Diagnosis and Management of Pericardial Effusion
Abstract
:Introduction
Discussions
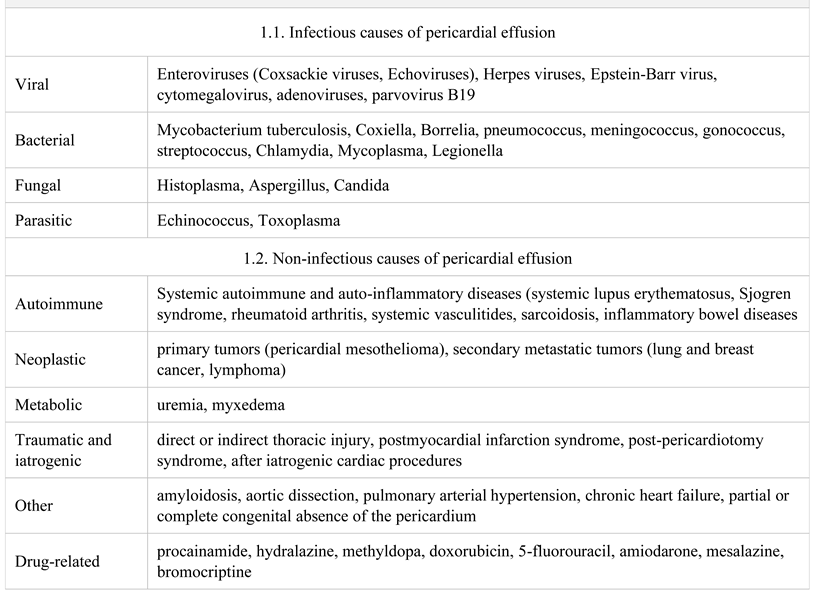
Etiology of pericardial effusion
Pathophysiology of pericardial effusion
Clinical presentation and paraclinical tests
Differential diagnosis of pericardial effusion
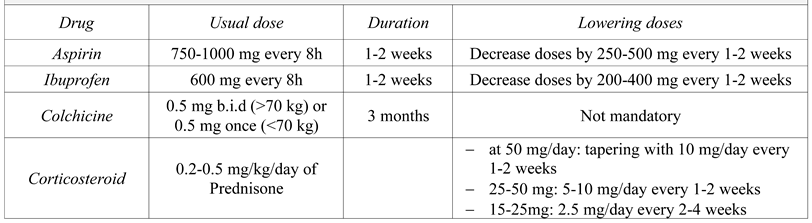
Treatment of pericardial effusion
Medical treatment
Pericardial drainage
Evolution and prognosis
Highlights
- ✓
- The etiology of pericardial effusion is diverse, from infectious diseases, malignancies, autoimmune diseases, chronic renal failure, to drug-related or after an invasive cardiac intervention.
- ✓
- Classical symptoms in pericardial effusion include pleuritic chest pain, associated with dyspnea.
- ✓
- Transthoracic echocardiography is the gold standard investigation for the diagnosis of pericardial effusions. The echocardiographic signs of cardiac tamponade are represented by the collapse of the right atrium and right ventricle, respiratory alteration of mitral and tricuspid flow, and changes of the inferior vena cava.
- ✓
- Pericardiocentesis guided by echocardiography is a life-saving procedure in cases of large pericardial effusions and cardiac tamponade.
Conclusions
Conflicts of Interest disclosure
Compliance with ethical standards
References
- Snyder, M.J.; Bepko, J.; White, M. Acute pericarditis: Diagnosis and management. Am Fam Physician. 2014, 89, 553–560. [Google Scholar] [PubMed]
- Sharma, N.K.; Waymack, J.R. Acute cardiac tamponade. [Updated 2019 Dec 17]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534806/.
- Adler, Y.; Charron, P.; Imazio, M.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015, 36, 2921–2964. [Google Scholar]
- Dababneh, E.; Siddique, M.S. Pericarditis. In StatPearlsPublishing [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28613734.
- Mayosi, B.M.; Burgess, L.J.; Doubell, A.F. Tuberculous pericarditis. Circulation. 2005, 112, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Ntsekhe, M.; Mayosi, B.M. Tuberculous pericarditis with and without HIV. Heart Fail Rev. 2013, 18, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zaha, D.C.; Bungau, S.; Aleya, S.; et al. What antibiotics for what pathogens? The sensitivity spectrum of isolated strains in an intensive care unit. Sci Total Environ. 2019, 687, 118–127. [Google Scholar] [CrossRef]
- Moisi, M.I.; Rus, M.; Bungau, S.; et al. Acute coronary syndromes in chronic kidney disease: Clinical and therapeutic characteristics. Medicina 2020, 56, 118. [Google Scholar] [CrossRef]
- Sagrista-Salueda, J.; Merce, A.S.; Soler-Soler, J. Diagnosis and management of pericardial effusion. World J Cardiol. 2011, 3, 135–143. [Google Scholar] [CrossRef]
- Imazio, M.; Adler, Y. Management of pericardial effusion. Eur Heart Journal. 2013, 34, 1186–1197. [Google Scholar] [CrossRef]
- Saito, Y.; Donohue, A.; Attai, S.; et al. The syndrome of cardiac tamponade with ‘small’ pericardial effusion. Echocardiography. 2008, 25, 321–327. [Google Scholar] [CrossRef]
- Shabetai, R. Pericardial effusion: Haemodynamic spectrum. Heart. 2004, 90, 255–256. [Google Scholar] [CrossRef]
- Stoicescu, M.; Csepento, C.; Mutiu, G.; et al. The role of increased level of plasma renin in etiopathogenic arterial hypertension in the young. Rom J Morphol Embryol 2011, 52 (Suppl. S1), 419–423. [Google Scholar] [PubMed]
- Stashko, E.; Meer, J.M. Cardiac Tamponade. [Updated 2019 Dec 5]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431090/.
- Maisch, B. Management of pericarditis and pericardial effusion, constrictive and effusive-constrictive pericarditis. Herz. 2018, 43, 663–678. [Google Scholar] [CrossRef]
- Imazio, M.; Gaita, F. Diagnosis and treatment of pericarditis. Heart. 2015, 101, 1159–1168. [Google Scholar] [CrossRef]
- Bodson, L.; Bouferrache, K.; Vieillard-Baron, A. Cardiac tamponade. Curr Opin Crit Care. 2011, 17, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Rosselo, X.; Wiegerinck, R.F.; Alguersuari, J.; et al. New electrocardiographic criteria to differentiate acute pericarditis and myocardial infarction. Am J Med. 2014, 127, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hae-Ok, J. Pericardial effusion and pericardiocentesis: Role of echocardiography. Korean Circ J. 2012, 42, 725–734. [Google Scholar]
- Little, W.C.; Freeman, G.L. Pericardial disease. Circulation. 2006, 113, 1622–1632. [Google Scholar] [CrossRef]
- Goodman, A.; Perera, P.; Mailhot, T.; Mandavia, D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012, 5, 72–75. [Google Scholar]
- Imazio, M.; Spodick, D.H.; Brucato, A.; et al. Controversial issues in the management of pericardial diseases. Circulation. 2010, 121, 916–928. [Google Scholar] [CrossRef]
- Cosyns, B.; Plein, S.; Nihoyanopoulos, P.; et al. Multimodality imaging in pericardial disease. Eur J Heart Cardiovasc Imaging. 2015, 16, 12–31. [Google Scholar] [CrossRef]
- Pepi, M.; Muratori, M. Echocardiography in the diagnosis and management of pericardial disease. J Cardiovasc Med. 2006, 7, 533–544. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; El-Tawil, O.S.; Bungau, S.G.; Atanasov, A.G. Applications of antioxidants in metabolic disorders and degenerative diseases: Mechanistic approach. Oxid Med Cell Longev. 2019, 2019, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, J.; Francone, M. Cardiovascular magnetic resonance in pericardial disease. J Cardiovasc Magn Reson. 2009, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S.; Bank, I.; Shinfeld, A. Diagnostic value of the biochemical composition of pericardial effusions in patients undergoing pericardiocentesis. Am J Cardiol. 2007, 99, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Brucato, A.; Trinchero, R. Colchicine for pericarditis: Hype or hope? Eur Heart J. 2009, 30, 532–539. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Trinchero, R.; et al. Individualized therapy for pericarditis. Expert Rev Cardiovasc Ther. 2009, 7, 965–975. [Google Scholar] [CrossRef]
- Suceveanu, A.I.; Pantea Stoian, A.; Parepa I et, a.l. Gut microbiota patterns in obese and type 2 Diabetes (T2D) patients from Romanian Black Sea coast region. Rev Chim. 2018, 69, 2260–2267. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Ristic, A.D.; Imazio, M.; et al. Management strategies in pericardial emergencies. Herz. 2006, 31, 891–900. [Google Scholar] [CrossRef]
- Mayosi, B.M.; Ntsekhe, M.; Volmink, J.A.; et al. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev 2002, CD000526. [Google Scholar]
- Kil, U.H.; Jung, H.O.; Koh, Y.S.; et al. Prognosis of large, symptomatic pericardial effusion treated by echo-guided percutaneous pericardiocentesis. Clin Cardiol. 2008, 31, 531–537. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.H.; Shin, J.D.; et al. Long-term follow-up results and clinical manifestations of patients with a moderate to large amount of pericardial effusion. Korean J Med. 2008, 74, 154–161. [Google Scholar]
- Halpern, D.G.; Argulian, E.; Briasoulis, A.; et al. A novel pericardial effusion scoring index to guide decision for drainage. Crit Pathw Cardiol. 2012, 11, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.; Enriquez-Sarano, M.; Freeman, W.K.; et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns and outcomes spanning 21 years. Mayo Clin Proc. 2002, 77, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Lindenberger, M.; Kjellberg, M.; Karlsson, E.; Wranne, B. Pericardiocentesis guided by 2-D echocardiography: The method of choice for treatment of pericardial effusion. J Intern Med. 2003, 253, 411–417. [Google Scholar] [CrossRef]
- Loukas, M.; Walters, A.; Boon, J.M.; et al. Pericardiocentesis: A clinical anatomy review. Clin Anat. 2012, 25, 872–881. [Google Scholar] [CrossRef]
- Manea, M.; Marcu, D.; Pantea Stoian, A.; et al. Heart failure with preserved ejection fraction and atrial fibrillation: A review. Rev Chim. 2018, 69, 41804184. [Google Scholar] [CrossRef]
- Khandaker, M.H.; Schaff, H.V.; Greason, K.L.; et al. Pericardiectomy vs medical management in patients with relapsing pericarditis. Mayo Clin Proc. 2012, 87, 1062–1070. [Google Scholar] [CrossRef]
- Hota, S.S.; Chow, C.M.; Bonneau, D. Surgical treatment for incessant pericarditis. Can J Cardiol. 2009, 25, 161162. [Google Scholar] [CrossRef]
 |
© 2020 by the author. 2020 Maria Manea, Ovidiu Gabriel Bratu, Nicolae Bacalbasa, Camelia Cristina Diaconu
Share and Cite
Manea, M.; Bratu, O.G.; Bacalbasa, N.; Diaconu, C.C. Diagnosis and Management of Pericardial Effusion. J. Mind Med. Sci. 2020, 7, 148-155. https://doi.org/10.22543/7674.72.P148155
Manea M, Bratu OG, Bacalbasa N, Diaconu CC. Diagnosis and Management of Pericardial Effusion. Journal of Mind and Medical Sciences. 2020; 7(2):148-155. https://doi.org/10.22543/7674.72.P148155
Chicago/Turabian StyleManea, Maria, Ovidiu Gabriel Bratu, Nicolae Bacalbasa, and Camelia Cristina Diaconu. 2020. "Diagnosis and Management of Pericardial Effusion" Journal of Mind and Medical Sciences 7, no. 2: 148-155. https://doi.org/10.22543/7674.72.P148155
APA StyleManea, M., Bratu, O. G., Bacalbasa, N., & Diaconu, C. C. (2020). Diagnosis and Management of Pericardial Effusion. Journal of Mind and Medical Sciences, 7(2), 148-155. https://doi.org/10.22543/7674.72.P148155