Predictable Peptide Conjugation Ratios by Activation of Proteins with Succinimidyl Iodoacetate (SIA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. MALDI-MS Characterization
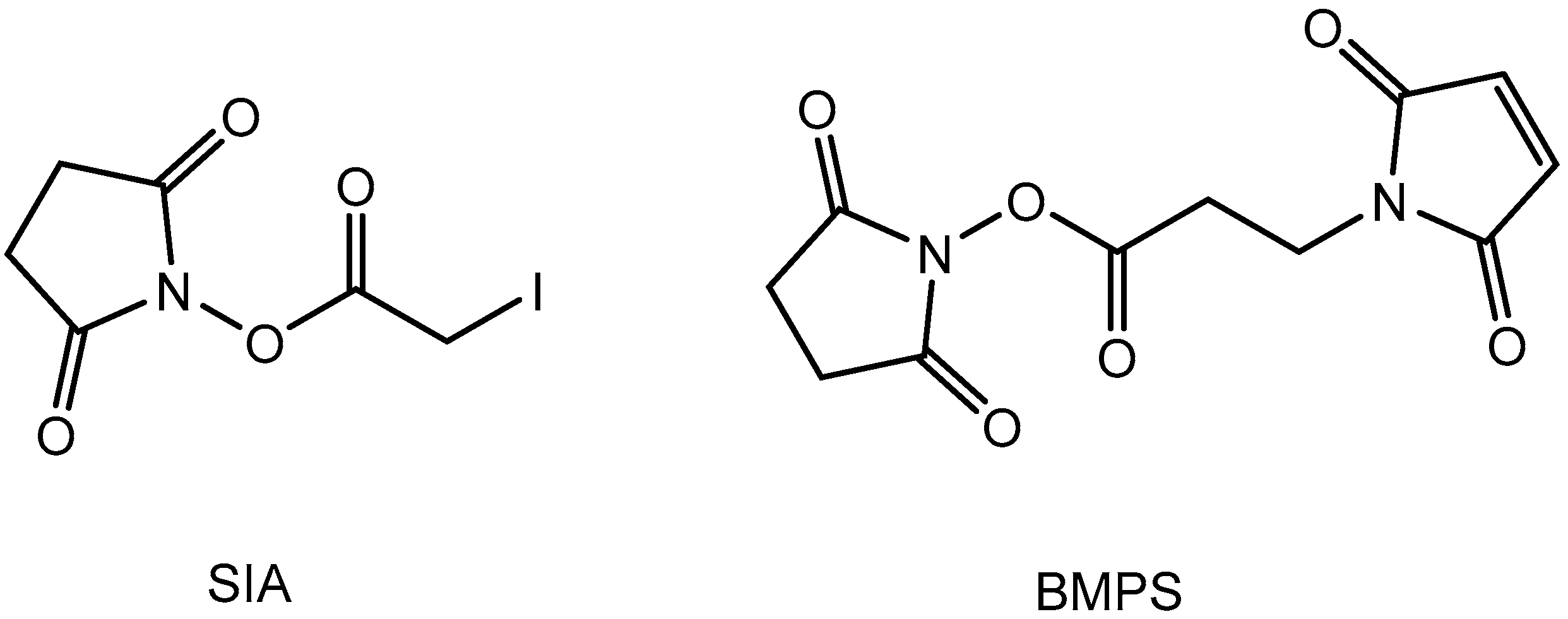
2.3. Solvolysis and Hydrolysis of SIA and BMPS
2.4. Activation of Proteins
2.5. Conjugation of Peptides to Activated Proteins
3. Results
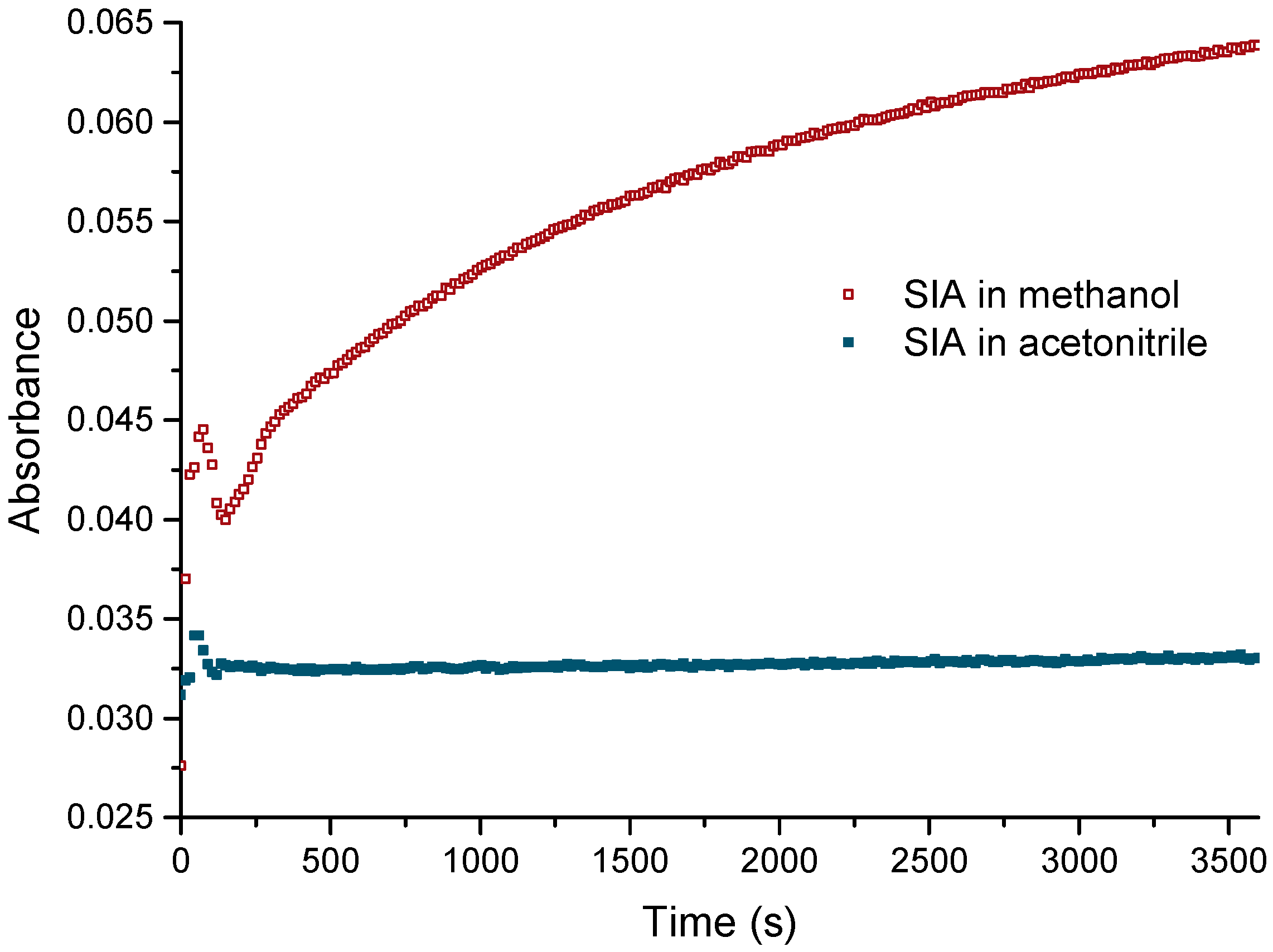
3.1. Solubilization of SIA in Organic Solvents
3.2. Hydrolysis of the Ester at Different pH Values
3.3. Activation of Proteins
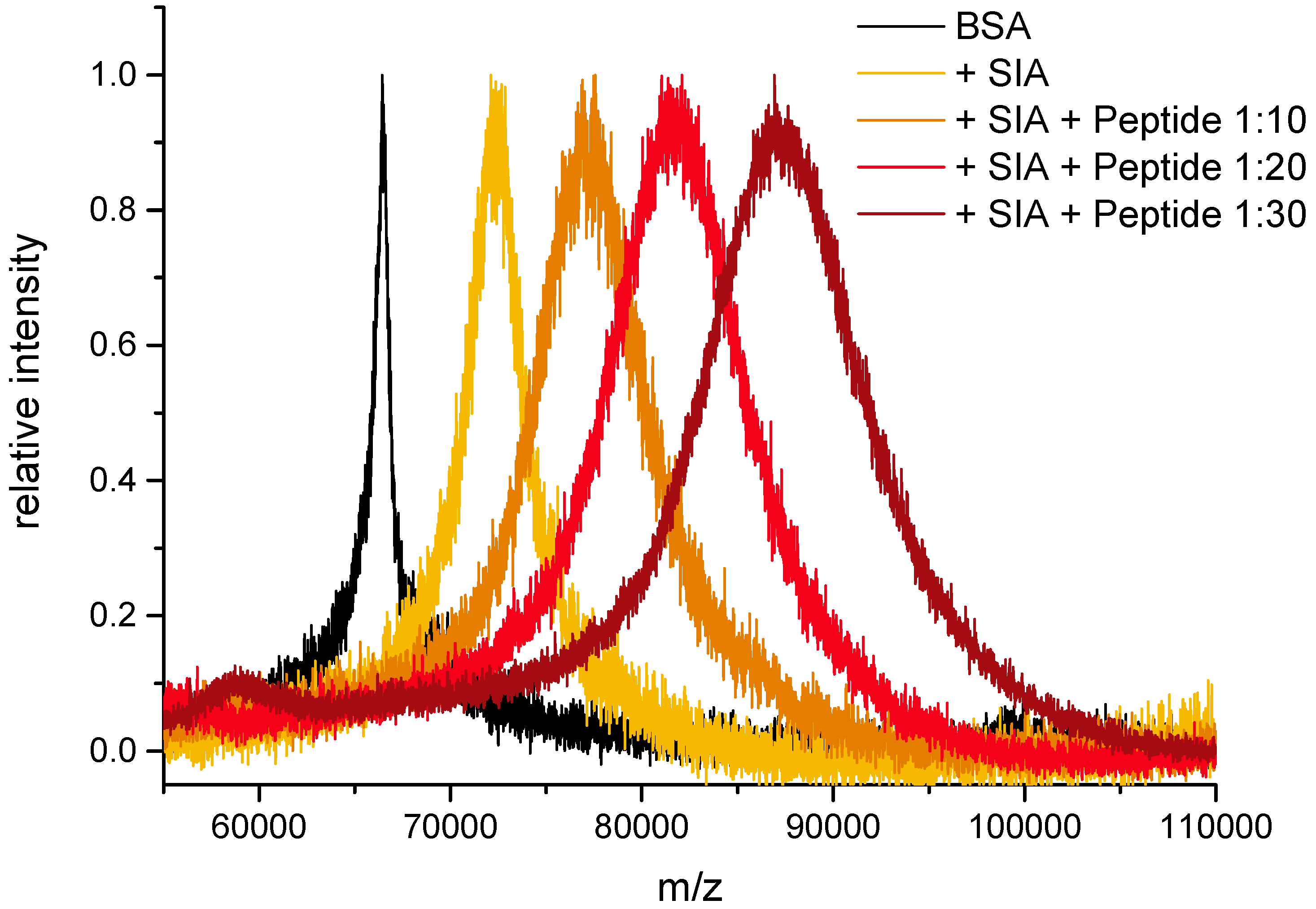
3.4. Peptide Conjugation, Excess of SIA (Protocol I)
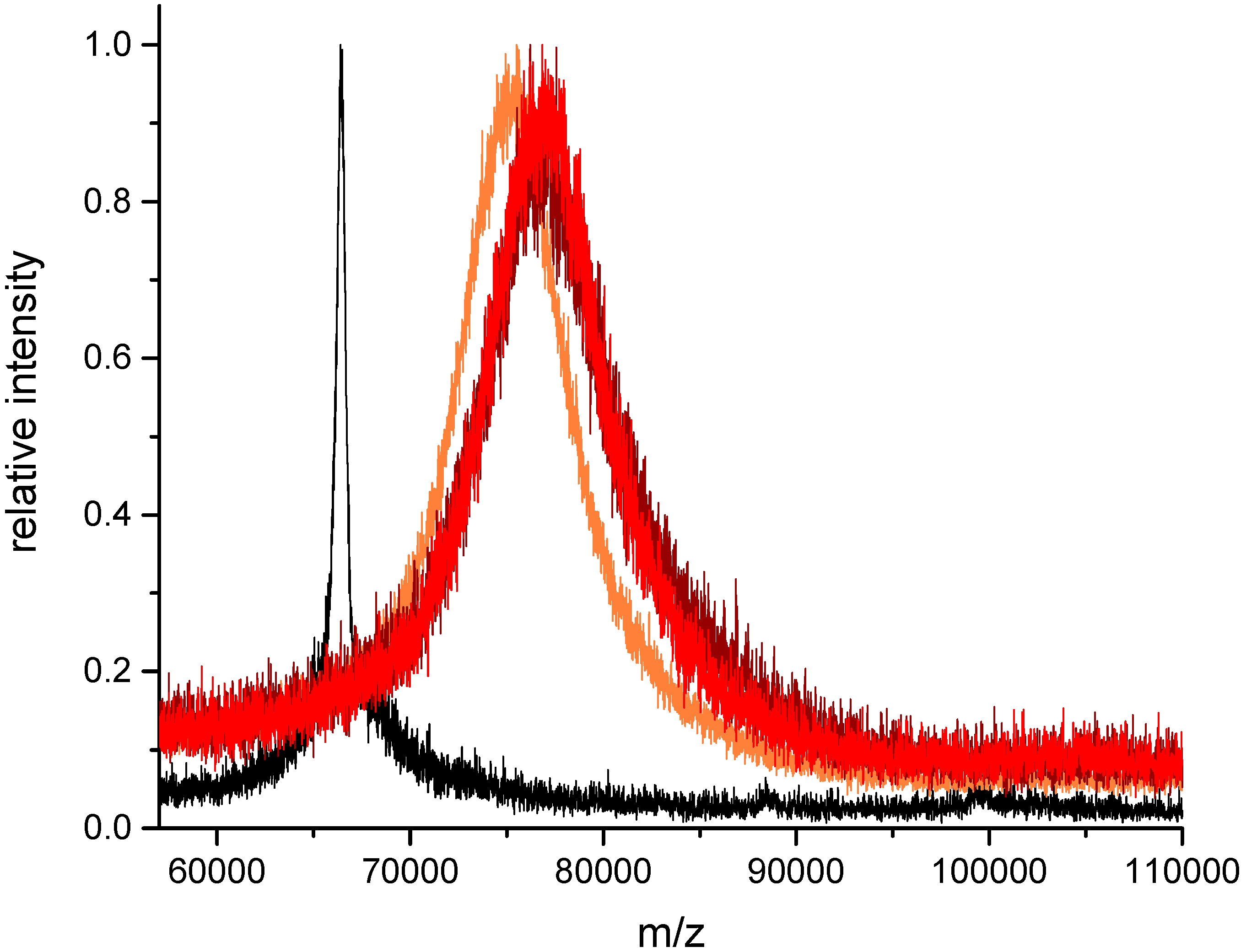
3.5. Peptide Conjugation, Excess of Peptide (Protocol II)
4. Discussion
- Protocol I: Excess of SIA
- + SIA conjugates are cheap and quick to prepare.
- + A single SIA conjugate can be used for peptide conjugates of different, controlled conjugation ratios.
- + Only low amounts of peptide are needed.
- − Peptide should be free of cysteine dimers and peptide concentration needs to be known.
- − Tris(2-carboxyethyl)phosphine (TCEP) and thiols are not tolerated.
- − Inactivation of residual SIA sites may be necessary in some cases.
- Protocol II: Excess of peptide
- + A limited fraction of cysteine dimers does not reduce the conjugation ratio.
- + Conjugates with different peptides can be prepared with equal, controlled conjugation ratios.
- +/− SIA conjugates of a very precise activation level are difficult to prepare. However, since most carrier proteins and SIA are relatively cheap reagents, these exploratory experiments (different ratios) do not consume any of the expensive peptide.
- +/− TCEP and thiols are not tolerated. However, in most cases, reductants are not needed.
- − Higher amounts of peptide are necessary.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Houen, G. Peptide Antibodies—Methods and Protocols; Methods Mol. Biol.; Humana Press; Springer: New York, NY, USA, 2015. [Google Scholar]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Hughes, B. Antibody-drug conjugates for cancer: Poised to deliver? Nat. Rev. Drug Discov. 2010, 9, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Gebleux, R.; Casi, G. Antibody-drug conjugates: Current status and future perspectives. Pharmacol. Ther. 2016, 167, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Teicher, B.A.; Hassan, R.T. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016, 17, E254–E262. [Google Scholar] [CrossRef]
- De Goeij, B.E.; Lambert, J.M. New developments for antibody-drug conjugate-based therapeutic approaches. Curr. Opin. Immunol. 2016, 40, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Rago, B.; Clark, T.; King, L.; Zhang, J.; Tumey, L.N.; Li, F.P.; Barletta, F.; Wei, C.; Leal, M.; Hansel, S.; et al. Calculated conjugated payload from immunoassay and LC-MS intact protein analysis measurements of antibody-drug conjugate. Bioanalysis 2016, 8, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.B.; Jalah, R.; Rice, K.C.; Li, F.Y.; Antoline, J.F.G.; Iyer, M.R.; Jacobson, A.E.; Boutaghou, M.N.; Alving, C.R.; Matyas, G.R. Characterization and optimization of heroin hapten-BSA conjugates: Method development for the synthesis of reproducible hapten-based vaccines. Anal. Bioanal. Chem. 2014, 406, 5927–5937. [Google Scholar] [CrossRef] [PubMed]
- Lateef, S.S.; Gupta, S.; Jayathilaka, L.P.; Krishnanchettiar, S.; Huang, J.S.; Lee, B.S. An improved protocol for coupling synthetic peptides to carrier proteins for antibody production using DMF to solubilize peptides. J. Biomol. Tech. 2007, 18, 173–176. [Google Scholar] [PubMed]
- Bobaly, B.; Randazzo, G.M.; Rudaz, S.; Guillarme, D.; Fekete, S. Optimization of non-linear gradient in hydrophobic interaction chromatography for the analytical characterization of antibody-drug conjugates. J. Chromatogr. A 2017, 1481, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Veuthey, J.L.; Beck, A.; Guillarme, D. Hydrophobic interaction chromatography for the characterization of monoclonal antibodies and related products. J. Pharm. Biomed. 2016, 130, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Becker, M.L. “Click” reactions: A versatile toolbox for the synthesis of peptide-conjugates. Chem. Soc. Rev. 2014, 43, 7013–7039. [Google Scholar] [CrossRef] [PubMed]
- Fodey, T.L.; Greer, N.M.; Crooks, S.R.H. Antibody production: Low dose immunogen vs. low incorporation hapten using salmeterol as a model. Anal. Chim. Acta 2009, 637, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Jalah, R.; Torres, O.B.; Mayorov, A.V.; Li, F.Y.; Antoline, J.F.G.; Jacobson, A.E.; Rice, K.C.; Deschamps, J.R.; Beck, Z.; Alving, C.R.; et al. Efficacy, but Not Antibody Titer or Affinity, of a Heroin Hapten Conjugate Vaccine Correlates with Increasing Hapten Densities on Tetanus Toxoid, but Not on CRM197 Carriers. Bioconj. Chem. 2015, 26, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Kitisripanya, T.; Jutathis, K.; Inyai, C.; Komaikul, J.; Udomsin, O.; Yusakul, G.; Tanaka, H.; Putalun, W. Anti-miroestrol polyclonal antibodies: A comparison of immunogen preparations used to obtain desired antibody properties. J. Nat. Med. 2016, 70, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.V.; Kaur, J.; Varshney, G.C.; Raje, M.; Suri, C.R. Synthesis and characterization of hapten-protein conjugates for antibody production against small molecules. Bioconj. Chem. 2004, 15, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Peeters, J.M.; Hazendonk, T.G.; Beuvery, E.C.; Tesser, G.I. Comparison of 4 Bifunctional Reagents for Coupling Peptides to Proteins and the Effect of the 3 Moieties on the Immunogenicity of the Conjugates. J. Immunol. Methods 1989, 120, 133–143. [Google Scholar] [CrossRef]
- Lee, A.C.; Powell, J.E.; Tregear, G.W.; Niall, H.D.; Stevens, V.C. A method for preparing beta-hCG COOH peptide-carrier conjugates of predictable composition. Mol. Immunol. 1980, 17, 749–756. [Google Scholar] [CrossRef]
- Lyon, R.P.; Setter, J.R.; Bovee, T.D.; Doronina, S.O.; Hunter, J.H.; Anderson, M.E.; Balasubramanian, C.L.; Duniho, S.M.; Leiske, C.I.; Li, F.; et al. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat. Biotechnol. 2014, 32, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Q.; Xu, K.Y.; Liu, L.N.; Raab, H.; Bhakta, S.; Kenrick, M.; Parsons-Reponte, K.L.; Tien, J.; Yu, S.F.; Mai, E.; et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 2012, 30, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Tumey, L.N.; Charati, M.; He, T.; Sousa, E.; Ma, D.S.; Han, X.G.; Clark, T.; Casavant, J.; Loganzo, F.; Barletta, F.; et al. Mild Method for Succinimide Hydrolysis on ADCs: Impact on ADC Potency, Stability, Exposure, and Efficacy. Bioconj. Chem. 2014, 25, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.P.M.; Morais, M.; Vassileva, V.; Robinson, E.; Rajkumar, V.S.; Smith, M.E.B.; Pedley, R.B.; Caddick, S.; Baker, J.R.; Chudasama, V. Functional native disulfide bridging enables delivery of a potent, stable and targeted antibody-drug conjugate (ADC). Chem. Commun. 2015, 51, 10624–10627. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.D.; Reid, R.; Robinson, L.; Ashley, G.W.; Santi, D.V. Long-Term Stabilization of Maleimide-Thiol Conjugates. Bioconj. Chem. 2015, 26, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Beck, S.; Weller, M.G.; Linscheid, M.W. MeCAT-new iodoacetamide reagents for metal labeling of proteins and peptides. Anal. Bioanal. Chem. 2011, 401, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Beck, S.; Weller, M.G.; Linscheid, M.W. Comparison of the fragmentation behavior of differentially metal-coded affinity tag (MeCAT)-labeled peptides. J. Mass Spectrom. 2012, 47, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Ahrends, R.; Pieper, S.; Neumann, B.; Scheler, C.; Linscheid, M.W. Metal-Coded Affinity Tag Labeling: A Demonstration of Analytical Robustness and Suitability for Biological Applications. Anal. Chem. 2009, 81, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Buskas, T.; Li, Y.H.; Boons, G.J. The immunogenicity of the tumor-associated antigen Lewisy may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chem. Eur. J. 2004, 10, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Wittrock, S.; Becker, T.; Kunz, H. Synthetic vaccines of tumor-associated glycopeptide antigens by immune-compatible thioether linkage to bovine serum albumin. Angew. Chem. Int. Ed. 2007, 46, 5226–5230. [Google Scholar] [CrossRef] [PubMed]
- Dovgan, I.; Kolodych, S.; Koniev, O.; Wagner, A. 2-(Maleimidomethyl)-1,3-Dioxanes (MD): A Serum-Stable Self-hydrolysable Hydrophilic Alternative to Classical Maleimide Conjugation. Sci. Rep. 2016, 6, 30835. [Google Scholar] [CrossRef] [PubMed]
- Dickens, F. Interaction of halogenacetates and sh compounds. The reaction of halogenacetic acids with glutathione and cysteine. The mechanism of iodoacetate poisoning of glyoxalase. Biochem. J. 1933, 27, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, L.; Schubert, M.P. The reaction of iodoacetic acid on mercaptans and amines. J. Biol. Chem. 1934, 106, 331–341. [Google Scholar]
- Sechi, S.; Chait, B.T. Modification of cysteine residues by alkylation. A tool in peptide mapping and protein identification. Anal. Chem. 1998, 70, 5150–5158. [Google Scholar] [PubMed]
- Gygi, S.P.; Rist, B.; Gerber, S.A.; Turecek, F.; Gelb, M.H.; Aebersold, R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 1999, 17, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Ahrends, R.; Pieper, S.; Kuhn, A.; Weisshoff, H.; Hamester, M.; Lindemann, T.; Scheler, C.; Lehmann, K.; Taubner, K.; Linscheid, M.W. A metal-coded affinity tag approach to quantitative proteomics. Mol. Cell. Proteom. 2007, 6, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Rector, E.S.; Tse, K.S.; Sehon, A.H.; Schwenk, R.J. Method for Preparation of Protein-Protein Conjugates of Predetermined Composition. J. Immunol. Methods 1978, 24, 321–336. [Google Scholar] [CrossRef]
- Inman, J.K. Syntheses of Macromolecular Immunomodulators and Conjugates Employing Haloacetyl Reagents. Ann. N.Y. Acad. Sci. 1993, 685, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Boeckler, C.; Frisch, B.; Muller, S.; Schuber, F. Immunogenicity of new heterobifunctional cross-linking reagents used in the conjugation of synthetic peptides to liposomes. J. Immunol. Methods 1996, 191, 1–10. [Google Scholar] [CrossRef]
- Houen, G.; Jensen, O.M. Conjugation to Preactivated Proteins Using Divinylsulfone and Iodoacetic Acid. J. Immunol. Methods 1995, 181, 187–200. [Google Scholar] [CrossRef]
- Houen, G.; Jakobsen, M.H.; Svaerke, C.; Koch, C.; Barkholt, V. Conjugation to preadsorbed preactivated proteins and efficient generation of anti peptide antibodies. J. Immunol. Methods 1997, 206, 125–134. [Google Scholar] [CrossRef]
- Houen, G.; Olsen, D.T.; Hansen, P.R.; Petersen, K.B.; Barkholt, V. Preparation of bioconjugates by solid-phase conjugation to ion exchange matrix-adsorbed carrier proteins. Bioconj. Chem. 2003, 14, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Hansen, P.R.; Houen, G. Production and characterization of peptide antibodies. Methods 2012, 56, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Houen, G.; Olsen, D.T. Solid-Phase Peptide-Carrier Conjugation. Methods Mol. Biol. 2015, 1348, 59–64. [Google Scholar]
- Jackson, D.Y. Processes for Constructing Homogeneous Antibody Drug Conjugates. Org. Process Res. Dev. 2016, 20, 852–866. [Google Scholar] [CrossRef]
- Chari, R.V.J.; Miller, M.L.; Widdison, W.C. Antibody- Drug Conjugates: An Emerging Concept in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 3796–3827. [Google Scholar] [CrossRef] [PubMed]
- Briand, J.P.; Muller, S.; Van Regenmortel, M.H. Synthetic peptides as antigens: Pitfalls of conjugation methods. J. Immunol. Methods 1985, 78, 59–69. [Google Scholar] [CrossRef]
- Frisch, B.; Boeckler, C.; Schuber, F. Synthesis of short polyoxyethylene-based heterobifunctional cross-linking reagents. Application to the coupling of peptides to liposomes. Bioconj. Chem. 1996, 7, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Le Sann, C. Maleimide spacers as versatile linkers in the synthesis of bioconjugates of anthracyclines. Nat. Prod. Rep. 2006, 23, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhang, G.D.; Clark, T.; Barletta, F.; Tumey, L.N.; Rago, B.; Hansel, S.; Han, X.G. Where Did the Linker-Payload Go? A Quantitative Investigation on the Destination of the Released Linker-Payload from an Antibody-Drug Conjugate with a Maleimide Linker in Plasma. Anal. Chem. 2016, 88, 4979–4986. [Google Scholar] [CrossRef] [PubMed]
- Zeck, A.; Eikenberg, A.; Weller, M.G.; Niessner, R. Highly sensitive immunoassay based on a monoclonal antibody specific for [4-arginine]microcystins. Anal. Chim. Acta 2001, 441, 1–13. [Google Scholar] [CrossRef]
- Skladal, P. Effect of methanol on the interaction of monoclonal antibody with free and immobilized atrazine studied using the resonant mirror-based biosensor. Biosens. Bioelectron. 1999, 14, 257–263. [Google Scholar] [CrossRef]
- Miron, T.; Wilchek, M. A Spectrophotometric Assay for Soluble and Immobilized N-Hydroxysuccinimide Esters. Anal. Biochem. 1982, 126, 433–435. [Google Scholar] [CrossRef]
- Klykov, O.; Weller, M.G. Quantification of N-hydroxysuccinimide and N-hydroxysulfosuccinimide by hydrophilic interaction chromatography (HILIC). Anal. Methods 2015, 7, 6443–6448. [Google Scholar] [CrossRef]


| pH | t1/2 [s] |
|---|---|
| (a) | |
| 5 | 180 |
| 6 | 110 |
| 7 | 36 |
| (b) | |
| 8 | 550 |
| 9 | 150 |
| Activation Step | m/z | Theoretical Ratio | Experimental Ratio |
|---|---|---|---|
| (Sequential Addition) | (Cumulative) | ||
| 0 | 66,441 | 0 | 0 |
| 1 | 69,417 | 60 | 17.7 |
| 2 | 70,942 | 60 | 26.8 |
| 3 | 71,640 | 60 | 30.9 |
| 4 | 72,095 | 60 | 33.7 |
| 5 | 72,316 | 60 | 35.0 |
| m/z | Theoretical Ratio | Experimental Ratio |
|---|---|---|
| 66,395 | 0 | 0 |
| 67,753 | 20 | 8.1 |
| 68,621 | 40 | 13.3 |
| 69,585 | 60 | 19.0 |
| BSA | BSA + SIA | Activ. Level | BSA + SIA + Peptide | calc. Ratio | exp. Ratio |
|---|---|---|---|---|---|
| (m/z) | (m/z) | SIA/BSA | (m/z) | Peptides/BSA | Peptides/BSA |
| 66,448 | 72,945 | 38.7 | 80,561 | 10 | 10.8 |
| 66,448 | 72,945 | 38.7 | 84,159 | 15 | 16.0 |
| 66,448 | 72,945 | 38.7 | 94,608 | 30 | 30.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, I.M.; Schwaar, T.; Bienwald, F.; Weller, M.G. Predictable Peptide Conjugation Ratios by Activation of Proteins with Succinimidyl Iodoacetate (SIA). Methods Protoc. 2018, 1, 2. https://doi.org/10.3390/mps1010002
Abbas IM, Schwaar T, Bienwald F, Weller MG. Predictable Peptide Conjugation Ratios by Activation of Proteins with Succinimidyl Iodoacetate (SIA). Methods and Protocols. 2018; 1(1):2. https://doi.org/10.3390/mps1010002
Chicago/Turabian StyleAbbas, Ioana M., Timm Schwaar, Frank Bienwald, and Michael G. Weller. 2018. "Predictable Peptide Conjugation Ratios by Activation of Proteins with Succinimidyl Iodoacetate (SIA)" Methods and Protocols 1, no. 1: 2. https://doi.org/10.3390/mps1010002
APA StyleAbbas, I. M., Schwaar, T., Bienwald, F., & Weller, M. G. (2018). Predictable Peptide Conjugation Ratios by Activation of Proteins with Succinimidyl Iodoacetate (SIA). Methods and Protocols, 1(1), 2. https://doi.org/10.3390/mps1010002