Laparoscopic Resection of Gastric GISTs. Where Do We Stand Now? A Single-Centered Experience
Highlights
- Complete surgical resection independent of tumor size represents the current optimal treatment of GISTs.
- Laparoscopic resection is possible, although the surgeon’s experience along with careful patient selection plays a critical role.
Abstract
:Highlights
- Complete surgical resection independent of tumor size represents the current optimal treatment of GISTs.
- Laparoscopic resection is possible, although the surgeon’s experience along with careful patient selection plays a critical role.
Abstract
Introduction
Materials and Methods
Results
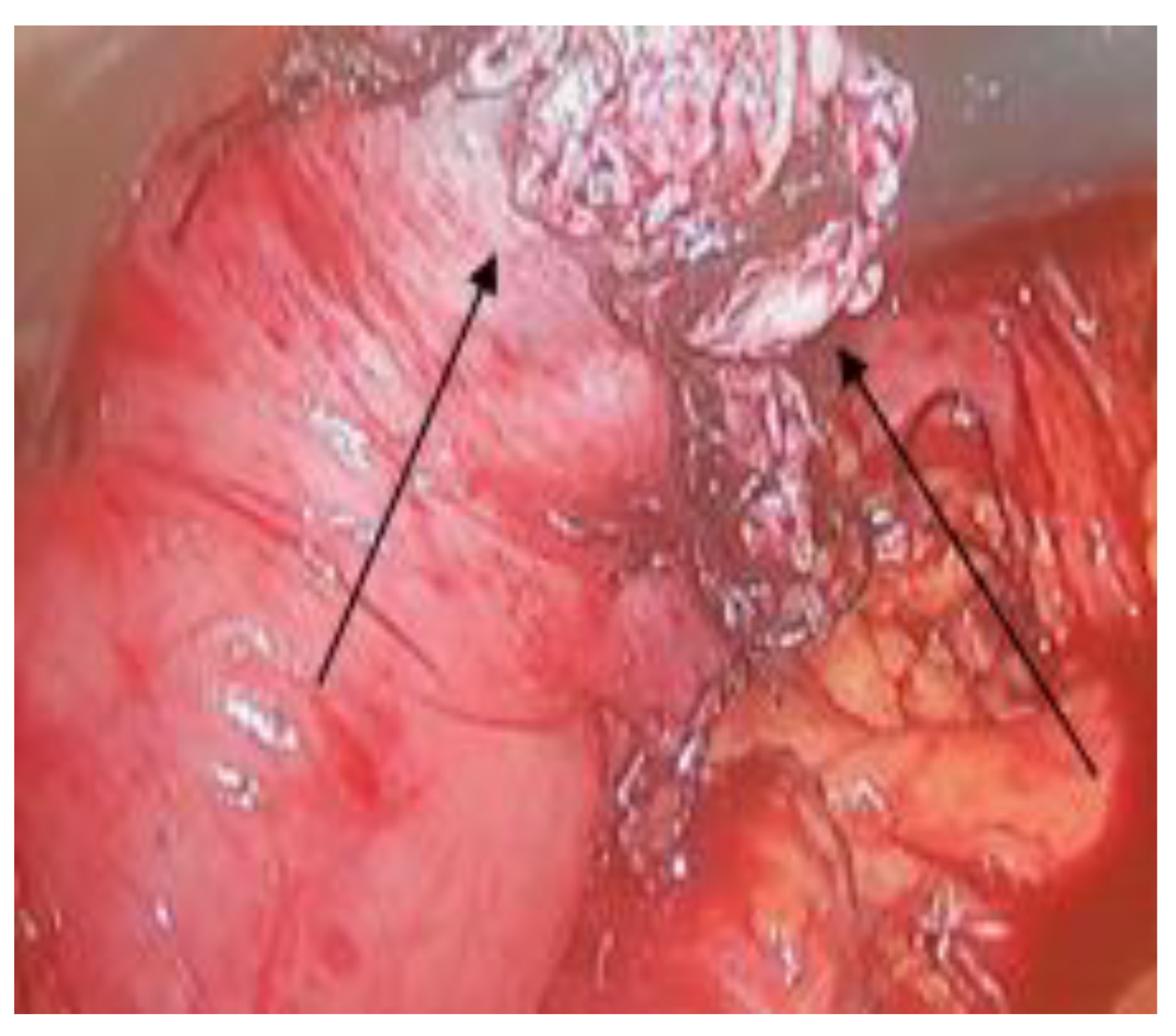
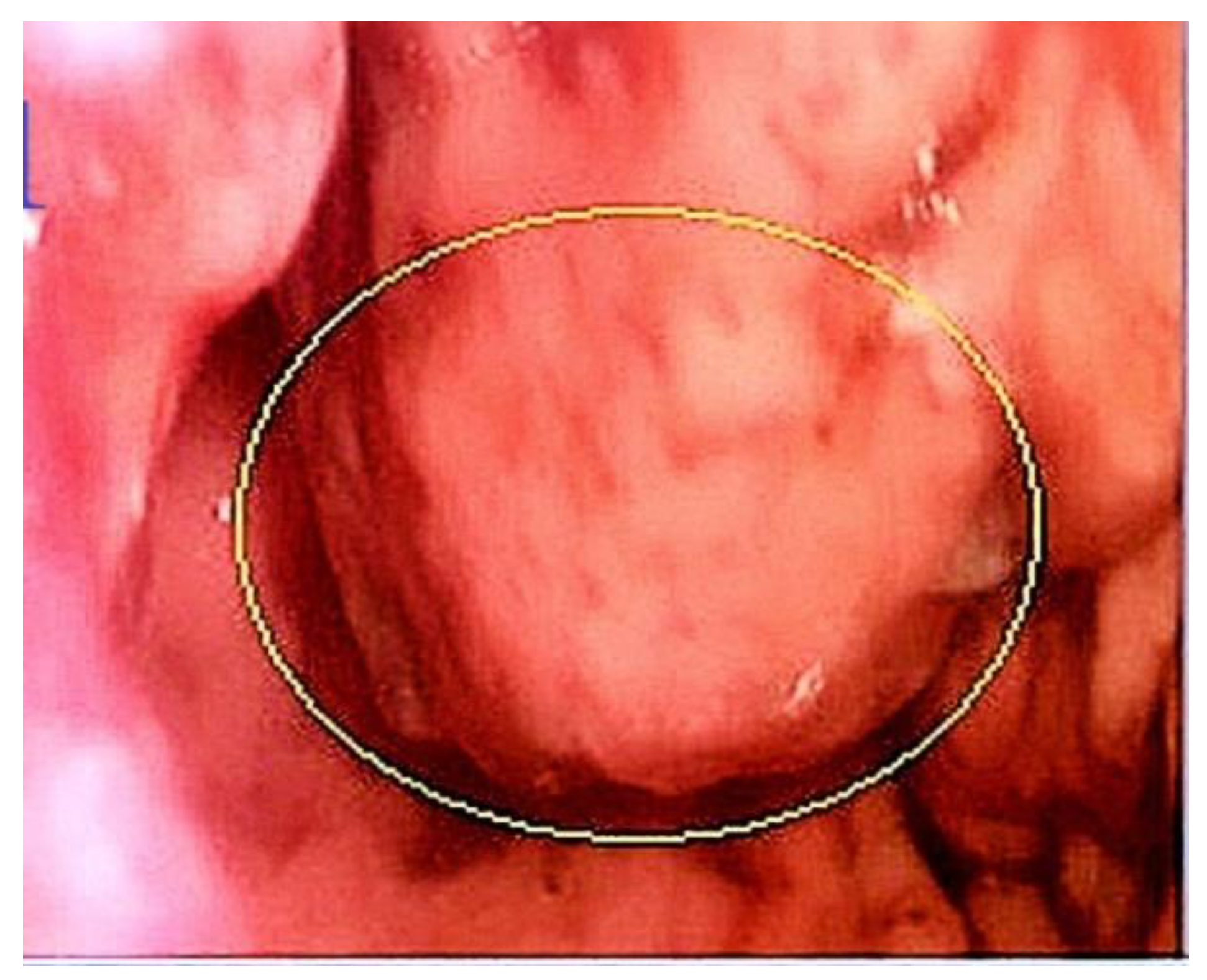


Discussions
Conclusions
Conflicts of Interest Disclosure
Compliance with Ethical Standards
References
- Corless, C.L.; Fletcher, J.A.; Heinrich, M.C. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004, 22, 3813–3825. [Google Scholar] [CrossRef] [PubMed]
- Nowain, A.; Bhakta, H.; Pais, S.; et al. Gastrointestinal stromal tumors: clinical profile, pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol. 2005, 20, 818–824. [Google Scholar] [CrossRef]
- Sircar, K.; Hewlett, B.R.; Huizinga, J.D.; Chorneyko, K.; Berezin, I.; Riddell, R.H. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999, 23, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.R.; Lee, W.J. SCY Minimally invasive surgery for gastric stromal cell tumors: intermediate follow-up results. J Gastrointest Surg. 2006, 10, 563–566. [Google Scholar] [CrossRef]
- Yang, J.; Feng, F.; Li, M.; Sun, L.; Hong, L.; Cai, L.; Wang, W.; Xu, G.; Zhang, H. Surgical resection should be taken into consideration for the treatment of small gastric gastrointestinal stromal tumors. Word J Surg Oncol. 2013, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, C.; Zheng, C.; Li, P.; Xie, J.; Wang, J.; Lu, J. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): a size-matched comparison. Surg Endosc. 2014, 28, 2577–2583. [Google Scholar] [CrossRef]
- Joensuu, H.; Fletcher, C.; Dimitrijevic, S.; Silberman, S.; Roberts, P.; Demetri, G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002, 3, 655–664. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Jim, J.; Nguyen, A.; Lee, J.; Chang, K. Laparoscopic resection of gastric stromal tumor: a tailored approach. Am Surg. 2003, 69, 946–950. [Google Scholar] [CrossRef]
- Koh, Y.X.; Chok, A.Y.; Zheng, H.L.; Tan, C.S.; Chow, P.K.; Wong, W.K.; Goh, B.K. A systematic review and meta- analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013, 20, 3549–3560. [Google Scholar] [CrossRef]
- Liang, J.W.; Zheng, Z.C.; Zhang, J.J.; Zhang, T.; Zhao, Y.; Yang, W.; Liu, Y.Q. Laparoscopic versus open gastric resections for gastric gastrointestinal stromal tumors: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2013, 23, 378–387. [Google Scholar] [CrossRef]
- De Vogelaere, K. 1.; Van Loo, I.; Peters, O.; Hoorens, A.; Haentjens, P.; Delvaux, G. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg Endosc. 2012, 26, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.D.; Walsh, R.M.; Kercher, K.W.; et al. Laparoscopic vs. open resection of gastric stromal tumors. Surg Endosc. 2002, 16, 803–807. [Google Scholar] [CrossRef]
- Mazur, M.T.; Clark, H.B. Gastric stromal tumors: reappraisal of histogenesis. Am J Surg Pathol. 1983, 7, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Bolocan, A.; Paduraru, D.N.; Nitipir, C.; et al. Mixed adenoneuroendocrine carcinoma of the gastrointestinal tract-features, diagnosis, management and prognostics. Romanian Biotechnological Letters. 2018, 23, 14193–14202. [Google Scholar]
- Ng, E.H.; Pollock, R.E.; Munsell, M.F.; et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas: implications for surgical management and staging. Ann Surg. 1992, 215, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H.; Yang, H.K. Surgical Treatment of Gastric Gastrointestinal Stromal Tumor. J Gastric Cancer. 2013, 13, 3–18. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Corless, C.L. Gastric GI stromal tumors (GISTs): the role of surgery in the era of targeted therapy. J Surg Oncol. 2005, 90, 195–207, discussion 207. [Google Scholar] [CrossRef]
- Yoshida, M.; Otani, Y.; Ohgami, M.; et al. Surgical management of gastric leiomyosarcoma: evaluation of the propriety of laparoscopic wedge resection. World J Surg. 1997, 21, 440–443. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Corless, C.L. Gastric GI stromal tumors (GISTs): the role of surgery in the era of targeted therapy. J Surg Oncol. 2005, 90, 195–207. [Google Scholar] [CrossRef]
- Gastrointestinal stromal tumours. Gastrointestinal stromal tumours. ESMO clinical practice guidelines for diagnosis, treatment and follow- up. Ann Oncol 2014, 25 (Suppl. S3), iii21–iii26. [Google Scholar] [CrossRef]
- Ye, L.; Wu, X.; Wu, T.; Wu, Q.; Liu, Z.; Liu, C.; Li, S.; Chen, T. Meta-analysis of laparoscopic vs. open resection of gastric gastrointestinal stromal tumors. PLoS One. 2017, 12, e177193. [Google Scholar] [CrossRef]
- Otani, Y.; Furukawa, T.; Yoshida, M.; Saikawa, Y.; Wada, N.; Ueda, M.; Kubota, T.; Mukai, M.; Kameyama, K.; Sugino, Y.; Kumai, K.; Kitajima, M. Operative indications for relatively small (2–5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. 2006, 139, 484–492. [Google Scholar] [CrossRef]
- Catena, F.; Di Battista, M.; Fusaroli, P.; Ansaloni, L.; Di Scioscio, V.; Santini, D.; Pantaleo, M.; Biasco, G.; Caletti, G.; Pinna, A. Laparoscopic treatment of gastric GIST: report of 21 cases and literature’s review. J Gastrointest Surg. 2008, 12, 561–568. [Google Scholar] [CrossRef]
- Novitsky, Y.W.; Kercher, K.W.; Sing, R.F.D.O.; Todd Heniford, B. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006, 243, 738–745. [Google Scholar] [CrossRef] [PubMed]
- DeMatteo, R.P.; Lewis, J.J.; Leung, D.; et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000, 231, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, D.C.; Ciucu, A.A.; Rabinca, A.A.; et al. An integrative medical perspective on novel dopamine detection. Rev Chim. (Bucharest) 2018, 69, 277–281. [Google Scholar] [CrossRef]
- Hainarosie, R.; Zainea, V.; Hainarosie, M.; et al. Methylene Blue Test in Assessing Disease Free Margins in Lingual Carcinoma Resection. Rev Chim. (Bucharest). 2017, 68, 2879–2880. [Google Scholar] [CrossRef]
- Ciocirlan, M.; Draghia, L.; Manuc, D.; et al. Nutritional status of patients with digestive cancers. Conference: 3rd International Conference on Interdisciplinary Management of Diabetes Mellitus and its Complications (INTERDIAB) Location: Bucharest, ROMANIA Date: MAR 02-04, 2017Sponsor(s): Assoc Renal Metab & Nutrit Studies; AstraZeneca Diabetes; MSD Diabetes; novo nordisk; SANOFI INTERDIAB 2017: DIABETES MELLITUS IN INTERNAL MEDICINE Book Series: International Conference on Interdisciplinary Management of Diabetes Mellitus and its Complications. 2017, 132–138.
- Miettinen, M.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005, 29, 52–68. [Google Scholar] [CrossRef]
- Poesina, N.D.; Bălălău, C.; Bârcă, M.; Ion, I.; Baconi, D.; Baston, C.; Băran Poesina, V. Testicular histopathological changes following sodium fluoride administration in mice. Rom J Morphol Embryol. 2013, 54, 1019–1024. [Google Scholar]
- Novitsky, Y.W.; Kercher, K.W.; Sing, R.F.; Heniford, B.T. Long-term Outcomes of Laparoscopic Resection of Gastric Gastrointestinal Stromal Tumors. Ann Surg. 2006, 243, 738–747. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Nakanishi, Y.; Yoshimura, K.; et al. Clinicopathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer. 2003, 6, 39–48. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. 2019 Iulian Slavu, Lucian Alecu, Adrian Tulin, Dana L. Stanculeanu, Cornelia Nitipir
Share and Cite
Slavu, I.; Alecu, L.; Tulin, A.; Stanculeanu, D.L.; Nitipir, C. Laparoscopic Resection of Gastric GISTs. Where Do We Stand Now? A Single-Centered Experience. J. Mind Med. Sci. 2019, 6, 334-339. https://doi.org/10.22543/7674.62.P334339
Slavu I, Alecu L, Tulin A, Stanculeanu DL, Nitipir C. Laparoscopic Resection of Gastric GISTs. Where Do We Stand Now? A Single-Centered Experience. Journal of Mind and Medical Sciences. 2019; 6(2):334-339. https://doi.org/10.22543/7674.62.P334339
Chicago/Turabian StyleSlavu, Iulian, Lucian Alecu, Adrian Tulin, Dana L. Stanculeanu, and Cornelia Nitipir. 2019. "Laparoscopic Resection of Gastric GISTs. Where Do We Stand Now? A Single-Centered Experience" Journal of Mind and Medical Sciences 6, no. 2: 334-339. https://doi.org/10.22543/7674.62.P334339
APA StyleSlavu, I., Alecu, L., Tulin, A., Stanculeanu, D. L., & Nitipir, C. (2019). Laparoscopic Resection of Gastric GISTs. Where Do We Stand Now? A Single-Centered Experience. Journal of Mind and Medical Sciences, 6(2), 334-339. https://doi.org/10.22543/7674.62.P334339


