, K. TelNet—A database for human and yeast genes involved in telomere maintenance. BMC Genet. 2018, 19, 32. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef] [Green Version]
- Ho Sui, S.J.; Mortimer, J.R.; Arenillas, D.J.; Brumm, J.; Walsh, C.J.; Kennedy, B.P.; Wasserman, W.W. oPOSSUM: Identification of over-represented transcription factor binding sites in co-expressed genes. Nucleic Acids Res. 2005, 33, 3154–3164. [Google Scholar] [CrossRef] [Green Version]
- Mathew, D.; Drury, J.A.; Valentijn, A.J.; Vasieva, O.; Hapangama, D.K. In silico, in vitro and in vivo analysis identifies a potential role for steroid hormone regulation of FOXD3 in endometriosis-associated genes. Hum. Reprod. 2016, 31, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Cotto, K.C.; Wagner, A.H.; Feng, Y.Y.; Kiwala, S.; Coffman, A.C.; Spies, G.; Wollam, A.; Spies, N.C.; Griffith, O.L.; Griffith, M. DGIdb 3.0: A redesign and expansion of the drug-gene interaction database. Nucleic Acids Res. 2018, 46, D1068–D1073. [Google Scholar] [CrossRef] [Green Version]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Wang, W.; Li, F.; Zhou, S.; Feng, X.; Xin, C.; Dai, Z.; Xiong, Y. Pan-cancer analysis identifies telomerase-associated signatures and cancer subtypes. Mol. Cancer 2019, 18, 106. [Google Scholar] [CrossRef] [Green Version]
- Cerone, M.A.; Burgess, D.J.; Naceur-Lombardelli, C.; Lord, C.J.; Ashworth, A. High-throughput RNAi screening reveals novel regulators of telomerase. Cancer Res. 2011, 71, 3328–3340. [Google Scholar] [CrossRef] [Green Version]
- Ramlee, M.K.; Wang, J.; Toh, W.X.; Li, S. Transcription Regulation of the Human Telomerase Reverse Transcriptase (hTERT) Gene. Genes 2016, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Uziel, O.; Yosef, N.; Sharan, R.; Ruppin, E.; Kupiec, M.; Kushnir, M.; Beery, E.; Cohen-Diker, T.; Nordenberg, J.; Lahav, M. The effects of telomere shortening on cancer cells: A network model of proteomic and microRNA analysis. Genomics 2015, 105, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Gay-Bellile, M.; Véronèse, L.; Combes, P.; Eymard-Pierre, E.; Kwiatkowski, F.; Dauplat, M.M.; Cayre, A.; Privat, M.; Abrial, C.; Bignon, Y.J.; et al. TERT promoter status and gene copy number gains: Effect on TERT expression and association with prognosis in breast cancer. Oncotarget 2017, 8, 77540–77551. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Matsuse, M.; Saenko, V.; Nakao, T.; Yamanouchi, K.; Sakimura, C.; Yano, H.; Nishihara, E.; Hirokawa, M.; Suzuki, K.; et al. TERT mRNA Expression as a Novel Prognostic Marker in Papillary Thyroid Carcinomas. Thyroid 2019, 29, 1105–1114. [Google Scholar] [CrossRef]
- Hugdahl, E.; Kalvenes, M.B.; Mannelqvist, M.; Ladstein, R.G.; Akslen, L.A. Prognostic impact and concordance of TERT promoter mutation and protein expression in matched primary and metastatic cutaneous melanoma. Br. J. Cancer 2018, 118, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Kulić, A.; Plavetić, N.D.; Gamulin, S.; Jakić-Razumović, J.; Vrbanec, D.; Sirotković-Skerlev, M. Telomerase activity in breast cancer patients: Association with poor prognosis and more aggressive phenotype. Med. Oncol. 2016, 33, 23. [Google Scholar] [CrossRef]
- Fernández-Marcelo, T.; Sánchez-Pernaute, A.; Pascua, I.; De Juan, C.; Head, J.; Torres-García, A.J.; Iniesta, P. Clinical Relevance of Telomere Status and Telomerase Activity in Colorectal Cancer. PLoS ONE 2016, 11, e0149626. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Casla, M.T.; Vidaurreta, M.; Sanchez-Rueda, D.; Maestro, M.L.; Arroyo, M.; Cerdán, F.J. Telomerase activity as a prognostic factor in colorectal cancer. Onkologie 2005, 28, 553–557. [Google Scholar] [CrossRef]
- Long, N.; Liu, N.; Liu, X.L.; Li, J.; Cai, B.Y.; Cai, X. Endometrial expression of telomerase, progesterone, and estrogen receptors during the implantation window in patients with recurrent implantation failure. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Lehner, R.; Enomoto, T.; McGregor, J.A.; Shroyer, A.L.; Haugen, B.R.; Pugazhenthi, U.; Shroyer, K.R. Quantitative analysis of telomerase hTERT mRNA and telomerase activity in endometrioid adenocarcinoma and in normal endometrium. Gynecol. Oncol. 2002, 84, 120–125. [Google Scholar] [CrossRef]
- Maida, Y.; Kyo, S.; Kanaya, T.; Wang, Z.; Tanaka, M.; Yatabe, N.; Nakamura, M.; Inoue, M. Is the telomerase assay useful for screening of endometrial lesions? Int. J. Cancer 2002, 100, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Delbono, O.; Wang, Z.M.; Messi, M.L.; Girard, T.; Urwyler, A.; Treves, S.; Zorzato, F. JP-45/JSRP1 variants affect skeletal muscle excitation-contraction coupling by decreasing the sensitivity of the dihydropyridine receptor. Hum. Mutat. 2013, 34, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.Y.; Horn, H.F.; Stewart, C.L.; Burke, B.; Bolcun-Filas, E.; Schimenti, J.C.; Dresser, M.E.; Pezza, R.J. Mechanism and regulation of rapid telomere prophase movements in mouse meiotic chromosomes. Cell Rep. 2015, 11, 551–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavropoulos, D.J.; Bradshaw, P.S.; Li, X.; Pasic, I.; Truong, K.; Ikura, M.; Ungrin, M.; Meyn, M.S. The Bloom syndrome helicase BLM interacts with TRF2 in ALT cells and promotes telomeric DNA synthesis. Hum. Mol. Genet. 2002, 11, 3135–3144. [Google Scholar] [CrossRef] [Green Version]
- Sobinoff, A.P.; Allen, J.A.; Neumann, A.A.; Yang, S.F.; Walsh, M.E.; Henson, J.D.; Reddel, R.R.; Pickett, H.A. BLM and SLX4 play opposing roles in recombination-dependent replication at human telomeres. EMBO J. 2017, 36, 2907–2919. [Google Scholar] [CrossRef]
- Saharia, A.; Stewart, S.A. FEN1 contributes to telomere stability in ALT-positive tumor cells. Oncogene 2009, 28, 1162–1167. [Google Scholar] [CrossRef] [Green Version]
- Gomez, D.E.; Armando, R.G.; Farina, H.G.; Menna, P.L.; Cerrudo, C.S.; Ghiringhelli, P.D.; Alonso, D.F. Telomere structure and telomerase in health and disease (review). Int. J. Oncol. 2012, 41, 1561–1569. [Google Scholar] [CrossRef] [Green Version]
- Cunniff, C.; Bassetti, J.A.; Ellis, N.A. Bloom’s Syndrome: Clinical Spectrum, Molecular Pathogenesis, and Cancer Predisposition. Mol. Syndromol. 2017, 8, 4–23. [Google Scholar] [CrossRef]
- Gruber, S.B.; Ellis, N.A.; Scott, K.K.; Almog, R.; Kolachana, P.; Bonner, J.D.; Kirchhoff, T.; Tomsho, L.P.; Nafa, K.; Pierce, H.; et al. BLM heterozygosity and the risk of colorectal cancer. Science 2002, 297, 2013. [Google Scholar] [CrossRef]
- Li, W.Q.; Hu, N.; Hyland, P.L.; Gao, Y.; Wang, Z.M.; Yu, K.; Su, H.; Wang, C.Y.; Wang, L.M.; Chanock, S.J.; et al. Genetic variants in DNA repair pathway genes and risk of esophageal squamous cell carcinoma and gastric adenocarcinoma in a Chinese population. Carcinogenesis 2013, 34, 1536–1542. [Google Scholar] [CrossRef]
- Yang, M.; Guo, H.; Wu, C.; He, Y.; Yu, D.; Zhou, L.; Wang, F.; Xu, J.; Tan, W.; Wang, G.; et al. Functional FEN1 polymorphisms are associated with DNA damage levels and lung cancer risk. Hum. Mutat. 2009, 30, 1320–1328. [Google Scholar] [CrossRef]
- Sang, Y.; Bo, L.; Gu, H.; Yang, W.; Chen, Y. Flap endonuclease-1 rs174538 G>A polymorphisms are associated with the risk of esophageal cancer in a Chinese population. Thorac. Cancer 2017, 8, 192–196. [Google Scholar] [CrossRef]
- Hurtado, A.; Holmes, K.A.; Ross-Innes, C.S.; Schmidt, D.; Carroll, J.S. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat. Genet. 2011, 43, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Jozwik, K.M.; Chernukhin, I.; Serandour, A.A.; Nagarajan, S.; Carroll, J.S. FOXA1 Directs H3K4 Monomethylation at Enhancers via Recruitment of the Methyltransferase MLL3. Cell Rep. 2016, 17, 2715–2723. [Google Scholar] [CrossRef] [Green Version]
- Qiu, M.; Bao, W.; Wang, J.; Yang, T.; He, X.; Liao, Y.; Wan, X. FOXA1 promotes tumor cell proliferation through AR involving the Notch pathway in endometrial cancer. BMC Cancer 2014, 14, 78. [Google Scholar] [CrossRef]
- Wang, J.; Bao, W.; Qiu, M.; Liao, Y.; Che, Q.; Yang, T.; He, X.; Qiu, H.; Wan, X. Forkhead-box A1 suppresses the progression of endometrial cancer via crosstalk with estrogen receptor α. Oncol. Rep. 2014, 31, 1225–1234. [Google Scholar] [CrossRef] [Green Version]
- Abe, Y.; Ijichi, N.; Ikeda, K.; Kayano, H.; Horie-Inoue, K.; Takeda, S.; Inoue, S. Forkhead box transcription factor, forkhead box A1, shows negative association with lymph node status in endometrial cancer, and represses cell proliferation and migration of endometrial cancer cells. Cancer Sci. 2012, 103, 806–812. [Google Scholar] [CrossRef]
- Shi, S.; Tan, Q.; Feng, F.; Huang, H.; Liang, J.; Cao, D.; Wang, Z. Identification of core genes in the progression of endometrial cancer and cancer cell-derived exosomes by an integrative analysis. Sci. Rep. 2020, 10, 9862. [Google Scholar] [CrossRef]
- Tong, X.; Wang, S.; Lei, Z.; Li, C.; Zhang, C.; Su, Z.; Liu, X.; Zhao, J.; Zhang, H.T. MYOCD and SMAD3/SMAD4 form a positive feedback loop and drive TGF-β-induced epithelial-mesenchymal transition in non-small cell lung cancer. Oncogene 2020, 39, 2890–2904. [Google Scholar] [CrossRef]
- Madonna, R.; De Caterina, R.; Willerson, J.T.; Geng, Y.J. Biologic function and clinical potential of telomerase and associated proteins in cardiovascular tissue repair and regeneration. Eur. Heart J. 2011, 32, 1190–1196. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhang, W.; Qian, J.; Wu, J.; Jiang, L.; Ling, C. R-spondin family members as novel biomarkers and prognostic factors in lung cancer. Oncol. Lett. 2019, 18, 4008–4015. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Chan, H.; Cash, D.D.; Miracco, E.J.; Ogorzalek Loo, R.R.; Upton, H.E.; Cascio, D.; O’Brien Johnson, R.; Collins, K.; Loo, J.A.; et al. Structure of Tetrahymena telomerase reveals previously unknown subunits, functions, and interactions. Science 2015, 350, aab4070. [Google Scholar] [CrossRef] [Green Version]
- Herold, T.; Jurinovic, V.; Mulaw, M.; Seiler, T.; Dufour, A.; Schneider, S.; Kakadia, P.M.; Feuring-Buske, M.; Braess, J.; Spiekermann, K.; et al. Expression analysis of genes located in the minimally deleted regions of 13q14 and 11q22-23 in chronic lymphocytic leukemia-unexpected expression pattern of the RHO GTPase activator ARHGAP20. Genes Chromosomes Cancer 2011, 50, 546–558. [Google Scholar] [CrossRef]
- Giannone, R.J.; McDonald, H.W.; Hurst, G.B.; Shen, R.F.; Wang, Y.; Liu, Y. The protein network surrounding the human telomere repeat binding factors TRF1, TRF2, and POT1. PLoS ONE 2010, 5, e12407. [Google Scholar] [CrossRef]
- Ohishi, T.; Hirota, T.; Tsuruo, T.; Seimiya, H. TRF1 Mediates Mitotic Abnormalities Induced by Aurora—A Overexpression. Cancer Res. 2010, 70, 2041. [Google Scholar] [CrossRef] [Green Version]
- Qi, D.L.; Ohhira, T.; Fujisaki, C.; Inoue, T.; Ohta, T.; Osaki, M.; Ohshiro, E.; Seko, T.; Aoki, S.; Oshimura, M.; et al. Identification of PITX1 as a TERT suppressor gene located on human chromosome 5. Mol. Cell. Biol. 2011, 31, 1624–1636. [Google Scholar] [CrossRef] [Green Version]
- Yoon, G.; Kim, Y.S.; Kim, B.G.; Bae, D.S.; Lee, J.W. Long-term recurrence-free survival in a patient with stage IVB uterine carcinosarcoma. J. Gynecol. Oncol. 2011, 22, 292–294. [Google Scholar] [CrossRef]
- Arora, A.; Abdel-Fatah, T.M.; Agarwal, D.; Doherty, R.; Moseley, P.M.; Aleskandarany, M.A.; Green, A.R.; Ball, G.; Alshareeda, A.T.; Rakha, E.A.; et al. Transcriptomic and Protein Expression Analysis Reveals Clinicopathological Significance of Bloom Syndrome Helicase (BLM) in Breast Cancer. Mol. Cancer 2015, 14, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Chen, Z.; Zheng, Y.; Liang, J.; Hu, Z.; Bian, Y.; Jiang, T.; Li, M.; Zhan, C.; Feng, M.; et al. Identification of cancer stem cell-related biomarkers in lung adenocarcinoma by stemness index and weighted correlation network analysis. J. Cancer Res. Clin. Oncol. 2020, 146, 1463–1472. [Google Scholar] [CrossRef]
- Li, D.; Zhu, J.; Firozi, P.F.; Abbruzzese, J.L.; Evans, D.B.; Cleary, K.; Friess, H.; Sen, S. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin. Cancer Res. 2003, 9, 991–997. [Google Scholar]
- Sen, S.; Zhou, H.; Zhang, R.D.; Yoon, D.S.; Vakar-Lopez, F.; Ito, S.; Jiang, F.; Johnston, D.; Grossman, H.B.; Ruifrok, A.C.; et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J. Natl. Cancer Inst. 2002, 94, 1320–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Zhao, C.; Jiang, L.; Lin, S.; Bi, J.; Wei, Q.; Yu, L.; Zhao, L.; Wei, M. High PITX1 expression in lung adenocarcinoma patients is associated with DNA methylation and poor prognosis. Pathol. Res. Pract. 2018, 214, 2046–2053. [Google Scholar] [CrossRef]
- Umene, K.; Yanokura, M.; Banno, K.; Irie, H.; Adachi, M.; Iida, M.; Nakamura, K.; Nogami, Y.; Masuda, K.; Kobayashi, Y.; et al. Aurora kinase A has a significant role as a therapeutic target and clinical biomarker in endometrial cancer. Int. J. Oncol. 2015, 46, 1498–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, O.H.; Kim, H.; He, Q.; Baek, H.J.; Yang, D.; Chen, L.Y.; Liang, J.; Chae, H.K.; Safari, A.; Liu, D.; et al. Genome-wide YFP fluorescence complementation screen identifies new regulators for telomere signaling in human cells. Mol. Cell. Proteom. 2011, 10, M110.001628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Hu, H.; Lin, F.; Lim, Y.P.; Hua, Y.; Tong, X.; Zhang, S. S100P is Overexpressed in Squamous Cell and Adenosquamous Carcinoma Subtypes of Endometrial Cancer and Promotes Cancer Cell Proliferation and Invasion. Cancer Investig. 2016, 34, 477–488. [Google Scholar] [CrossRef]
- El Hajj, J.; Nguyen, E.; Liu, Q.; Bouyer, C.; Adriaenssens, E.; Hilal, G.; Ségal-Bendirdjian, E. Telomerase regulation by the long non-coding RNA H19 in human acute promyelocytic leukemia cells. Mol. Cancer 2018, 17, 85. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Ramzi, N.H.; Yin-Ling, W.; Rose, I.M.; Hatta Mohd Dali, A.Z.; Jamal, R. Laser capture microdissection with genome-wide expression profiling displayed gene expression signatures in endometrioid endometrial cancer. Cancer Investig. 2012, 30, 156–164. [Google Scholar] [CrossRef]
- Ning, Y.; Xu, J.F.; Li, Y.; Chavez, L.; Riethman, H.C.; Lansdorp, P.M.; Weng, N.P. Telomere length and the expression of natural telomeric genes in human fibroblasts. Hum. Mol. Genet. 2003, 12, 1329–1336. [Google Scholar] [CrossRef]
- Ma, H.; Urquidi, V.; Wong, J.; Kleeman, J.; Goodison, S. Telomerase reverse transcriptase promoter regulation during myogenic differentiation of human RD rhabdomyosarcoma cells. Mol. Cancer Res. 2003, 1, 739–746. [Google Scholar]
- Cessna, M.H.; Zhou, H.; Perkins, S.L.; Tripp, S.R.; Layfield, L.; Daines, C.; Coffin, C.M. Are myogenin and myoD1 expression specific for rhabdomyosarcoma? A study of 150 cases, with emphasis on spindle cell mimics. Am. J. Surg. Pathol. 2001, 25, 1150–1157. [Google Scholar] [CrossRef]
- Michelagnoli, M.P.; Burchill, S.A.; Cullinane, C.; Selby, P.J.; Lewis, I.J. Myogenin—A more specific target for RT-PCR detection of rhabdomyosarcoma than MyoD1. Med. Pediatr. Oncol. 2003, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arbajian, E.; Köster, J.; Vult von Steyern, F.; Mertens, F. Inflammatory leiomyosarcoma is a distinct tumor characterized by near-haploidization, few somatic mutations, and a primitive myogenic gene expression signature. Mod. Pathol. 2018, 31, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Idilli, A.I.; Pagani, F.; Kerschbamer, E.; Berardinelli, F.; Bernabé, M.; Cayuela, M.L.; Piazza, S.; Poliani, P.L.; Cusanelli, E.; Mione, M.C. Changes in the Expression of Pre-Replicative Complex Genes in hTERT and ALT Pediatric Brain Tumors. Cancers 2020, 12, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovejoy, C.A.; Li, W.; Reisenweber, S.; Thongthip, S.; Bruno, J.; de Lange, T.; De, S.; Petrini, J.H.; Sung, P.A.; Jasin, M.; et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012, 8, e1002772. [Google Scholar] [CrossRef]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Li, Z.; Wan, Z.; Shao, M.; Wu, S.; Wang, G. Potential Prognostic and Diagnostic Values of CDC6, CDC45, ORC6 and SNHG7 in Colorectal Cancer. OncoTargets Ther. 2019, 12, 11609–11621. [Google Scholar] [CrossRef] [Green Version]
- Chudasama, D.; Bo, V.; Hall, M.; Anikin, V.; Jeyaneethi, J.; Gregory, J.; Pados, G.; Tucker, A.; Harvey, A.; Pink, R.; et al. Identification of cancer biomarkers of prognostic value using specific gene regulatory networks (GRN): A novel role of RAD51AP1 for ovarian and lung cancers. Carcinogenesis 2018, 39, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, H.; Qiao, L.; Jin, X.; Dong, H.; Wang, Y. Silencing of RAD51AP1 suppresses epithelial-mesenchymal transition and metastasis in non-small cell lung cancer. Thorac. Cancer 2019, 10, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Resnick, M.B.; Sabo, E.; Kondratev, S.; Kerner, H.; Spagnoli, G.C.; Yakirevich, E. Cancer-testis antigen expression in uterine malignancies with an emphasis on carcinosarcomas and papillary serous carcinomas. Int. J. Cancer 2002, 101, 190–195. [Google Scholar] [CrossRef]
- Srdelić, S.; Kuzmić-Prusac, I.; Spagnoli, G.C.; Juretić, A.; Čapkun, V. MAGE-A4 and MAGE-A1 Immunohistochemical Expression in High-grade Endometrial Cancer. Int. J. Gynecol. Pathol. 2019, 38, 59–65. [Google Scholar] [CrossRef]
- de Almeida, B.P.; Apolónio, J.D.; Binnie, A.; Castelo-Branco, P. Roadmap of DNA methylation in breast cancer identifies novel prognostic biomarkers. BMC Cancer 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladner, R.D.; Lynch, F.J.; Groshen, S.; Xiong, Y.P.; Sherrod, A.; Caradonna, S.J.; Stoehlmacher, J.; Lenz, H.J. dUTP nucleotidohydrolase isoform expression in normal and neoplastic tissues: Association with survival and response to 5-fluorouracil in colorectal cancer. Cancer Res. 2000, 60, 3493–3503. [Google Scholar] [PubMed]
- Takatori, H.; Yamashita, T.; Honda, M.; Nishino, R.; Arai, K.; Yamashita, T.; Takamura, H.; Ohta, T.; Zen, Y.; Kaneko, S. dUTP pyrophosphatase expression correlates with a poor prognosis in hepatocellular carcinoma. Liver Int. 2010, 30, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Déjardin, J.; Kingston, R.E. Purification of proteins associated with specific genomic Loci. Cell 2009, 136, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, P.F.; Jiang, T.; Chen, F.; Shi, P.C.; Li, H.Q.; Bai, J.; Song, J. KIF4A facilitates cell proliferation via induction of p21-mediated cell cycle progression and promotes metastasis in colorectal cancer. Cell Death Dis. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Arndt, G.M.; MacKenzie, K.L. New prospects for targeting telomerase beyond the telomere. Nat. Rev. Cancer 2016, 16, 508–524. [Google Scholar] [CrossRef]
- Lu, S.; Sung, T.; Amaro, M.; Hirakawa, B.; Jessen, B.; Hu, W. Phenotypic Characterization of Targeted Knockdown of Cyclin-Dependent Kinases in the Intestinal Epithelial Cells. Toxicol. Sci. 2020. [Google Scholar] [CrossRef]
- Chen, F.; Shen, C.; Wang, X.; Wang, H.; Liu, Y.; Yu, C.; Lv, J.; He, J.; Wen, Z. Identification of genes and pathways in nasopharyngeal carcinoma by bioinformatics analysis. Oncotarget 2017, 8, 63738–63749. [Google Scholar] [CrossRef]
- Berus, T.; Markiewicz, A.; Zwierzchowska, K.; Biecek, P.; Orlowska-Heitzman, J.; Romanowska-Dixon, B.; Donizy, P. Downregulation of Polo-like kinase-1 (PLK-1) expression is associated with poor clinical outcome in uveal melanoma patients. Folia Histochem. Cytobiol. 2020. [Google Scholar] [CrossRef]
- Yi, Z.Y.; Meng, T.G.; Ma, X.S.; Li, J.; Zhang, C.H.; Ouyang, Y.C.; Schatten, H.; Qiao, J.; Sun, Q.Y.; Qian, W.P. CDC6 regulates both G2/M transition and metaphase-to-anaphase transition during the first meiosis of mouse oocytes. J. Cell. Physiol. 2020, 235, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yi, Y.; Wu, W.; Wu, K.; Zhang, W. Bioinformatics prediction and analysis of hub genes and pathways of three types of gynecological cancer. Oncol. Lett. 2019, 18, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Lin, J.; He, H. Identification of Potential Crucial Genes Associated with the Pathogenesis and Prognosis of Endometrial Cancer. Front. Genet. 2019, 10, 373. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Li, X.; Li, D.; Zhang, X.; Yin, Y.; Deng, X.; Sheng, X. Prognostic factors and genes associated with endometrial cancer based on gene expression profiling by bioinformatics analysis. Arch. Gynecol. Obs. 2016, 293, 1287–1295. [Google Scholar] [CrossRef]
- Lv, S.; Xu, X.; Wu, Z. Identification of key candidate genes and pathways in endometrial cancer: Evidence from bioinformatics analysis. Oncol. Lett. 2019, 18, 6679–6689. [Google Scholar] [CrossRef] [Green Version]
- Huo, X.; Sun, H.; Cao, D.; Yang, J.; Peng, P.; Yu, M.; Shen, K. Identification of prognosis markers for endometrial cancer by integrated analysis of DNA methylation and RNA-Seq data. Sci. Rep. 2019, 9, 9924. [Google Scholar] [CrossRef]
- Oki, S.; Sone, K.; Oda, K.; Hamamoto, R.; Ikemura, M.; Maeda, D.; Takeuchi, M.; Tanikawa, M.; Mori-Uchino, M.; Nagasaka, K.; et al. Oncogenic histone methyltransferase EZH2: A novel prognostic marker with therapeutic potential in endometrial cancer. Oncotarget 2017, 8, 40402–40411. [Google Scholar] [CrossRef] [Green Version]
- Eskander, R.N.; Ji, T.; Huynh, B.; Wardeh, R.; Randall, L.M.; Hoang, B. Inhibition of enhancer of zeste homolog 2 (EZH2) expression is associated with decreased tumor cell proliferation, migration, and invasion in endometrial cancer cell lines. Int. J. Gynecol. Cancer 2013, 23, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Ihira, K.; Dong, P.; Xiong, Y.; Watari, H.; Konno, Y.; Hanley, S.J.; Noguchi, M.; Hirata, N.; Suizu, F.; Yamada, T.; et al. EZH2 inhibition suppresses endometrial cancer progression via miR-361/Twist axis. Oncotarget 2017, 8, 13509–13520. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Guan, H. Expression of EZH2 in endometrial carcinoma and its effects on proliferation and invasion of endometrial carcinoma cells. Oncol. Lett. 2017, 14, 7191–7196. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.W.; Choi, J.E.; Han, H.D.; Hu, W.; Matsuo, K.; Nishimura, M.; Lee, J.S.; Kwon, S.Y.; Cho, C.H.; Kim, J.; et al. Clinical and biological significance of EZH2 expression in endometrial cancer. Cancer Biol. 2020, 21, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, Y.W.; Li, Y.P. Over-expression of miR-1271 inhibits endometrial cancer cells proliferation and induces cell apoptosis by targeting CDK1. Eur. Rev. Med. Pharm. Sci. 2017, 21, 2816–2822. [Google Scholar]
- Meng, X.; Laidler, L.L.; Kosmacek, E.A.; Yang, S.; Xiong, Z.; Zhu, D.; Wang, X.; Dai, D.; Zhang, Y.; Wang, X.; et al. Induction of mitotic cell death by overriding G2/M checkpoint in endometrial cancer cells with non-functional p53. Gynecol. Oncol. 2013, 128, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.Q.; Yang, X.; Weber, G.; Liu, X. Plk1 phosphorylation of TRF1 is essential for its binding to telomeres. J. Biol. Chem. 2008, 283, 25503–25513. [Google Scholar] [CrossRef] [Green Version]
- Oliviero, G.; Brien, G.L.; Waston, A.; Streubel, G.; Jerman, E.; Andrews, D.; Doyle, B.; Munawar, N.; Wynne, K.; Crean, J.; et al. Dynamic Protein Interactions of the Polycomb Repressive Complex 2 during Differentiation of Pluripotent Cells. Mol. Cell. Proteom. 2016, 15, 3450–3460. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.P.; Cifuentes-Rojas, C.; Kesner, B.; Aeby, E.; Lee, H.G.; Wei, C.; Oh, H.J.; Boukhali, M.; Haas, W.; Lee, J.T. TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell 2017, 170, 86–101. [Google Scholar] [CrossRef] [Green Version]
- Marión, R.M.; Montero, J.J.; López de Silanes, I.; Graña-Castro, O.; Martínez, P.; Schoeftner, S.; Palacios-Fábrega, J.A.; Blasco, M.A. TERRA regulate the transcriptional landscape of pluripotent cells through TRF1-dependent recruitment of PRC2. Elife 2019, 8. [Google Scholar] [CrossRef]
- Townsend, M.H.; Ence, Z.E.; Felsted, A.M.; Parker, A.C.; Piccolo, S.R.; Robison, R.A.; O’Neill, K.L. Potential new biomarkers for endometrial cancer. Cancer Cell Int. 2019, 19, 19. [Google Scholar] [CrossRef]
- Michalska, M.M.; Samulak, D.; Romanowicz, H.; Smolarz, B. Association of polymorphisms in the 5’ untranslated region of RAD51 gene with risk of endometrial cancer in the Polish population. Arch. Gynecol. Obs. 2014, 290, 985–991. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Yang, L.; Xu, H.; Zhang, T.; An, R.; Zhu, K. Association between RAD51 135 G/C polymorphism and risk of 3 common gynecological cancers: A meta-analysis. Medicine 2018, 97, e11251. [Google Scholar] [CrossRef]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Jiang, X.; Lee, W.H.; Chen, P.L. Assembly of functional ALT-associated promyelocytic leukemia bodies requires Nijmegen Breakage Syndrome 1. Cancer Res. 2003, 63, 2589–2595. [Google Scholar] [PubMed]
- Olivier, M.; Charbonnel, C.; Amiard, S.; White, C.I.; Gallego, M.E. RAD51 and RTEL1 compensate telomere loss in the absence of telomerase. Nucleic Acids Res. 2018, 46, 2432–2445. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.S.; Zhou, H.; Yang, L.; Wang, L.; Jiang, Z.S.; Wang, S.M. CCNA2 facilitates epithelial-to-mesenchymal transition via the integrin αvβ3 signaling in NSCLC. Int. J. Clin. Exp. Pathol. 2017, 10, 8324–8333. [Google Scholar]
- Zhou, L.; Li, J.; Zhao, Y.P.; Cui, Q.C.; Zhou, W.X.; Guo, J.C.; You, L.; Wu, W.M.; Zhang, T.P. The prognostic value of Cyclin B1 in pancreatic cancer. Med. Oncol. 2014, 31, 107. [Google Scholar] [CrossRef]
- Bie, L.; Zhao, G.; Ju, Y.; Zhang, B. Integrative genomic analysis identifies CCNB1 and CDC2 as candidate genes associated with meningioma recurrence. Cancer Genet. 2011, 204, 536–540. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, L.; Wang, Y.; Xi, Q.; Zhong, J.; Liu, J.; Yang, S.; Liu, R.; Wang, J.; Huang, M.; et al. High expression of CDC6 is associated with accelerated cell proliferation and poor prognosis of epithelial ovarian cancer. Pathol. Res. Pract. 2016, 212, 239–246. [Google Scholar] [CrossRef]
- Jernman, J.; Välimäki, M.J.; Hagström, J.; Louhimo, J.; Haapasalo, H.; Arola, J.; Haglund, C. Cyclin A predicts metastatic potential of rectal neuroendocrine tumors. Hum. Pathol. 2014, 45, 1605–1609. [Google Scholar] [CrossRef]
- Ben Younes, K.; Doghri, R.; Mrad, K.; Ben Romdhane, N.; Ben Aissa-Fennira, F. Cyclin A2 as a potential differential marker of splenic diffuse red pulp small B-cell lymphoma: A report of the first case. Ann. Hematol. 2017, 96, 511–512. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, H.; Liang, X.; Xu, J.; Cai, X. Chk1-induced CCNB1 overexpression promotes cell proliferation and tumor growth in human colorectal cancer. Cancer Biol. 2014, 15, 1268–1279. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Chen, X.; Xie, G.; He, Y.; Yan, D.; Zheng, D.; Li, S.; Fu, X.; Li, Y.; Pang, X.; et al. Cdc6 contributes to cisplatin-resistance by activation of ATR-Chk1 pathway in bladder cancer cells. Oncotarget 2016, 7, 40362–40376. [Google Scholar] [CrossRef]
- Schek, N.; Hall, B.L.; Finn, O.J. Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in human pancreatic adenocarcinoma. Cancer Res. 1988, 48, 6354–6359. [Google Scholar]
- Révillion, F.; Pawlowski, V.; Hornez, L.; Peyrat, J.P. Glyceraldehyde-3-phosphate dehydrogenase gene expression in human breast cancer. Eur. J. Cancer 2000, 36, 1038–1042. [Google Scholar] [CrossRef]
- Tokunaga, K.; Nakamura, Y.; Sakata, K.; Fujimori, K.; Ohkubo, M.; Sawada, K.; Sakiyama, S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987, 47, 5616–5619. [Google Scholar] [PubMed]
- Hao, L.; Zhou, X.; Liu, S.; Sun, M.; Song, Y.; Du, S.; Sun, B.; Guo, C.; Gong, L.; Hu, J.; et al. Elevated GAPDH expression is associated with the proliferation and invasion of lung and esophageal squamous cell carcinomas. Proteomics 2015, 15, 3087–3100. [Google Scholar] [CrossRef] [PubMed]
- Brzozowa-Zasada, M.; Kurek, J.; Piecuch, A.; Stęplewska, K. Correlation study of GAPDH, Bcl-2, and Bax protein immunoexpression in patients with colorectal adenocarcinoma. Przegla̜d Gastroenterol. 2018, 13, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Ezura, K.; Yoshida, K.; Yugawa, T.; Narisawa-Saito, M.; Kiyono, T.; Ohta, S.; Obuse, C.; Fujita, M. Involvement of human ORC and TRF2 in pre-replication complex assembly at telomeres. Genes Cells 2008, 13, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Przyborski, S.; Cooke, M.J.; Zhang, X.; Stewart, R.; Anyfantis, G.; Atkinson, S.P.; Saretzki, G.; Armstrong, L.; Lako, M. A key role for telomerase reverse transcriptase unit in modulating human embryonic stem cell proliferation, cell cycle dynamics, and in vitro differentiation. Stem Cells 2008, 26, 850–863. [Google Scholar] [CrossRef]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharm. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef]
- Demarse, N.A.; Ponnusamy, S.; Spicer, E.K.; Apohan, E.; Baatz, J.E.; Ogretmen, B.; Davies, C. Direct binding of glyceraldehyde 3-phosphate dehydrogenase to telomeric DNA protects telomeres against chemotherapy-induced rapid degradation. J. Mol. Biol. 2009, 394, 789–803. [Google Scholar] [CrossRef] [Green Version]
- Sundararaj, K.P.; Wood, R.E.; Ponnusamy, S.; Salas, A.M.; Szulc, Z.; Bielawska, A.; Obeid, L.M.; Hannun, Y.A.; Ogretmen, B. Rapid shortening of telomere length in response to ceramide involves the inhibition of telomere binding activity of nuclear glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 2004, 279, 6152–6162. [Google Scholar] [CrossRef] [Green Version]
- The Human Protein Atlas. The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 5 July 2020).
- Wan, C.; Borgeson, B.; Phanse, S.; Tu, F.; Drew, K.; Clark, G.; Xiong, X.; Kagan, O.; Kwan, J.; Bezginov, A.; et al. Panorama of ancient metazoan macromolecular complexes. Nature 2015, 525, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, M.Y.; Hubner, N.C.; Poser, I.; Cox, J.; Nagaraj, N.; Toyoda, Y.; Gak, I.A.; Weisswange, I.; Mansfeld, J.; Buchholz, F.; et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 2015, 163, 712–723. [Google Scholar] [CrossRef] [Green Version]
- Kustatscher, G.; Grabowski, P.; Schrader, T.A.; Passmore, J.B.; Schrader, M.; Rappsilber, J. Co-regulation map of the human proteome enables identification of protein functions. Nat. Biotechnol. 2019, 37, 1361–1371. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Yang, S.; Fang, D. E2F1: A potential negative regulator of hTERT transcription in normal cells upon activation of oncogenic c-Myc. Med. Sci. Monit. 2012, 18, RA12–RA15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, A.; Shen, C.; Zhang, B.; Rao, Z.; Wang, R.; Yang, S.; Ning, S.; Mao, G.; Fang, D. E2F1 acts as a negative feedback regulator of c-Myc-induced hTERT transcription during tumorigenesis. Oncol. Rep. 2014, 32, 1273–1280. [Google Scholar] [CrossRef]
- Yu, P.; Shen, X.; Yang, W.; Zhang, Y.; Liu, C.; Huang, T. ZEB1 stimulates breast cancer growth by up-regulating hTERT expression. Biochem. Biophys. Res. Commun. 2018, 495, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tang, B.; Hu, C.J.; Xiao, Y.F.; Xie, R.; Yong, X.; Wu, Y.Y.; Dong, H.; Yang, S.M. An hTERT/ZEB1 complex directly regulates E-cadherin to promote epithelial-to-mesenchymal transition (EMT) in colorectal cancer. Oncotarget 2016, 7, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Kyo, S.; Takakura, M.; Kanaya, T.; Kitagawa, Y.; Itoh, H.; Takahashi, M.; Inoue, M. Identification and characterization of negative regulatory elements of the human telomerase catalytic subunit (hTERT) gene promoter: Possible role of MZF-2 in transcriptional repression of hTERT. Nucleic Acids Res. 2000, 28, 2557–2562. [Google Scholar] [CrossRef]
- Peterson, M.J.; Morris, J.F. Human myeloid zinc finger gene MZF produces multiple transcripts and encodes a SCAN box protein. Gene 2000, 254, 105–118. [Google Scholar] [CrossRef]
- Ertosun, M.G.; Hapil, F.Z.; Osman Nidai, O. E2F1 transcription factor and its impact on growth factor and cytokine signaling. Cytokine Growth Factor Rev. 2016, 31, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shen, J.; Sun, J. CDK4/RB/E2Fs axis as potential therapeutic target of endometrial cancer. Biomed. Pharm. 2020, 125, 109870. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Ye, L.; Zhenyu, H.; Li, F.; Xiong, Y.; Lin, C.; Wu, X.; Deng, G.; Shi, W.; Song, L.; et al. ANP32E induces tumorigenesis of triple-negative breast cancer cells by upregulating E2F1. Mol. Oncol. 2018, 12, 896–912. [Google Scholar] [CrossRef]
- Mallik, I.; Davila, M.; Tapia, T.; Schanen, B.; Chakrabarti, R. Androgen regulates Cdc6 transcription through interactions between androgen receptor and E2F transcription factor in prostate cancer cells. Biochim. Biophys. Acta 2008, 1783, 1737–1744. [Google Scholar] [CrossRef] [Green Version]
- Mints, M.; Mushtaq, M.; Iurchenko, N.; Kovalevska, L.; Stip, M.C.; Budnikova, D.; Andersson, S.; Polischuk, L.; Buchynska, L.; Kashuba, E. Mitochondrial ribosomal protein S18-2 is highly expressed in endometrial cancers along with free E2F1. Oncotarget 2016, 7, 22150–22158. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Q.T.; He, Q.Q. Identification of key transcription factors in endometrial cancer by systems bioinformatics analysis. J. Cell. Biochem. 2019, 120, 15443–15454. [Google Scholar] [CrossRef]
- Palomer, X.; Álvarez-Guardia, D.; Davidson, M.M.; Chan, T.O.; Feldman, A.M.; Vázquez-Carrera, M. The interplay between NF-kappaB and E2F1 coordinately regulates inflammation and metabolism in human cardiac cells. PLoS ONE 2011, 6, e19724. [Google Scholar] [CrossRef] [Green Version]
- De Siervi, A.; De Luca, P.; Byun, J.S.; Di, L.J.; Fufa, T.; Haggerty, C.M.; Vazquez, E.; Moiola, C.; Longo, D.L.; Gardner, K. Transcriptional autoregulation by BRCA1. Cancer Res. 2010, 70, 532–542. [Google Scholar] [CrossRef] [Green Version]
- Millour, J.; de Olano, N.; Horimoto, Y.; Monteiro, L.J.; Langer, J.K.; Aligue, R.; Hajji, N.; Lam, E.W. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Mol. Cancer 2011, 10, 1046–1058. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, T.; Durieux, E.; Morel, A.P.; de Saint Hilaire, P.; Ray-Coquard, I.; Puisieux, A.; Devouassoux-Shisheboran, M. Role of epithelial-mesenchymal transition factors in the histogenesis of uterine carcinomas. Virchows Arch. 2019, 475, 85–94. [Google Scholar] [CrossRef]
- Caramel, J.; Ligier, M.; Puisieux, A. Pleiotropic Roles for ZEB1 in Cancer. Cancer Res. 2018, 78, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.Y.; Lin, L.; Li, Y.H.; Jiang, H.P.; Zhu, L.T.; Deng, Y.R.; Lin, D.; Chen, W.; Zeng, C.Y.; Wang, L.J.; et al. ZEB1 promotes invasion and metastasis of endometrial cancer by interacting with HDGF and inducing its transcription. Am. J. Cancer Res. 2019, 9, 2314–2330. [Google Scholar]
- Romero-Pérez, L.; López-García, M.; Díaz-Martín, J.; Biscuola, M.; Castilla, M.; Tafe, L.J.; Garg, K.; Oliva, E.; Matias-Guiu, X.; Soslow, R.A.; et al. ZEB1 overexpression associated with E-cadherin and microRNA-200 downregulation is characteristic of undifferentiated endometrial carcinoma. Mod. Pathol. 2013, 26, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Spoelstra, N.S.; Jean, A.; Howe, E.; Torkko, K.C.; Clark, H.R.; Darling, D.S.; Shroyer, K.R.; Horwitz, K.B.; Broaddus, R.R.; et al. ZEB1 expression in type I vs type II endometrial cancers: A marker of aggressive disease. Mod. Pathol. 2008, 21, 912–923. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Wang, X.; Cao, X.; Shen, L.; Zhu, J. ZEB1 expression in endometrial biopsy predicts lymph node metastases in patient with endometrial cancer. Dis. Markers 2014, 2014, 680361. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Li, A.; Han, X. The roles of ZEB1 in tumorigenic progression and epigenetic modifications. Biomed. Pharm. 2019, 110, 400–408. [Google Scholar] [CrossRef]
- Liu, Y.; Nan, F.; Lu, K.; Wang, Y.; Liu, Y.; Wei, S.; Wu, R.; Wang, Y. Identification of key genes in endometrioid endometrial adenocarcinoma via TCGA database. Cancer Biomark. 2017, 21, 11–21. [Google Scholar] [CrossRef]
- Lindner, P.; Paul, S.; Eckstein, M.; Hampel, C.; Muenzner, J.K.; Erlenbach-Wuensch, K.; Ahmed, H.P.; Mahadevan, V.; Brabletz, T.; Hartmann, A.; et al. EMT transcription factor ZEB1 alters the epigenetic landscape of colorectal cancer cells. Cell Death Dis. 2020, 11, 147. [Google Scholar] [CrossRef]
- Vannier, C.; Mock, K.; Brabletz, T.; Driever, W. Zeb1 regulates E-cadherin and Epcam (epithelial cell adhesion molecule) expression to control cell behavior in early zebrafish development. J. Biol. Chem. 2013, 288, 18643–18659. [Google Scholar] [CrossRef] [Green Version]
- Maturi, V.; Enroth, S.; Heldin, C.H.; Moustakas, A. Genome-wide binding of transcription factor ZEB1 in triple-negative breast cancer cells. J. Cell. Physiol. 2018, 233, 7113–7127. [Google Scholar] [CrossRef] [Green Version]
- Brix, D.M.; Bundgaard Clemmensen, K.K.; Kallunki, T. Zinc Finger Transcription Factor MZF1-A Specific Regulator of Cancer Invasion. Cells 2020, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Lheureux, S.; Oza, A.M. Treatment strategies for endometrial cancer: Current practice and perspective. Curr. Opin. Obs. Gynecol. 2017, 29, 47–58. [Google Scholar] [CrossRef]
- Meireles, C.G.; Pereira, S.A.; Valadares, L.P.; Rêgo, D.F.; Simeoni, L.A.; Guerra, E.N.S.; Lofrano-Porto, A. Effects of metformin on endometrial cancer: Systematic review and meta-analysis. Gynecol. Oncol. 2017, 147, 167–180. [Google Scholar] [CrossRef]
- Chu, D.; Wu, J.; Wang, K.; Zhao, M.; Wang, C.; Li, L.; Guo, R. Effect of metformin use on the risk and prognosis of endometrial cancer: A systematic review and meta-analysis. BMC Cancer 2018, 18, 438. [Google Scholar] [CrossRef] [Green Version]
- Slomovitz, B.M.; Jiang, Y.; Yates, M.S.; Soliman, P.T.; Johnston, T.; Nowakowski, M.; Levenback, C.; Zhang, Q.; Ring, K.; Munsell, M.F.; et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol. 2015, 33, 930–936. [Google Scholar] [CrossRef]
- Acevedo-Gadea, C.; Santin, A.D.; Higgins, S.A.; Urva, S.; Ratner, E.; Silasi, D.A.; Azodi, M.; Rutherford, T.; Schwartz, P.E.; Abu-Khalaf, M.M. Phase I clinical trial of the mammalian target of rapamycin inhibitor everolimus in combination with oral topotecan for recurrent and advanced endometrial cancer. Int. J. Gynecol. Cancer 2014, 24, 528–533. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Favier, L.; Weber, B.; Roemer-Becuwe, C.; Bougnoux, P.; Fabbro, M.; Floquet, A.; Joly, F.; Plantade, A.; Paraiso, D.; et al. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. Br. J. Cancer 2013, 108, 1771–1777. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Lin, C.Y.; Wu, R.C.; Lee, Y.S.; Lee, L.Y.; Tsai, C.L.; Yang, L.Y.; Liu, H.; Chen, S.J.; Wang, T.H.; et al. The combination of everolimus and terameprocol exerts synergistic antiproliferative effects in endometrial cancer: Molecular role of insulin-like growth factor binding protein 2. J. Mol. Med. (Berl.) 2018, 96, 1251–1266. [Google Scholar] [CrossRef]
- Fong, P.; Ao, C.N.; Tou, K.I.; Huang, K.M.; Cheong, C.C.; Meng, L.R. Experimental and In Silico Analysis of Cordycepin and its Derivatives as Endometrial Cancer Treatment. Oncol. Res. 2019, 27, 237–251. [Google Scholar] [CrossRef]
- Malloy, K.M.; Wang, J.; Clark, L.H.; Fang, Z.; Sun, W.; Yin, Y.; Kong, W.; Zhou, C.; Bae-Jump, V.L. Novasoy and genistein inhibit endometrial cancer cell proliferation through disruption of the AKT/mTOR and MAPK signaling pathways. Am. J. Transl. Res. 2018, 10, 784–795. [Google Scholar]
- Taylor, C.W.; Lui, R.; Fanta, P.; Salmon, S.E. Effects of suramin on in vitro growth of fresh human tumors. J. Natl. Cancer Inst. 1992, 84, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Nishida, J.; Horiuchi, S.; Rong, F.; Ueoka, Y.; Matsuda, T.; Kato, H.; Furugen, Y.; Yoshida, K.; Kato, K.; et al. Sodium butyrate induces growth arrest and senescence-like phenotypes in gynecologic cancer cells. Int. J. Cancer 2001, 94, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Higa, A.; Hoshi, H.; Hiyama, G.; Takahashi, N.; Ryufuku, M.; Morisawa, G.; Yanagisawa, Y.; Ito, E.; Imai, J.I.; et al. Evaluation of anticancer agents using patient-derived tumor organoids characteristically similar to source tissues. Oncol. Rep. 2018, 40, 635–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrauwen, S.; Depreeuw, J.; Coenegrachts, L.; Hermans, E.; Lambrechts, D.; Amant, F. Dual blockade of PI3K/AKT/mTOR (NVP-BEZ235) and Ras/Raf/MEK (AZD6244) pathways synergistically inhibit growth of primary endometrioid endometrial carcinoma cultures, whereas NVP-BEZ235 reduces tumor growth in the corresponding xenograft models. Gynecol. Oncol. 2015, 138, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Chung, J.S.; Jo, J.C.; Cho, S.H.; Shin, H.J. Phase II study of safety and efficacy of BEB (bendamustine, etoposide, and busulfan) conditioning regimen for autologous stem cell transplantation in non-Hodgkin lymphoma. Ann. Hematol. 2020, 99, 819–828. [Google Scholar] [CrossRef]
- Bogeljić Patekar, M.; Milunović, V.; Mišura Jakobac, K.; Perica, D.; Mandac Rogulj, I.; Kursar, M.; Planinc-Peraica, A.; Ostojić Kolonić, S. Bendamustine: An old drug in the new era for patients with non-hodgkin lymphomas and chronic lymphocytic leukemia. Acta Clin. Croat. 2018, 57, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Park, S.Y.; Wu, Z.; Liu, K.H.; Seo, Y.H. Hybrid inhibitors of DNA and HDACs remarkably enhance cytotoxicity in leukaemia cells. J. Enzym. Inhib. Med. Chem. 2020, 35, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Yerram, P.; Reiss, S.N.; Modelevsky, L.; Gavrilovic, I.T.; Kaley, T. Evaluation of toxicity of carmustine with or without bevacizumab in patients with recurrent or progressive high grade gliomas. J. Neurooncol. 2019, 145, 57–63. [Google Scholar] [CrossRef]
- Simone, M.; Erba, E.; Damia, G.; Vikhanskaya, F.; Di Francesco, A.M.; Riccardi, R.; Bailly, C.; Cuevas, C.; Fernandez Sousa-Faro, J.M.; D’Incalci, M. Variolin B and its derivate deoxy-variolin B: New marine natural compounds with cyclin-dependent kinase inhibitor activity. Eur. J. Cancer 2005, 41, 2366–2377. [Google Scholar] [CrossRef]
- Jarry, M.; Lecointre, C.; Malleval, C.; Desrues, L.; Schouft, M.T.; Lejoncour, V.; Liger, F.; Lyvinec, G.; Joseph, B.; Loaëc, N.; et al. Impact of meriolins, a new class of cyclin-dependent kinase inhibitors, on malignant glioma proliferation and neo-angiogenesis. Neuro Oncol. 2014, 16, 1484–1498. [Google Scholar] [CrossRef] [Green Version]
- Faria, C.C.; Agnihotri, S.; Mack, S.C.; Golbourn, B.J.; Diaz, R.J.; Olsen, S.; Bryant, M.; Bebenek, M.; Wang, X.; Bertrand, K.C.; et al. Identification of alsterpaullone as a novel small molecule inhibitor to target group 3 medulloblastoma. Oncotarget 2015, 6, 21718–21729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Sato, Y.; Masud, H.; Takayama, M.; Matsuda, H.; Hara, Y.; Yanagi, Y.; Yoshida, M.; Goshima, F.; Murata, T.; et al. Antitumor activity of cyclin-dependent kinase inhibitor alsterpaullone in Epstein-Barr virus-associated lymphoproliferative disorders. Cancer Sci. 2020, 111, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wei, Y.; Yao, J.; Chu, Y.Y.; Li, C.W.; Hsu, J.L.; Nie, L.; Hung, M.C. Inhibition of CDK2 reduces EZH2 phosphorylation and reactivates ERα expression in high-grade serous ovarian carcinoma. Am. J. Cancer Res. 2020, 10, 1194–1206. [Google Scholar] [PubMed]
- Leshem, Y.; King, E.M.; Mazor, R.; Reiter, Y.; Pastan, I. SS1P Immunotoxin Induces Markers of Immunogenic Cell Death and Enhances the Effect of the CTLA-4 Blockade in AE17M Mouse Mesothelioma Tumors. Toxins 2018, 10, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, R.; Lerner, M.R.; Benbrook, D.; Lightfoot, S.A.; Brackett, D.J.; Wang, Q.C.; Pastan, I. Antitumor activity of SS(dsFv)PE38 and SS1(dsFv)PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic culture in vitro. Clin. Cancer Res. 2002, 8, 3520–3526. [Google Scholar]
- Xu, P.F.; Yang, J.A.; Liu, J.H.; Yang, X.; Liao, J.M.; Yuan, F.E.; Liu, B.H.; Chen, Q.X. PI3Kβ inhibitor AZD6482 exerts antiproliferative activity and induces apoptosis in human glioblastoma cells. Oncol. Rep. 2019, 41, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Berchuck, A.; Rodriguez, G.; Kinney, R.B.; Soper, J.T.; Dodge, R.K.; Clarke-Pearson, D.L.; Bast, R.C., Jr. Overexpression of HER-2/neu in endometrial cancer is associated with advanced stage disease. Am. J. Obs. Gynecol. 1991, 164, 15–21. [Google Scholar] [CrossRef]
- Morrison, C.; Zanagnolo, V.; Ramirez, N.; Cohn, D.E.; Kelbick, N.; Copeland, L.; Maxwell, G.L.; Fowler, J.M. HER-2 is an independent prognostic factor in endometrial cancer: Association with outcome in a large cohort of surgically staged patients. J. Clin. Oncol. 2006, 24, 2376–2385. [Google Scholar] [CrossRef]
- Saffari, B.; Jones, L.A.; el-Naggar, A.; Felix, J.C.; George, J.; Press, M.F. Amplification and overexpression of HER-2/neu (c-erbB2) in endometrial cancers: Correlation with overall survival. Cancer Res. 1995, 55, 5693–5698. [Google Scholar]
- Zhang, Y.; Zhao, D.; Gong, C.; Zhang, F.; He, J.; Zhang, W.; Zhao, Y.; Sun, J. Prognostic role of hormone receptors in endometrial cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2015, 13, 208. [Google Scholar] [CrossRef] [Green Version]
- Vageli, D.; Ioannou, M.G.; Koukoulis, G.K. Transcriptional activation of hTERT in breast carcinomas by the Her2-ER81-related pathway. Oncol. Res. 2009, 17, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Goueli, B.S.; Janknecht, R. Upregulation of the Catalytic Telomerase Subunit by the Transcription Factor ER81 and Oncogenic HER2/Neu, Ras, or Raf. Mol. Cell. Biol. 2004, 24, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, Q.; Sun, C.; Huang, Y.; Zhang, J.; Wang, Q. Clinical relevance of ARF/ARL family genes and oncogenic function of ARL4C in endometrial cancer. Biomed. Pharm. 2020, 125, 110000. [Google Scholar] [CrossRef]
- Jiang, T.; Sui, D.; You, D.; Yao, S.; Zhang, L.; Wang, Y.; Zhao, J.; Zhang, Y. MiR-29a-5p inhibits proliferation and invasion and induces apoptosis in endometrial carcinoma via targeting TPX2. Cell Cycle 2018, 17, 1268–1278. [Google Scholar] [CrossRef] [Green Version]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the human interactome defines protein communities and disease networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef]
- Huang, D.H.; Jian, J.; Li, S.; Zhang, Y.; Liu, L.Z. TPX2 silencing exerts anti-tumor effects on hepatocellular carcinoma by regulating the PI3K/AKT signaling pathway. Int. J. Mol. Med. 2019, 44, 2113–2122. [Google Scholar] [CrossRef]
- Yang, Y.; Li, D.P.; Shen, N.; Yu, X.C.; Li, J.B.; Song, Q.; Zhang, J.H. TPX2 promotes migration and invasion of human breast cancer cells. Asian Pac. J. Trop. Med. 2015, 8, 1064–1070. [Google Scholar] [CrossRef]
- Tomica, D.; Ramić, S.; Danolić, D.; Šušnjar, L.; Perić-Balja, M.; Puljiz, M. Impact of oestrogen and progesterone receptor expression in the cancer cells and myometrium on survival of patients with endometrial cancer. J. Obs. Gynaecol. 2018, 38, 96–102. [Google Scholar] [CrossRef]
- Yang, W.J.; Wang, H.B.; Wang, W.D.; Bai, P.Y.; Lu, H.X.; Sun, C.H.; Liu, Z.S.; Guan, D.K.; Yang, G.W.; Zhang, G.L. A network-based predictive gene expression signature for recurrence risks in stage II colorectal cancer. Cancer Med. 2020, 9, 179–193. [Google Scholar] [CrossRef]
- Yu, G.; Lee, Y.C.; Cheng, C.J.; Wu, C.F.; Song, J.H.; Gallick, G.E.; Yu-Lee, L.Y.; Kuang, J.; Lin, S.H. RSK promotes prostate cancer progression in bone through ING3, CKAP2, and PTK6-mediated cell survival. Mol. Cancer Res. 2015, 13, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Czaplinska, D.; Gorska, M.; Mieczkowski, K.; Peszynska-Sularz, G.; Zaczek, A.J.; Romanska, H.M.; Sadej, R. RSK1 promotes murine breast cancer growth and metastasis. Folia Histochem. Cytobiol. 2018, 56, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Salhi, A.; Farhadian, J.A.; Giles, K.M.; Vega-Saenz de Miera, E.; Silva, I.P.; Bourque, C.; Yeh, K.; Chhangawala, S.; Wang, J.; Ye, F.; et al. RSK1 activation promotes invasion in nodular melanoma. Am. J. Pathol. 2015, 185, 704–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotla, S.; Vu, H.T.; Ko, K.A.; Wang, Y.; Imanishi, M.; Heo, K.S.; Fujii, Y.; Thomas, T.N.; Gi, Y.J.; Mazhar, H.; et al. Endothelial senescence is induced by phosphorylation and nuclear export of telomeric repeat binding factor 2-interacting protein. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Ungar, L.; Yosef, N.; Sela, Y.; Sharan, R.; Ruppin, E.; Kupiec, M. A genome-wide screen for essential yeast genes that affect telomere length maintenance. Nucleic Acids Res. 2009, 37, 3840–3849. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.Y.; Chen, Y.; Jia, J.S.; Zhou, C.; Lian, M.; Wen, Y.T.; Li, X.Y.; Chen, H.W.; Lin, X.L.; Zhang, X.L.; et al. Loss of Cirbp expression is correlated with the malignant progression and poor prognosis in nasopharyngeal carcinoma. Cancer Manag. Res. 2019, 11, 6959–6969. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.W.; Ellenson, L.H. Molecular Genetics of Endometrial Carcinoma. Annu. Rev. Pathol. 2019, 14, 339–367. [Google Scholar] [CrossRef]
- Kim, S.R.; Cloutier, B.T.; Leung, S.; Cochrane, D.; Britton, H.; Pina, A.; Storness-Bliss, C.; Farnell, D.; Huang, L.; Shum, K.; et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecol. Oncol. 2020, 158, 3–11. [Google Scholar] [CrossRef]

| TF | Fisher Score |
|---|---|
| E2F1 | 49.853 |
| MZF1_5-13 | 54.086 |
| ZEB1 | 50.209 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradfield, A.; Button, L.; Drury, J.; Green, D.C.; Hill, C.J.; Hapangama, D.K. Investigating the Role of Telomere and Telomerase Associated Genes and Proteins in Endometrial Cancer. Methods Protoc. 2020, 3, 63. https://doi.org/10.3390/mps3030063
Bradfield A, Button L, Drury J, Green DC, Hill CJ, Hapangama DK. Investigating the Role of Telomere and Telomerase Associated Genes and Proteins in Endometrial Cancer. Methods and Protocols. 2020; 3(3):63. https://doi.org/10.3390/mps3030063
Chicago/Turabian StyleBradfield, Alice, Lucy Button, Josephine Drury, Daniel C. Green, Christopher J. Hill, and Dharani K. Hapangama. 2020. "Investigating the Role of Telomere and Telomerase Associated Genes and Proteins in Endometrial Cancer" Methods and Protocols 3, no. 3: 63. https://doi.org/10.3390/mps3030063
APA StyleBradfield, A., Button, L., Drury, J., Green, D. C., Hill, C. J., & Hapangama, D. K. (2020). Investigating the Role of Telomere and Telomerase Associated Genes and Proteins in Endometrial Cancer. Methods and Protocols, 3(3), 63. https://doi.org/10.3390/mps3030063





