Paroxysmal Nocturnal Hemoglobinuria: Pandora’s Box?
Abstract
:Introduction
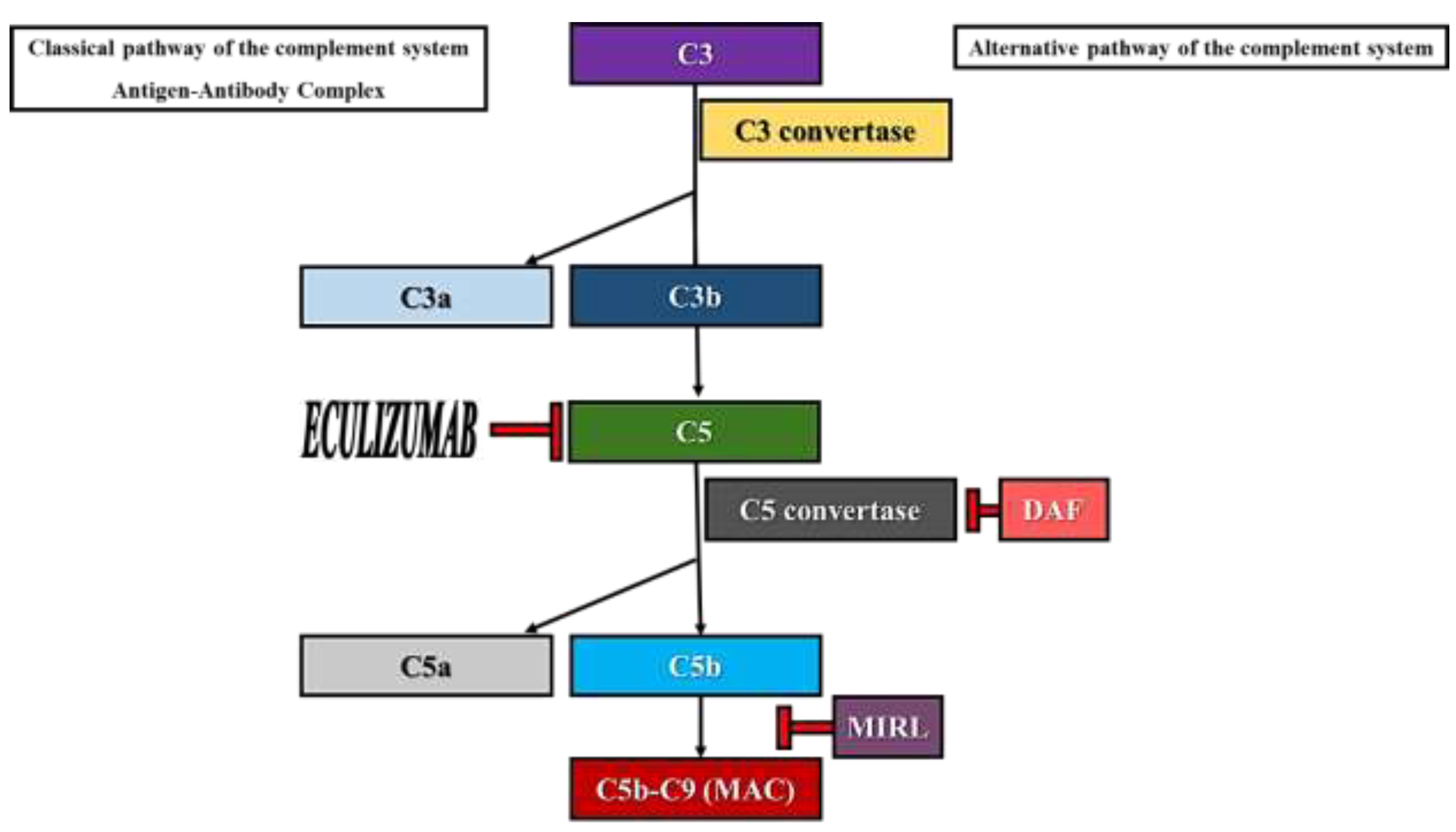
- the sensitivity of type I clones to complement- mediated lysis is normal. The expression of MIRL (CD59) and DAF (CD55) on the surface of erythrocytes is normal.
- the sensitivity of type II clones to complement- mediated lysis is 2-4 times higher than normal. These cells have a partial deficiency in MIRL and DAF. The clinical course of PNH patients whose erythrocytes’ phenotype corresponds to type II clones is benign, suffering from hemolytic crises only when supervening events (infections, trauma etc.) lead to the brisk activation of the complement system.
- the sensitivity of type III clones to complement- mediated lysis is 15-25 times higher than normal. These cells are totally deficient in MIRL and DAF and, thus, a higher representation of this clone will lead to a severe clinical PNH course.
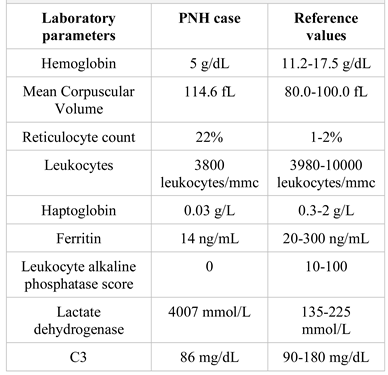
Case Presentation
- spontaneous cortico-subcortical hypodensity with loss of grey-white matter differentiation located in the right frontal area (compatible with a subacute ischemic stroke).
- small spontaneous hypodense lesion similar in density to the cerebrospinal fluid depicted as a craniocaudal band in the frontal white matter on the right side (possible sequelae lesion).
- minimal calcified atheromata of the parasellar segment of the internal carotid artery and left vertebral artery.
Discussions
- ✔
- Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired non-malignant hematological disorder which affects the pluripotent hematopoietic stem cell.
- ✔
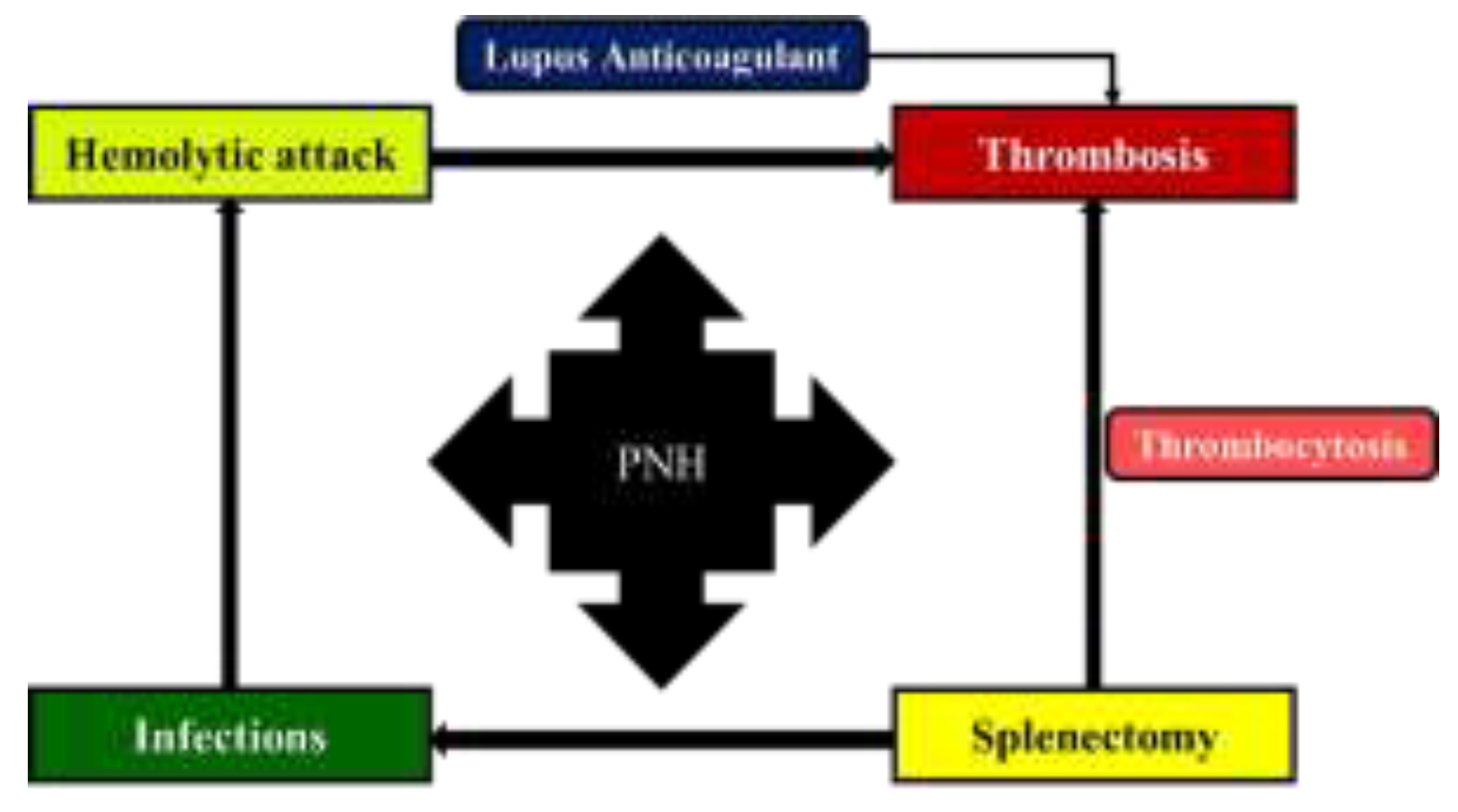
- PNH is characterized by intravascular hemolysis, thrombosis, and (or) bone marrow failure.
- ✔
- Although the clinical picture in PNH is dominated by the presence of hemolytic attacks, sometimes the pattern of the disease can change towards the predominance of thromboembolic episodes.
Conclusions
Author Contributions
Compliance with ethical standards
Acknowledgments
Conflicts of interest
Abbreviations
- Anti-DNAds antibodies, anti-double-stranded deoxyribonucleic acid antibodies.
- Anti-Ro/SSA antibodies, anti-Sjögren’s-syndrome- related antigen A antibodies.
- Anti-LA/SSB antibodies, anti-Sjögren’s-syndrome- related antigen B antibodies.
- CD, cluster of differentiation.
- DAF, decay-accelerating factor.
- GPI, glycosylphosphatidylinositol.
- LMWH, low molecular weight heparin.
- MAC, membrane attack complex.
- MIRL, membrane inhibitor of reactive lysis.
- PIGA, phosphatidylinositol glycan-A.
- PNH, paroxysmal nocturnal hemoglobinuria.
References
- Patriquin, C.J.; Kiss, T.; Caplan, S.; et al. How we treat paroxysmal nocturnal hemoglobinuria: A consensus statement of the Canadian PNH Network and review of the national registry. Eur J Haematol 2019, 102, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; DeZern, A.E.; Kinoshita, T.; et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017, 3, 17028. [Google Scholar] [CrossRef] [PubMed]
- Devalet, B.; Mullier, F.; Chatelain, B.; et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: A review. Eur J Haematol 2015, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.J. Update on the diagnosis and management of paroxysmal nocturnal hemoglobinuria. Hematology Am Soc Hematol Educ Program 2016, 2016, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Pérez, J.C.; Aguilar-Calderón, P.E.; Salazar-Cavazos, L.; et al. Evans syndrome: Clinical perspectives, biological insights and treatment modalities. J Blood Med 2018, 9, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, Q.; Luo, B.; et al. Prevalence of prothrombotic factors in patients with Budd-Chiari syndrome or non-cirrhotic nonmalignant portal vein thrombosis: A hospital-based observational study. J Gastroenterol Hepatol 2020, 35, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.U.; Saleem, H.; Iqbal, S.; Ghayas, R. A Case of Thrombosis Due To Paroxysmal Nocturnal Haemoglobinuria Presenting at An Early Age. J Ayub Med Coll Abbottabad 2018, 30, 138–139. [Google Scholar] [PubMed]
- Gad, M.M.; Ya’qoub, L.; Mahmoud, A.N.; et al. Echocardiography, Transcranial Doppler, and Oximetry for Imaging and Quantification of PFO-Mediated. In Patent Foramen Ovale Closure for Stroke, Myocardial Infarction, Peripheral Embolism, Migraine, and Hypoxemia; Academic Press: Cambridge, MA, USA, 2020; pp. 15–28. [Google Scholar] [CrossRef]
- Miranda, B.; Fonseca, A.C.; Ferro, J.M. Patent foramen ovale and stroke. J Neurol 2018, 265, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Tiu, R.; Yacoub, H.; Maciejewski, J.; et al. Recurrent ischemic stroke in paroxysmal nocturnal hemoglobinuria: Paroxysmal nocturnal hemoglobinuria or missed patent foramen ovale? J Stroke Cerebrovasc Dis 2009, 18, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, A.; Kleinstern, G.; Spectre, G.; et al. Thromboembolic Events Following Splenectomy: Risk Factors, Prevention, Management and Outcomes. World J Surg 2018, 42, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.J.; Ishtiaq, R.; Ishtiaq, D. Ischemic Stroke Presenting as the First Symptom in a Setting of Paroxysmal Nocturnal Hemoglobinuria. Cureus 2017, 9, e1439. [Google Scholar] [CrossRef] [PubMed]
- Laslo, C.L.; Stoian, A.P.; Socea, B.; et al. New oral anticoagulants and their reversal agents. J Mind Med Sci. 2018, 5, 195–201. [Google Scholar] [CrossRef]
- Gaman, M.A.; Epingeac, M.E.; Gaman, A.M. The relationship between oxidative stress markers, age, neutrophil-to-lymphocyte ration and obesity. HemaSphere 2019, 3 (Suppl. S1). [Google Scholar] [CrossRef]

 |
© 2020 by the author. 2020 Mihnea-Alexandru Găman, Iulia Ursuleac, Daniel Coriu
Share and Cite
Găman, M.-A.; Ursuleac, I.; Coriu, D. Paroxysmal Nocturnal Hemoglobinuria: Pandora’s Box? J. Mind Med. Sci. 2020, 7, 245-249. https://doi.org/10.22543/7674.72.P245249
Găman M-A, Ursuleac I, Coriu D. Paroxysmal Nocturnal Hemoglobinuria: Pandora’s Box? Journal of Mind and Medical Sciences. 2020; 7(2):245-249. https://doi.org/10.22543/7674.72.P245249
Chicago/Turabian StyleGăman, Mihnea-Alexandru, Iulia Ursuleac, and Daniel Coriu. 2020. "Paroxysmal Nocturnal Hemoglobinuria: Pandora’s Box?" Journal of Mind and Medical Sciences 7, no. 2: 245-249. https://doi.org/10.22543/7674.72.P245249
APA StyleGăman, M.-A., Ursuleac, I., & Coriu, D. (2020). Paroxysmal Nocturnal Hemoglobinuria: Pandora’s Box? Journal of Mind and Medical Sciences, 7(2), 245-249. https://doi.org/10.22543/7674.72.P245249



