Abstract
According to statistical data published in 2019, breast cancer is among the leading causes of death in women worldwide. The serine/threonine kinase (AKT) or protein kinase B (PkB) signaling pathway is activated by phosphorylation processes, which further is associated with cell growth, proliferation, and survival, but also with activation of glucose metabolism. Mutations of the AKT signaling pathway components (especially PI3KCA and PTEN) have been observed in breast cancer patients, which are associated with resistance to hormonal treatment. Many clinical trials are testing the effect of AKT inhibition in order to block the growth and proliferation of breast cancer cells. The purpose of this review is to present the incidence of this neoplastic disease, to describe AKT signaling pathways activation, mutations that occur at its level, and inhibitors that can block this protein kinase.
Introduction
Following a systemic analysis conducted over a period of 27 years (1990–2017) regarding the incidence of cancer, published online in September 2019, there were 24.5 million cases and 9.6 million deaths registered worldwide [1]. Skin, breast, and colorectal cancer were the most common neoplastic disorders reported among women, representing 54% of all cancers [1]. In the Global top 10 cancers, breast cancer was the third most common cancer overall, with 2 million incident cases registered in 2017. Of the 143 countries that participated in the systemic analysis, in 112 countries breast cancer was the most common cause of death among women [1].
According to statistical data from Romania, the incidence of neoplastic diseases in 2018 registered at 83,461 cases for both sexes, with 50,902 deaths [2]. In Romanian women, the 5 most common types of cancer are breast, colorectal, cervix uteri, lung, and corpus uteri. In 2018, 38,439 cases of cancer were diagnosed in women, of which 9625 were breast cancer [2]. Last year, Romania registered 30,100 deaths among men and 20,200 among women. In the case of women of Romanian origin, as well as in other countries, breast cancer represents the most common neoplastic disease, with 8,981 cases reported.
Breast palpation and clinical breast examination are the classic methods available to all women for detecting tumors, as mammography is not generally available at the public health care level in many areas [3].
Discussions
Breast Cancer Subtypes
Currently, for a more accurate diagnosis of breast cancer, a number of factors have been proposed, such as tumor size and degree, histological subtype, lymphovascular invasion of tumor cells, presence of tumor cell receptors, and axial lymph nodes.
Ki-67 is currently used as a valuable parameter that expresses the potential for proliferation and division of tumor cells. By combining genetic tests with histological analyses (macroscopic and microscopic) and immunohistochemistry, it is possible to detect the presence of cell surface receptors [estrogen, progesterone, and human epidermal growth factor receptor 2 (HER2)] [4].
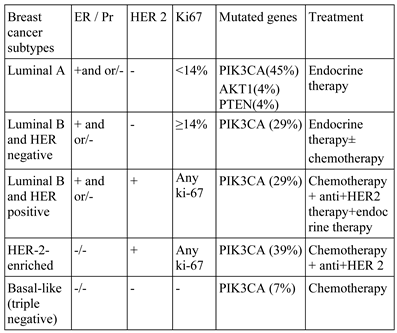
Luminal A, Luminal B with HER 2 negative, Luminal B with Her 2 positive, HER 2 enriched, and basal-like (triple negative) are the most recent classifications (in 2013) for breast cancer, according to the St Gallen classification system (Table 1) [4].

Table 1.
According to St. Gallen classification (adapted from [4,5,6,7]).
Pi3k signaling pathway
Phosphatidylinositol-3 kinases (PI3K) are a family of lipid structure kinases involved in the regulation of numerous biological processes, such as cell proliferation, survival and differentiation, metabolism, and migration [8]. Depending on their structure and kinetic differences, these protein kinases can be divided into 3 classes: class I, II, and III. PI3Kα, PI3Kβ, PI3KŸ and PI3Kچ belong to class I and are unfortunately abnormally activated in breast cancer [9]. These kinases contain a regulatory subunit and a catalytic subunit, so for class IA, there are 3 catalytic subunits (p110α, p110β, p110چ) that are encoded by PIK3CA, PIK3CB, PIK3CD. The regulatory subunits of class IA, p85α, p85β and p55Ÿ, are encoded by PIK3R1, PIKR2 and PIKR3 [8,9]. In the case of IB class of protein kinases, which are heterodimers, the catalytic subunit p110Ÿ is encoded by PIK3CG, presenting one or two regulatory subunits, namely p101 encoded by PIK3CR5 or p87 encoded by PIK3R6 [10]. All cell types express the catalytic subunits p110α and p110β, while p110Ÿ and p110چ are expressed only by leukocytes [10].
Class I PI3Ks are recruited to the plasma membrane when the activator binds to the specific receptor, so the catalytic subunit phosphorylates phosphatidylinositol 4,5 bisphosphate (PIP2) to phosphatidyl inositol 3,4,5 triphosphate (PIP3). PIP3 acts as a secondary messenger and coordinates AKT localization at the plasma membrane and then phosphorylation by PDK1 (phosphoinositide dependent protein kinase-1) [10]. PDK1 phosphorylates AKT at threonine residue 308, and for complete activation, serine residue 473 is phosphorylated by mTOR (mammalian target of rapamycin) [10,11].
mTOR is a serine / threonine protein kinase complex that is composed of mTORC 1 and mTORC 2, these having similar structure but different functions. mTORC2 phosphorylates AKT for full activation at serine. Following activation by phosphorylation of AKT, this protein kinase phosphorylates and thus inhibits tuberous sclerosis complex 1 and 2 (TSC 1 and 2), this inhibition leading to the activation of mTORC1. Once activated mTORC1, it activates in turn 40 S ribosomal protein S6 kinase (S6K) and eukaryotic initiation factor 4E binding protein (4EBP1) which lead to cell growth and activation of glucose metabolism [12,13].
Phosphatase and tensin homolog (PTEN) negatively regulate AKT by dephosphorylating PIP3 to PIP2. Apart from PTEN, it has recently been observed that there may be another negative regulator for PIP3, polyphosphate 4-phosphatase type II (INPP4B) [14].
AKT Activation in Cancer
The incidence of mutations that occur in the AKT signaling pathway in breast cancer is 25%. Most of these mutations are found in the PIKCA that encodes the p110α catalytic subunit, with the substitution of a single amino acid such as E545K and E542K in the helical domain (exon 9) and H1047R in the kinase domain (exon20) [15]. Mutations occurring both in the helical and kinase domains will increase the enzymatic activity, of components of AKT signaling pathways, which will promote oncogenic transformation. Depending on the breast cancer subtype, AKT mutations occur around 20-25% [16].
An increase of the mutations of over 30% is registered in the case of the tumor that has hormonal receptors, for HER2+, is registered 25% AKT mutations. In contrast, triple negative breast cancer has the lowest rate of AKT mutations [16]. A number of somatic gene mutations have already been observed within this signaling pathway, including PI3CA, PIKR1, PTEN, AKT1, which will result in either increased PI3K activity or loss of PTEN functionality [16].
In mammals, class 1 protein kinases are divided into 1A and 1B, the first class being activated by tyrosine kinase receptor (RTKs), and the second class by receptors coupled with G proteins.
The involvement of PI3K/ AKT signaling pathways in breast cancer pathogenesis can be achieved through several mechanisms:
- The occurrence of mutations or amplification of PI3KCA, PIK3CB, PIK3R1 leads to increased activity of AKT signaling pathways.
- Overexpression of some target molecules or activating signals such as HER 2, epidermal growth factor receptor (EGFR), insulin-like growth factor receptor 1(IGF-R1).
- Loss of negative regulators for PIP3, PTEN and INPP4B.
- Overexpression of AKT1, AKT2 and PDK1 [16].
In breast cancer, the most common mutations of AKT which determine the overactivation of this signaling pathway are the loss of the negative PTEN regulator and the PIK3CA mutation [17]. These changes cause cell transformation, progression, and chair formation of the tumor mass. In 2004, PIK3A, which encodes the p110α catalytic subunit, was discovered to be the most common genetic mutation being considered a highly oncogenic gene [17]. Other mutations have been identified, for example, in the kinase domain H1047R, in the catalytic domain E542K and E545K [16]. She and co-workers identified a 25% reduction for PTEN in breast cancer; the same team of researchers observed in the case of triple negative breast cancer, characterized by a low survival rate, alteration with 30% of PTEN [18].
Endocrine Resistance and PI3K/AKT
Mutations in AKT signaling pathways may be responsible for resistance to hormonal treatment. Hyperactivation of receptors such as HER1, HER2, EGFR via AKT may contribute to the resistance of anti-estrogen therapy [19].
Several mechanisms have been described as responsible for endocrine resistance in breast cancer, such as disorders of the ER/PgR pathway components, modifications of signaling molecules involved in the cell cycle or survival, or activation of signaling pathways that ensure cell replication [20]. ER is composed of 2 subunits, namely ERα and ERβ, being a nuclear receptor. Estrogen activation of ERα is responsible in many types of breast cancer for cell proliferation. In contrast, ERβ exhibits opposite effects of ERα, inhibiting the stimulatory effects of estradiol (E2) on cell proliferation. Alpha and beta receptors contain two transcriptional domains, namely AF1 and AF2, both of which have activation functions [21]. Binding of E2 to the ER leads to the activation of a number of processes that result in the translocation of chaperone proteins from the Erα receptor, receptor dimerization, phosphorylation and finally the binding of ER to DNA [22].
AF1 regulates gene transcription, even in cells that exhibit ERα deletion, being independent of ligand, AF2 instead binds to coactivators or corepressors [23]. Activation of AF1 is mediated by crosslinks and crosstalk among the RAS/B-RAF, AKT and CDK2 (cyclin-dependent kinase 2/7) pathways. This activation mechanism unfortunately leads to resistance observed in different endocrine therapies [24]. At the molecular level, estrone activity leads to activation of insulin-like growth factor (IGF), AKT, and MAPK signaling pathways. This activation will downregulate ER and PgR expression at the cell surface level [25].
In the case of metastatic breast cancer, the AKT signaling pathway is most frequently altered, upregulation of this molecules promotes the transcriptional activity of ER that will contribute to anti-estrogen resistance, leading to tumor growth, motility, metabolism and survival [26].
Breast Cancer Biomarkers and Therapy
Currently, a number of AKT signaling pathway inhibitors are being studied, which generally focus on the simultaneous blocking of PI3K and mTOR. As already noted, there is a link between increased activity of PI3K and breast cancer tumorigenesis or drug resistance. Recent studies have revealed the importance of testing and using PI3K inhibitors in the tumor microenvironment [27].
Beneficial effects of PI3K inhibition have been observed in the treatment of leukemia, improving immunotherapy in solid cancer where it selectively blocks T cell-mediated immune tolerance [28]. For example, agent EL147 is a PI3K selective inhibitor that can block IPI3K class. This drug, which can be administered orally, cannot inhibit mTOR but a preclinical efficacy has been observed on PI3K and PTEN. Among the first drugs that were studied were those that were analogues of rapamycin, so mTOR inhibition may play a crucial role in the therapy of breast cancer. In a phase II randomized trial, patients diagnosed with breast tumors > 2cm ER+ received letrozole plus placebo for 16 weeks or letrozole and everolimus (rapamycin analog).
The administration of everolimus had a better response rate to treatment. The use of mTOR inhibitors has beneficial effects in breast cancer therapy [29].
Breast cancer classification is currently performed according to histological evaluation, immunohistochemistry and genetic testing, and biomarker detection can be of real use in the most accurate diagnosis of this neoplastic disease. Increased expression of cytokeratin 5/6 and 17, laminin, and fatty acid binding protein was observed in basal-like tumors. These biomarkers show a 15% increase in invasive cancers, being associated with breast cancer and BRCA mutations [30].
Depending on the molecular classification, Luminal A is a hormone receptor-positive breast cancer (estrogen receptor and / or progesterone receptor positive), HER2 negative, and is associated with low levels of Ki-67 protein, which helps in rapid growth of cancer cells. Luminal A breast cancer has a lower degree, the proliferation rate is slow, and it has the best prognosis. Luminal B breast cancer is positive for hormone receptors (estrogen receptor and / or progesterone receptor positive) and HER2 positive or HER2 negative, associated with increased Ki-67 levels. The rate of proliferation of Luminal cancer B is faster than Luminal A breast cancer [30].
Negative/ basal triple breast cancer is the type of neoplasia without hormonal receptors (estrogen receptor and progesterone receptor negative) and HER2 negative. It unfortunately develops in women with BRCA1 gene mutations, among younger and African-American women. HER2-enriched breast cancer is hormone receptor negative (estrogen and progesterone) and HER2 positive. These cancers have a faster proliferation rate than Luminal breast cancers and a poorer prognosis, although they may be successfully treated with therapies targeting the HER2 protein, such as Herceptin (trastuzumab), Perjeta (pertuzumab), Tykerb (lapatinib) and Nerlynx (neratinib) [30].
pS6K (S6 kinase) and pAKT are biomarkers of response to inhibitors for mTOR pathway (rapamycin analogues), tested on cell lines and tumors. Increased levels of pS6K are associated with a reserved survival prognosis [30]. Increased levels of phosphorylated proteins such as AKT, GSK3β, and TSC2 have been observed in various tumor cell lines [31].
INPP4B is a potential tumor suppressor biomarker that regulates AKT, so deletion of this phosphatase results in PTEN loss that will correlate with poor survival prognosis [31,32,33].
An inhibitor of mTORC 1, everolimus was tested on breast cancer cell lines that had the PIKCA mutation where mTORC1 inhibition could be observed [31].
Ribosomal S6 kinases (RPS6KA2) and S4 (RSK4) and c-myc activation are mutations of the mitogen-activated protein kinase signaling pathways (MAPK) that may be the key of resistance to AKT inhibitors. Positive effects have been reported for basal-like triple negative breast cancer where inhibitors for AKT and MAPK have been used [31].
Highlights
- ✓
- Mutations of the AKT signaling pathway components (especially PI3KCA and PTEN) have been observed in breast cancer patients, which are associated with resistance to hormonal treatment.
- ✓
- Mutations that occur at this signaling pathway cause AKT overactivation which will further lead to the activation of target molecules responsible for cell growth, survival and proliferation and protein synthesis of breast tumor cells.
Conclusions
AKT is an extremely complicated intracellular signaling pathway that has an important role in the pathogenesis of breast cancer. Mutations that occur at this signaling pathway cause AKT overactivation which will further lead to the activation of target molecules responsible for cell growth, survival, and proliferation and protein synthesis of breast tumor cells.
Conflicts of Interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability- Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study [published online ahead of print, 2019 Sep 27]. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- World Health Organisation. Romania, Blobocan. 2018. Available online: https://gco.iarc.fr/.
- World Health Organisation. The global cancer observatory 2018. 2018. Available online: https://gco.iarc.fr/today/home.
- Prat, A.; Pineda, E.; Adamo, B.; et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015, 24 Suppl 2, S26–S35. [Google Scholar] [CrossRef]
- Hashmi, A.A.; Aijaz, S.; Khan, S.M.; et al. Prognostic parameters of luminal A and luminal B intrinsic breast cancer subtypes of Pakistani patients. World J Surg Oncol. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, C.S.; Gómez, H.L.; Cruz, W.R.; et al. Breast cancer classification according to immunohistochemistry markers: Subtypes and association with clinicopathologic variables in a peruvian hospital database. Clin Breast Cancer. 2010, 10, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Yang, S.X.; Polley, E.; Lipkowitz, S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev. 2016, 45, 87–96. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stefani, C.; et al. Structure, activation and biological effects of AKT or protein kinase B. Rev Medicala Romana 2019, 14, 233–237. [Google Scholar] [CrossRef]
- Holz, M.K. The role of S6K1 in ER-positive breast cancer. Cell Cycle 2012, 11, 3159–3165. [Google Scholar] [CrossRef][Green Version]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Woolley, J.F.; Dzneladze, I.; Salmena, L. Phosphoinositide signaling in cancer: INPP4B Akt(s) out. Trends Mol Med. 2015, 21, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016, 67, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Samuels, Y.; Wang, Z.; Bardelli, A.; et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef] [PubMed]
- She, Q.B.; Gruvberger-Saal, S.K.; Maurer, M.; et al. Integrated molecular pathway analysis informs a synergistic combination therapy targeting PTEN/PI3K and EGFR pathways for basal-like breast cancer. BMC Cancer. 2016, 16, 587. [Google Scholar] [CrossRef]
- Di Cosimo, S.; Baselga, J. Management of breast cancer with targeted agents: Importance of heterogenicity. Nat Rev Clin Oncol. 2010, 7, 139–147. [Google Scholar] [CrossRef]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011, 62, 233–247. [Google Scholar] [CrossRef]
- Eyster, K.M. The estrogen receptors: An overview from different perspectives. Methods Mol Biol. 2016, 1366, 1–10. [Google Scholar]
- Arnal, J.F.; Fontaine, C.; Abot, A.; et al. Lessons from the dissection of the activation functions (AF-1 and AF- 2) of the estrogen receptor alpha in vivo. Steroids 2013, 78, 576–582. [Google Scholar] [CrossRef]
- Bostner, J.; Skoog, L.; Fornander, T.; et al. Estrogen receptor-alpha phosphorylation at serine 305, nuclear p21-activated kinase 1 expression, and response to tamoxifen in postmenopausal breast cancer. Clin Cancer Res. 2010, 16, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Stebbing, J.; Giamas, G.; Murphy, J. Endocrine resistance in hormone receptor positive breast cancer- from mechanism to therapy. Front Endocrinol. 2019, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, T.J.; Meier, J.B.; Jansen, V.M. Current landscape of targeted therapies for hormone-receptor positive, HER2 negative metastatic breast cancer. Front Oncol. 2018, 8, 308. [Google Scholar] [CrossRef]
- Miller, T.W.; Rexer, B.N.; Garrett, J.T.; Arteaga, C.L. Mutations in the phosphatidylinositol 3-kinase pathway: Role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011, 13, 224. [Google Scholar] [CrossRef]
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; et al. A phase Ib study of alpelisib (BYL719), a PI3Ka- specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef]
- Ahmad, S.; Abu-Eid, R.; Shrimali, R.; et al. Differential PI3Kd signaling in CD4+ T-cell subsets enables selective targeting of T regulatory cells to enhance cancer immunotherapy. Cancer Res. 2017, 77, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Motofei, I.G.; Rowland, D.L.; Popa, F.; et al. A Pilot Study on Tamoxifen Sexual Side Effects and Hand Preference in Male Breast Cancer. Arch Sex Behav. 2015, 44, 1589–1594. [Google Scholar] [CrossRef]
- Baselga, J.; Semiglazov, V.; van Dam, P.; et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009, 27, 2630–2637. [Google Scholar] [CrossRef]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR pathway in brest cancer: Targets, trials and biomarkers. Ther Adv Med Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Mazilu, L.; Stanculeanu, D.L.; Gheorghe, A.D.; et al. Chemotherapy and other factors affecting quality of life in non-small cell lung cancer (NSCLC) patients. Rev Chim (Bucharest). 2019, 70, 33–35. [Google Scholar] [CrossRef]
- Suceveanu, A.I.; Mazilu, L.; Nitipir, C.; Pantea Stoian, A.; Parepa, I.; Voinea, C.; Suceveanu, A.P. Diabetes mellitus raise the risk for interval colorectal cancer and advanced adenomas. Rev Chim (Bucharest). 2019, 70, 1808–1811. [Google Scholar] [CrossRef]
© 2020 by the author. 2020 Daniela Miricescu, Camelia Cristina Diaconu, Constantin Stefani, Ana Maria Alexandra Stanescu, Alexandra Totan, Ioana Ruxandra Rusu, Ovidiu Gabriel Bratu, Dan Spinu, Maria Greabu