Immunohistochemical Pattern—A Prognostic Factor for Synchronous Gastrointestinal Cancer
Abstract
:Introduction
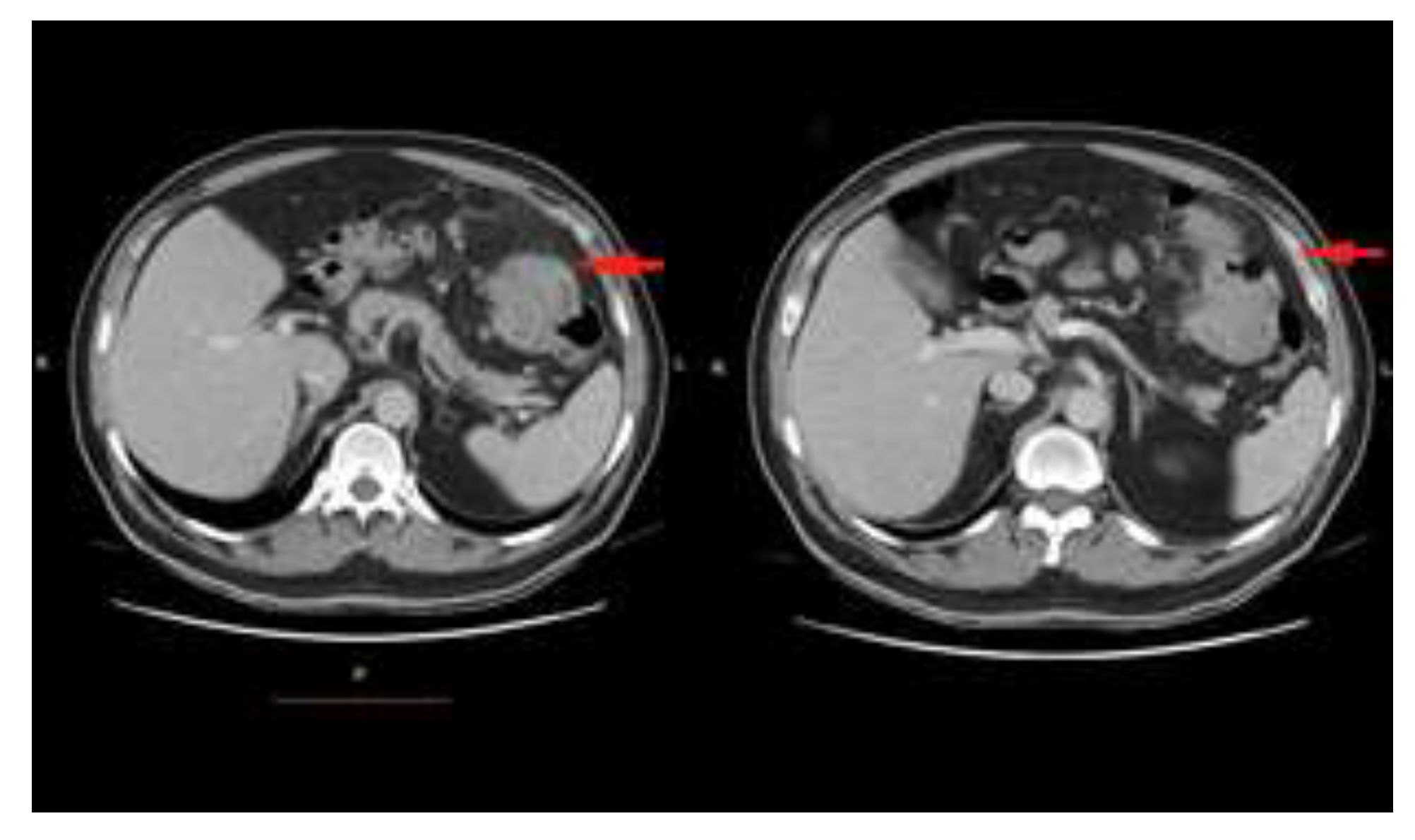
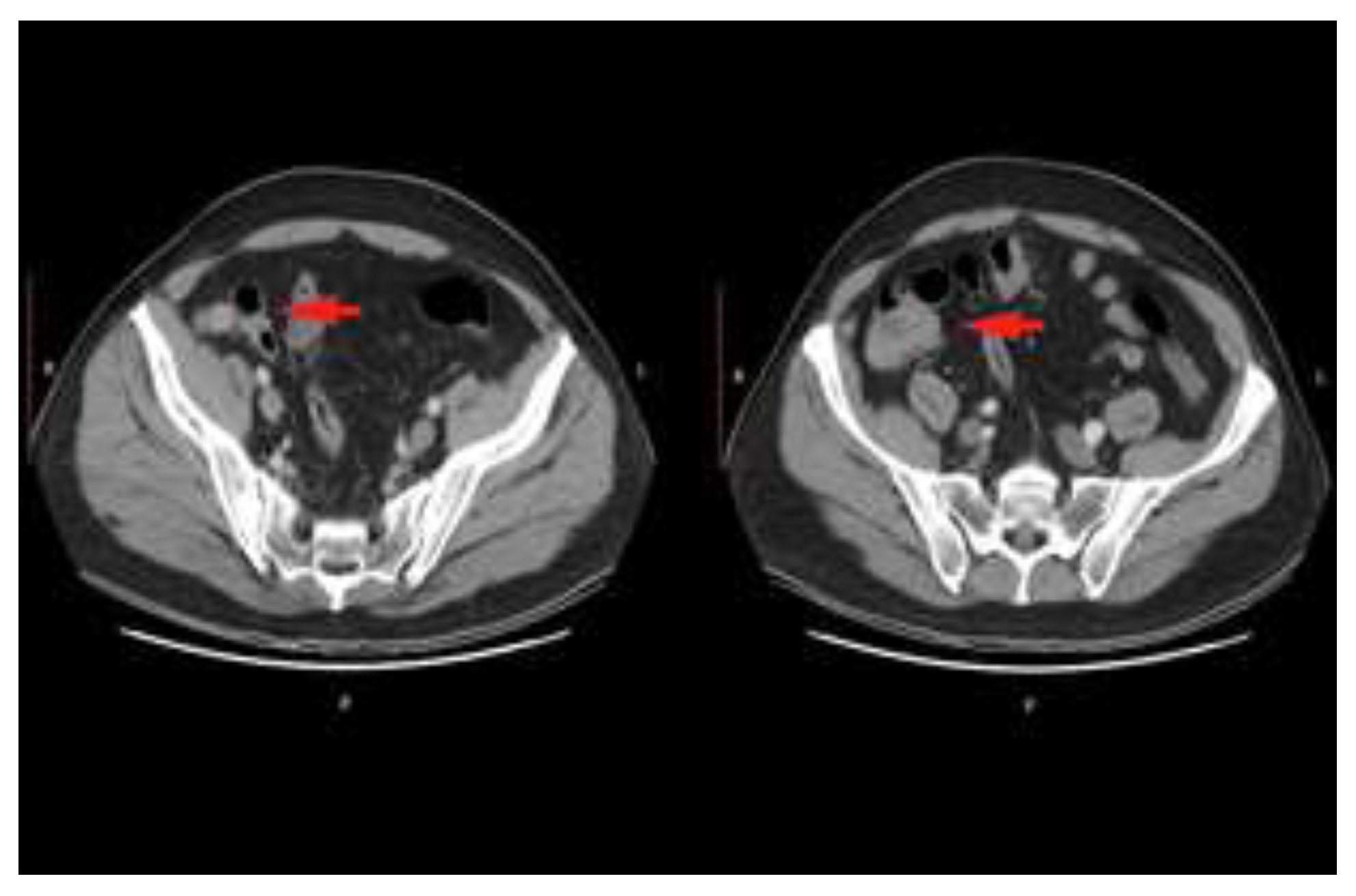
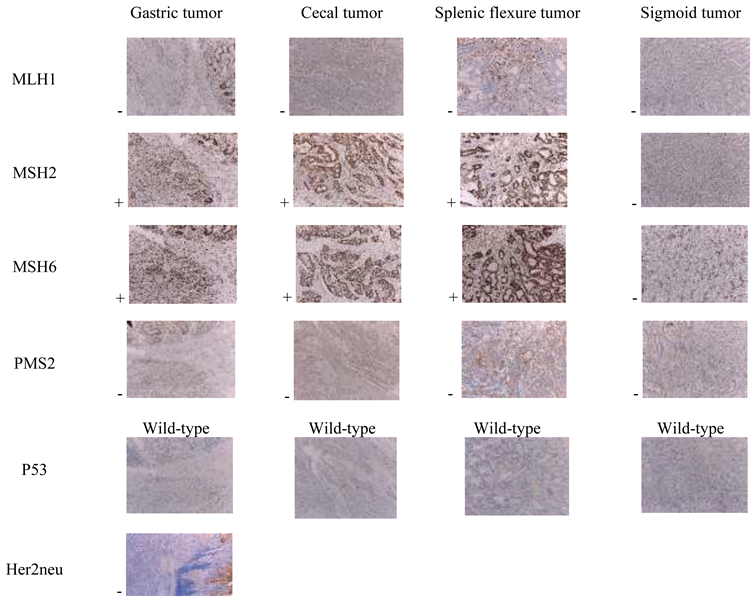
Case presentation

Discussions
Molecular events associated with colon carcinogenesis
Mismatch repair (MMR) genes and Microsatellite instability (MSI)
- CRC or endometrial carcinoma before the age of 50 years.
- A second CRC before the age of 70 years.
- CRC before the age of 70 years AND another synchronous or previous Lynch Syndrome associated tumor.
Clinical and prognostic features of MSI-H colorectal tumors
Highlights
- ✔
- Careful pre- and intraoperative evaluation of patients with suspected synchronous cancers is particularly important in therapeutic planning.
- ✔
- MSI represents a promising disease marker for colorectal cancers because of the favorable prognosis associated with MSI-H and particularities of oncological management.
Conclusions
Compliance with ethical standards
Conflict of Interest
References
- Bittorf, B.; Kessler, H.; Merkel, S.; Brückl, W.; Wein, A.; Ballhausen, W.G.; Hohenberger, W.; Günther, K. Multiple primary malignancies: An epidemiological and pedigree analysis of 57 patients with at least three tumours. Eur J Surg Oncol 2001, 27, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Suceveanu, A.I.; Mazilu, L.; Nitipir, C.; et al. Diabetes Mellitus raise the Risk for Interval Colorectal Cancer and Advanced Colorectal Adenomas. Revista Chimie 2019, 70, 1808–1811. [Google Scholar] [CrossRef]
- Nitipir, C.; Barbu, M.A.; Orlov, C.; et al. Type II Diabetes Mellitus—Associated Risk Factor in the Onset and Evolution of Digestive Tract Carcinoma. Romanian Biotechnological Letters 2019, 24, 140–146. [Google Scholar] [CrossRef]
- Şavlovschi, C.; Comandaşu, M.; Şerban, D. Specifics of diagnosis and treatment in synchronous colorectal cancers (SCC). Chirurgia 2013, 108, 43–45. [Google Scholar] [PubMed]
- Lam, A.K.; Chan, S.S.; Leung, M. Synchronous colorectal cancer: Clinical, pathological and molecular implications. World J Gastroenterol 2014, 20, 6815–6820. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Yu, C.S.; Kim, J.; et al. Clinicopathological features and surgical options for synchronous colorectal cancer. Medicine 2017, 96, e6224. [Google Scholar] [CrossRef]
- Kato, T.; Alonso, S.; Muto, Y.; et al. Clinical characteristics of synchronous colorectal cancers in Japan. World J Surg Oncol 2016, 14, 272. [Google Scholar] [CrossRef]
- Mazilu, L.; Suceveanu, A.I.; Tomescu, D.; et al. Optimizing the indication for breast-conservative surgery (BCS) in patients with locally-advanced breast cancer. Chirurgia 2013, 108, 478–481. [Google Scholar] [PubMed]
- Savlovschi, C.; Serban, D.; Trotea, T.; Borcan, R.; Dumitrescu, D. Post-surgery morbidity and mortality in colorectal cancer in elderly subjects. Chirurgia 2013, 108, 177–179. [Google Scholar]
- Nguyen, H.T.; Duong, H.Q. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol Lett. 2018, 16, 9–18. [Google Scholar] [CrossRef]
- van Lier, M.G.; Wagner, A.; van Leerdam, M.E.; et al. A review on the molecular diagnostics of Lynch syndrome: A central role for the pathology laboratory. J Cell Mol Med. 2010, 14, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Gafà, R.; Maestri, I.; Santini, A.; Matteuzzi, M.; Cavazzini, L. Immunohistochemical pattern of MLH1/MSH2 expression is related to clinical and pathological features in colorectal adenocarcinomas with microsatellite instability. Mod Pathol 2002, 15, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.C.; Lin, M.T.; Le, D.T.; Eshleman, J.R. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res. 2016, 22, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Lynch, P.M.; Lanspa, S.J.; Snyder, C.L.; Lynch, J.F.; Boland, C.R. Review of the Lynch syndrome: History, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 2009, 76, 1–18. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef]
- Lanza, G.; Gafà, R.; Maestri, I.; Santini, A.; Matteuzzi, M.; Cavazzini, L. Immunohistochemical pattern of MLH1/MSH2 expression is related to clinical and pathological features in colorectal adenocarcinomas with microsatellite instability. Mod Pathol 2002, 15, 741–749. [Google Scholar] [CrossRef]
- Jo, W.S.; Carethers, J.M. Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer Biomark. 2006, 2, 51–60. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Zamfir-Chiru-Anton, A.; Stanciu, M.M.; Pantea-Stoian, A.; Nitipir, C.; Gheorghe, D.C. Serum melatonin is inversely associated with matrix metalloproteinase-9 in oral squamous cell carcinoma. Oncol Lett 2020, 19, 3011–3020. [Google Scholar] [CrossRef]
- Smyrk, T.C.; Watson, P.; Kaul, K.; Lynch, H.T. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001, 91, 2417–2422. [Google Scholar] [CrossRef]
- Savlovschi, C.; Serban, D.; Andreescu, C.; Dascalu, A.; Pantu, H. Economic analysis of medical management applied for left colostomy. Chirurgia 2013, 108, 666–669. [Google Scholar]
- Canna, K.; McArdle, P.A.; McMillan, D.C.; et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005, 92, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Savlovschi, C.; Brănescu, C.; Serban, D.; et al. Hernia Amyand—caz clinic [Amyand's hernia—a clinical case]. Chirurgia 2010, 105, 409–414. [Google Scholar] [PubMed]
- Kiss, L.; Kiss, R.; Porr, P.J.; et al. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Chirurgia 2011, 106, 347–352. [Google Scholar]
- Jo, W.S.; Carethers, J.M. Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer Biomark. 2006, 2, 51–60. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef]
- Jover, R.; Zapater, P.; Castells, A.; et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut 2006, 55, 848–855. [Google Scholar] [CrossRef]
- McGivern, A.; Wynter, C.V.; Whitehall, V.L.; et al. Promoter hypermethylation frequency and BRAF mutations distinguish hereditary non-polyposis colon cancer from sporadic MSI-H colon cancer. Fam Cancer 2004, 3, 101–107. [Google Scholar] [CrossRef]
- Tulin, A.; Slavu, I.; Tulin, R.; et al. Does sex of the patient play a role in survival for MSI colorectal cancer? J Mind Med Sci. 2018, 5, 278–283. [Google Scholar] [CrossRef]
- Serban, D.; Smarandache, A.M.; Cristian, D.; Tudor, C.; Duta, L.; Dascalu, A.M. Medical errors and patient safety culture-shifting the healthcare paradigm in Romanian hospitals. Rom J Leg Med 2020, 28, 195–201. [Google Scholar] [CrossRef]
- Mazilu, L.; Stanculeanu, D.L.; Gheorghe, A.D.; Voinea, F.; Suceveanu, A.P.; Pituru, S.; Diaconu, C.C.; Parepa, I.R.; Pantea Stoian, A.; Pop, C.S.; et al. Incidenced of Chemotherapy Induced Peripheral Neuropathy in Cancer Patients in clinical Practice. Farmacia. 2018, 66, 904–908. [Google Scholar]
- Dumitrescu, D.; Savlovschi, C.; Borcan, R.; et al. Caz clinic—hernie diafragmatică voluminoasă—abdomen acut chirurgical: Dificultăţi diagnostice şi terapeutice [Clinical case—voluminous diaphragmatic hernia—Surgically acute abdomen: Diagnostic and therapeutical challenges]. Chirurgia 2011, 106, 657–660. [Google Scholar] [PubMed]
- Ciuhu, A.N.; Pantea-Stoian, A.M.; Nitipir, C.; et al. Assessment of cachexia in cancer patients with advanced disease. In Proceedings of the 3rd International Conference on Interdisciplinary Management of Diabetes Mellitus and its Complications (INTERDIAB), Sponsor(s): Assoc Renal Metab & Nutrit Studies; AstraZeneca Diabetes; MSD Diabetes; novo nordisk; Sanofi Interdiab 2017: Diabetes Mellitus in Internal Medicine Book Series: International Conference on Interdisciplinary Management of Diabetes Mellitus and its Complications. Bucharest, Romania, 2–4 March 2017; 2017; pp. 139–147. [Google Scholar]
 |
© 2020 by the author. 2020 Catalin Alius, Catalin Gabriel Cirstoveanu, Cristinel Dumitru Badiu, Valeriu Ardeleanu, Vasile Adrian Dumitru
Share and Cite
Alius, C.; Cirstoveanu, C.G.; Badiu, C.D.; Ardeleanu, V.; Dumitru, V.A. Immunohistochemical Pattern—A Prognostic Factor for Synchronous Gastrointestinal Cancer. J. Mind Med. Sci. 2020, 7, 250-256. https://doi.org/10.22543/7674.72.P250256
Alius C, Cirstoveanu CG, Badiu CD, Ardeleanu V, Dumitru VA. Immunohistochemical Pattern—A Prognostic Factor for Synchronous Gastrointestinal Cancer. Journal of Mind and Medical Sciences. 2020; 7(2):250-256. https://doi.org/10.22543/7674.72.P250256
Chicago/Turabian StyleAlius, Catalin, Catalin Gabriel Cirstoveanu, Cristinel Dumitru Badiu, Valeriu Ardeleanu, and Vasile Adrian Dumitru. 2020. "Immunohistochemical Pattern—A Prognostic Factor for Synchronous Gastrointestinal Cancer" Journal of Mind and Medical Sciences 7, no. 2: 250-256. https://doi.org/10.22543/7674.72.P250256
APA StyleAlius, C., Cirstoveanu, C. G., Badiu, C. D., Ardeleanu, V., & Dumitru, V. A. (2020). Immunohistochemical Pattern—A Prognostic Factor for Synchronous Gastrointestinal Cancer. Journal of Mind and Medical Sciences, 7(2), 250-256. https://doi.org/10.22543/7674.72.P250256



